Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and magnetic characterisation of Fe1−xMgxSb2O4 (x = 0.25, 0.50, 0.75) and their oxygen-excess derivatives, Fe1−xMgxSb2O4+y
Benjamin P.
de Laune
a,
Mariana J.
Whitaker
a,
Jose F.
Marco
b,
Michael F.
Thomas
c,
Frank J.
Berry
a,
Martin R.
Lees
 d and
Colin
Greaves
d and
Colin
Greaves
 *a
*a
aSchool of Chemistry, University of Birmingham, Birmingham B15 2TT, UK. E-mail: c.greaves@bham.ac.uk
bInstituto de Quimica Fisica “Rocasolano”, CSIC, Serrano 119, 28006 Madrid, Spain
cDepartment of Physics, University of Liverpool, Liverpool L69 3BX, UK
dDepartment of Physics, University of Warwick, Coventry, CV4 7AL, UK
First published on 5th May 2017
Abstract
Three new materials of composition Fe1−xMgxSb2O4 (x = 0.25, 0.50, 0.75) with the tetragonal schafarzikite structure have been synthesised. Magnetic susceptibility measurements suggest that Fe1−xMgxSb2O4 (x = 0.25, 0.50) are canted antiferromagnets whilst Fe0.25Mg0.75Sb2O4 is paramagnetic. The magnetic ordering temperatures decrease as the Mg2+ concentration increases. The materials form oxygen-excess phases when heated in oxygen-rich atmospheres at temperatures of ∼350 °C. 57Fe Mössbauer spectroscopy shows that the oxidation process involves the oxidation of Fe2+ to Fe3+. Powder neutron diffraction confirms the location of the excess oxygen within the structural channels and reveals a change in magnetic order at low temperatures from A-type (magnetic moments along 〈100〉) for Fe1−xMgxSb2O4 to C-type (magnetic moments along [001]) for the oxidised materials. The change is attributed to a weakening of the antiferromagnetic exchange interactions between edge-linked FeO6 octahedra for the Fe3+-containing materials.
Introduction
The compound FeSb2O4 occurs naturally as the mineral schafarzikite and is isostructural with the tetragonal form of Pb3O4.1 Its structure,2 shown in Fig. 1, consists of chains of edge-linked FeO6 octahedra along the c-axis; the chains are linked by trigonal pyramidal Sb3+ cations which are bonded to three oxide ions and possess lone pairs of electrons (E) directed into the empty channels that exist between the chains of FeO6 octahedra. If the lone pairs are regarded as ligands, they provide pseudo-tetrahedral SbO3E geometry for the Sb3+ ions. The Fe–Fe distance within the chains (2.96 Å) is shorter than the nearest Fe–Fe distance perpendicular to the chains (6.07 Å) and provides some one-dimensional character to FeSb2O4. The material undergoes an antiferromagnetic transition around 45 K.3,4 A variety of structurally-related MSb2O4 (M = Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Mg2+) have been reported.5–9One unsuccessful attempt to substitute on the Sb site has been reported,10 but we subsequently demonstrated that this is possible by successfully synthesising and characterising materials with composition FeSb2−xPbxO4.11 We have also prepared new phases that contain mixed magnetic cations on the M site and determined their magnetic properties.12 Two principal types of antiferromagnetic order are observed for magnetic MSb2O4 compounds: A-type and C-type13 with the magnetic moments aligned as shown in Fig. 2. Only a small energy difference separates these types of order, and subtle changes to the existing magnetic exchange interactions can change the magnetic ground state. Low temperature powder neutron diffraction (PND) experiments, for example, revealed that the A-type magnetic ground state found for both FeSb2O4 and MnSb2O4 is gradually converted to C-type with increasing Co2+ substitution in the series with compositions Fe1−xCoxSb2O4 and Mn1−xCoxSb2O4.12 In addition, the A-type order in FeSb2O4 can also be converted to C-type by partial oxidation of Fe2+ to Fe3+ which occurs in FeSb2−xPbxO4.11 Of particular interest is the ferromagnetic ground state observed below ∼7.4 K in the isostructural CuAs2O4,14 where both intrachain and interchain magnetic exchange interactions are ferromagnetic. The synthesis and low temperature behaviour of highly conducting ferromagnetic materials of this type would be extremely interesting and a better understanding, initially, of how to create strong intrachain ferromagnetism would be beneficial. Although FeSb2−xPbxO4 has chain ferromagnetism,11 it is not clear to what extent this relates to the formation of Fe3+ since the large Pb2+ ion causes significant structural changes. Indeed, the Fe3+-containing material FePbBiO4 has A-type magnetic order.15
 | ||
| Fig. 2 The common types of magnetic order observed for magnetic MSb2O4 phases: (a) A-type, (b) C-type. | ||
The 57Fe Mössbauer spectrum of FeSb2O4 at ca. 4.2 K is unusual, being the result of combined magnetic hyperfine and electric quadrupole interactions of comparable strength;16,17 combining this spectral information with data recorded above TN has been used to determine the splitting of the 5T2g ground state of Fe2+ caused by its distorted octahedral environment.18 We reinvestigated the Mössbauer spectra recorded from FeSb2O4 and reported on how, in FeSb2−xPbxO4, the anisotropic magnetic interactions give rise to situations in which weakly coupled Fe2+ ions appear to coexist in a non-magnetic state alongside Fe3+ ions in a magnetically ordered state.19
We report here an investigation of the nature of the transition from A- to C-type magnetic order by exploring materials in which the overall magnetic exchange is weakened by the substitution of non-magnetic Mg2+ ions on the Fe sites to form Fe1−xMgxSb2O4; in particular, we explored whether this caused a change in the magnetic order. A particularly interesting feature of Fe2+-containing materials is their ability to undergo partial oxidation associated with the accommodation of additional oxide ions within the channels and bonded to Sb ions20 (see Fig. 1). In this report, we highlight topotactic oxidation of this type for the new Fe1−xMgxSb2O4 compounds. We report on the low-temperature magnetic properties of the oxidised phases Fe1−xMgxSb2O4+y revealed by magnetic susceptibility measurements, 57Fe Mössbauer spectroscopy and PND to determine whether oxidation of this type also causes a change in magnetic order, as was observed for FeSb2−xPbxO4.11 This is important to determine the extent to which the presence of Fe3+ influences the magnetic order, since the framework structure suffers little change compared with the substitution of Pb2+ ions on the Sb3+ sites previously reported.11
Experimental details
All Fe1−xMgxSb2O4 samples were prepared by heating stoichiometric amounts of Fe2O3, MgO, Sb2O3 and Sb metal in alumina crucibles contained within evacuated sealed silