Photochromism of a spirooxazine in the single crystalline phase†
Dinesh G.
Patel
b,
Jason B.
Benedict
b,
Roni A.
Kopelman
b and
Natia L.
Frank
*a
aDepartment of Chemistry, PO Box 3065, University of Victoria, Victoria, BC V8W 3V6, Canada. E-mail: nlfrank@uvic.ca; Fax: (+1) 250-721-7149; Tel: (+1) 250-721-7154
bDepartment of Chemistry, Box 351700, University of Washington, Seattle, WA 98195-1700, USA
First published on 29th March 2005
Abstract
The single crystals of a closed form spirooxazine spiro[azahomoadamantane-isoquinolinoxazine] were found for the first time to undergo photocoloration processes consistent with photochromism in the single crystalline phase.
Photochromic materials have potential applications in destructive and non-destructive optical data storage that arise from the dramatic change in absorption spectra associated with photoisomerization processes.1–3 The optically-driven changes in electronic structure associated with photochromism can be coupled with emission processes,4 optical rotation,5 or magnetic properties,6 giving rise to non-destructive read–write materials. Challenges associated with incorporating photochromic materials into optical storage technologies arise from creating thermally irreversible photochromic systems that are capable of photoisomerization in the solid state or polymer films. While several classes of photochromic compounds have been reported, compounds that exhibit photochromic activity in the single crystalline phase are extremely rare.7–9 Diarylethenes have been shown to exhibit photochemically reversible isomerization in the single crystalline phase with very small changes in volume dictated by the “reaction cavity”.9 The investigation of solid state photochemical systems which undergo significant volume changes upon photoisomerization is therefore of great interest toward understanding the structural and energetic requirements for solid-state reactivity.
Spirooxazines and spiropyrans form a class of photochromes that undergo reversible photoinduced isomerization in solution from a colorless closed spirooxazine form (SO) with λmax ∼ 350 nm to a colored photomerocyanine form (PMC) (Scheme 1).10–12 Delocalization of the π system in the PMC form leads to a bathochromic shift in the absorption spectra to λmax ∼ 600 nm. Investigations into spirooxazine photochromism in confined environments have led to the prediction that spiroooxazines are not likely to undergo photoisomerization in severely constrained environments due to the large volume changes associated with isomerization.13,14 Herein we report evidence to support the photochemically reversible, thermally irreversible isomerization of a spirooxazine in the single crystalline phase obtained from spiro[azahomoadamantane-isoquinolinoxazine] (1).
Compound 1 was synthesized as an equilibrium mixture of 1a and 1b by base-promoted condensation of 7-hydroxy-8-nitrosoisoquinoline with 5-methyl-4-azahomoadamantyl-4-enium iodide15 in methylene chloride to give a purple microcrystalline solid. The thermal equilibrium state in solution (KT = [PMC]/[SO]) lies toward the open form (KT = 0.17) in acetonitrile at 298 K relative to parent spirooxazines in which KT is typically 0.001.16,17 Steady-state UV irradiation of 1 in solution (280 nm < λ < 400 nm) at 298 K leads to an increase in absorption at λmax = 564 nm, consistent with ring opening to the photomerocyanine form (1b) (Fig. 1). The colorability of 1, defined here as the difference between the photostationary state and the thermal equilibrium state (ΔK = KUV − KT = 9.0), is extremely large relative to that of the parent spirooxazines (ΔK ∼ 0.02) 18 in CH3CN at 298 K. Thermal relaxation to the equilibrium state occurs with a rate constant three orders of magnitude slower (kT = 3.34 × 10−4 s−1) than that of the parent spirooxazine spiro[indoline-naphthoxazine] (kT = 5.02 × 10−1 s−1)19 in CH3CN at 298 K, consistent with a stabilization of the PMC form. The solution photochemical and thermal behavior of 1a is therefore characteristic of the larger family of spirooxazines and spiropyrans with a significant enhancement in the colorability and stabilization of the photomerocyanine form.
 | ||
| Scheme 1 Photochromism of 1. | ||
 | ||
| Fig. 1 Time-resolved spectra of steady state UV irradiation (λexc 250–400 nm) of 1 in acetonitrile (4 × 10−5 M at 298 K). | ||
The effect of constrained media on the photoisomerization and thermal relaxation behavior of 1 was investigated in both polymer films and the microcrystalline state. A polystyrene thin film of 1 (2.1% w/w) cast from a dichloromethane solution exhibited intense coloration upon irradiation with an absorption band at λmax = 578 nm, a bathochromic shift of 14 nm relative to 1 in acetonitrile. In addition, a shoulder appeared on the blue edge of the band due to either PMC aggregation (H aggregate) or formation of an alternative isomeric PMC form, both of which have been proposed to form in polymer films.10 Similar to other spirooxazines, the thermal back reaction of 1 in polystyrene was fit to a biexponential function with rate constants k1 = 2.28 × 10−4 s−1 and k2 = 2.15 × 10−3 s−1 at 298 K indicating multiple pathways for reversion to the SO form. In addition, irradiation of 1 in the microcrystalline state leads to coloration followed by thermal reversion on the order of hours upon removal of the light source. Thus, the photoisomerization and thermal relaxation behavior of 1 is consistent with other spirooxazines in partially constrained environments.20–22
X-ray quality single crystals of 1a were obtained by recrystallization from hexane to give colorless monoclinic P21/c prisms.23 The closed form (SO) crystallizes with four molecules in the unit cell in pairs of head-to-tail dimers that sit orthogonal to each other in the crystal lattice (Fig. 2). Intermolecular π–π contacts within each dimer are weak, with an intermolecular mean plane distance of 3.818 Å. Analysis of bond lengths and angles reveals little deviation from typical bond lengths and angles of other spirooxazines.15,24–26
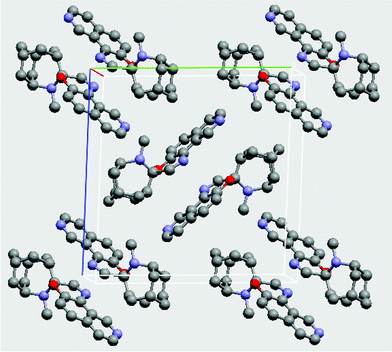 | ||
| Fig. 2 Packing diagram of spirooxazine 1 as viewed down the a-axis. | ||
Steady state UV irradiation with an Hg-arc lamp of colorless single crystals leads to coloration with no loss of transparency or crystalline order, as determined by X-ray diffraction experiments. In the absence of light at 298 K, the coloration of the single crystal does not decay, indicating that the stability of the photomerocyanine form in the single crystalline phase is extremely high. On the other hand, pulverized single crystals decolorize within hours, indicating that while in the microcrystalline state thermal reversion to the closed form occurs rapidly, consistent with other studies of spirooxazine photoisomerization in the microcrystalline state.13,20,21 The increased stability of the photomerocyanine form in the crystalline state suggests that photoisomerization is occurring in the single crystalline phase and is not simply a surface effect.27 Steady state visible irradiation (λex > 520 nm) of photocolored single crystals leads to reversion and rapid decoloration in seconds to regenerate colorless crystals of 1a (kdec ∼ 10−1 s−1). The cycle of photoisomerization is photochemically reversible, thermally irreversible, and fatigue resistant, as evidenced by numerous cycles of irradiation (> 10 cycles).
In order to determine the extent of alignment of the photocolored species in the crystalline state, a single crystal of 1a was irradiated with the UV light from a Kr–Ar mixed-gas ion laser for two hours (λex = 333–363 nm) and its absorption band measured as a function of the direction of polarized light before and after UV irradiation. The single crystal was rotated with respect to the polarizer until complete extinction was observed at 45° relative to [100] in (011). This direction was defined as 0° with respect to the polarizer. A very slight change in the intensity of the PMC band was detected as the crystal was rotated with respect to the direction of polarized light (Fig. 3). Thus, polarized absorption spectra of the spirooxazine crystal before and after UV irradiation suggest little anisotropy at polarizer angles of 0° and 90°. This could in principle be due either to a photocolored disordered phase or to head-to-tail packing of 1 in the crystal lattice. The direction of the spirooxazine molecular dipole is not expected to change appreciably in direction after photoisomerization, independent of the conformation of the PMC form. The latter effect would then lead to a cancelling of molecular dipoles and little difference in intensity with polarizer angle, as is observed. Similar results were obtained for all faces of the crystal.
![Polarized absorption spectra of spirooxazine 1 before UV irradiation with the polarizer at 0°
(red circles) and 90°
(black circles), and after irradiation with the polarizer at 0°
(purple squares) and 90°
(blue squares) relative to the [011] plane.](/image/article/2005/CC/b417026a/b417026a-f3.gif) | ||
| Fig. 3 Polarized absorption spectra of spirooxazine 1 before UV irradiation with the polarizer at 0° (red circles) and 90° (black circles), and after irradiation with the polarizer at 0° (purple squares) and 90° (blue squares) relative to the [011] plane. | ||
In an attempt to determine the structure of the photogenerated form, a full X-ray data set of 1 was obtained before and after irradiation. The difference Fourier electron density map, defined as the electron density of an irradiated (UV light) single crystal of 1 minus the electron density of a non-irradiated single crystal of 1, was generated and visualized with the Maxus program (Fig. 4). The electron density difference map exhibits small changes in the vicinity of the azahomoadamantyl group, with little change in other regions of the molecule. This suggests that (i) either the photogenerated form is disordered, (ii) little change in structure occurs upon photoisomerization, or (iii) that the percentage of molecules undergoing conversion under these conditions is not large enough to allow full or partial structure determination. DSC experiments on irradiated crystals of 1a support the latter interpretation. The high optical density of the photocolored form (ε ∼ 30,000) may in fact preclude high conversion efficiency after initial irradiation, suggesting the need for two-photon conversion experiments.
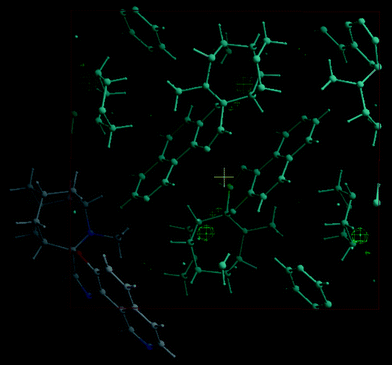 | ||
| Fig. 4 Fourier electron density difference map showing the electron density of an irradiated (UV light) single crystal of 1a minus the electron density of a non-irradiated single crystal of 1a as viewed down the a-axis. | ||
As topochemical reactions are diffusionless, sufficient space in the “reaction cavity” and sufficient flexibility in the overall crystal packing are required for spatial reorganization to occur. It is possible that in this case, the azahomoadamantyl group provides sufficient free space in the crystal lattice to allow single crystalline phase photoisomerization. Consistent with this is the observation that steady state visible irradiation of single crystals of indolyl analog, spiro[indoline-isoquinolinoxazine], does not lead to photocoloration. Both spirooxazines, however, undergo photoisomerization in the microcrystalline state and in KBr pellets with comparable rates, suggesting that microcrystalline state photoisomerization does not necessarily correlate with single crystalline behavior.
In conclusion, we have synthesized an azahomoadamantyl spirooxazine that undergoes photochemically reversible and thermally irreversible coloration in the single crystalline phase. While investigations of thermal and light-induced isomerization processes for 1 reveal behavior typical of spirooxazines in solution, the thermal reversion in the single crystal is extraordinarily slow, consistent with generation of a PMC form in a constrained environment. Reversible photocoloration in the single crystalline phase was observed only from the closed SO form of 1, and not from single crystals of other spirooxazines. Future studies will involve structural analysis of the photocolored form generated in the single crystalline phase through two-photon processes and X-ray crystallography.
This work was supported by the National Science Foundation (NSF-STC/MDITR), PRF (41492-AC3), AFOSR, and the University of Washington. We are grateful to Dr Bart Kahr for X-ray crystallographic analysis and for the use of a U-Pole spectrophotometer.
Notes and references
- R. C. Bertelson, in Photochromism, ed. G. H. Brown, Wiley-Interscience, New York, 1971 Search PubMed.
- G. H. Brown, in Photochromism, Wiley-Interscience, New York, 1971 Search PubMed.
- H. Dürr and H. Bouas-Laurent, in Photochromism: Molecules and Systems, Elsevier, New York, 1990 Search PubMed.
- A. S. Dvornikov, Y. C. Liang, C. S. Cruse and P. M. Rentzepis, J. Phys. Chem. B, 2004, 108, 8652 CrossRef CAS.
- E. Murguly, T. B. Norsten and N. R. Branda, Angew. Chem., Int. Ed., 2001, 40, 1752 CrossRef CAS.
- S. Bénard, A. Léaustic, E. Riviére, P. Yu and R. Clément, Chem. Mater., 2001, 13, 3709 CrossRef.
- M. Irie, S. Kobatake and M. Horichi, Science, 2001, 291, 1769 CrossRef CAS.
- J. R. Scheffer and P. R. Pokkuluri, in Unimolecular Photoreactions of Organic Crystals, ed. V. Ramamurthy, Wiley-VCH, New York, 1990 Search PubMed.
- S. Yamamoto, K. Matsuda and M. Irie, Angew. Chem., Int. Ed., 2003, 42, 1636 CrossRef CAS.
- G. Berkovic, V. Krongauz and V. Weiss, Chem. Rev., 2000, 100, 1741 CrossRef CAS.
- A. K. Chibisov and H. Gorner, J. Phys. Chem. A, 1999, 102, 5211 CrossRef CAS.
- N. Y. C. Chu, in 4n + 2 Systems: Spirooxazines, ed. H. B.-L. Durr, Elsevier, Amsterdam, 1990 Search PubMed.
- M. Suzuki, T. Asahi, K. Takahashi and H. Masuhara, Chem. Phys. Lett., 2003, 368, 384 CrossRef CAS.
- N. Tamai and H. Miyasaka, Chem. Rev., 2000, 100, 1875 CrossRef CAS.
- K. Chamontin, V. Lokshin, A. Samat, R. Guglielmetti, R. Dubest and J. Aubard, Dyes Pigments, 1999, 43, 119 CrossRef CAS.
- A. V. Metelitsa, J. C. Micheau, N. A. Voloshin, E. N. Voloshina and V. I. Minkin, J. Phys. Chem. A, 2001, 105, 8417 CrossRef CAS.
- S. Maeda, in Spirooxazines, ed. J. C. Crano and R. Gugliemetti, Plenum Press, New York, 1999 Search PubMed.
- G. Favaro, V. Malatesta, U. Mazzucato, G. Ottavi and A. Roamni, J. Photochem. Photobiol. A, 1995, 87, 235 CrossRef CAS.
- A. Kellmann, F. Tfibel and R. Guglielmetti, J. Photochem. Photobiol. A, 1995, 91, 131 CrossRef CAS.
- S. Bénard and Y. Pei, Adv. Mater., 2000, 12, 48 CrossRef CAS.
- Y. R. Yi and I. J. Lee, J. Photochem. Photobiol. A, 2002, 151, 89 CrossRef CAS.
- S. Bénard and Y. Pei, Chem. Commun., 2000, 65 RSC.
- C21H23N3O M = 333.42, a = 6.6340(2), b = 15.3270(4), c = 16.2050 (6) Å, U = 1619.82(9) Å3, space group P21/c (No. 14), Z = 4, Wavelength = 0.71073 Å, 4354 relections measured, 2381 unique (Rint = 0.0259) which were used in all calculations. The final wR(F2) was 0.1038 (all data). CCDC 256171. See http://www.rsc.org/suppdata/cc/b4/b417026a/ for crystallographic data in CIF or other electronic format.
- W. Clegg, N. C. Norman, T. Flood, L. Sallans, W. S. Kwak, P. L. Kwiatkowski and J. G. Lasch, Acta Crystallogr., Sect. C, 1991, 47, 817 CrossRef.
- R. Millini, G. del Piero, P. Allegrini, L. Crisci and V. Malatesta, Acta Crystallogr., Sect. C, 1991, 47, 2567 CrossRef.
- J.-P. Reboul, A. Samat, P. Lareginie, V. Lokshin and R. Guglielmetti, Acta Crystallogr., Sect. C, 1995, 51, 1614 CrossRef.
- M. Suzuki, T. Asahi and H. Masuhara, Phys. Chem. Chem. Phys., 2002, 4, 185 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic data and spectroscopy of compound 1. See http://www.rsc.org/suppdata/cc/b4/b417026a/ |
| This journal is © The Royal Society of Chemistry 2005 |
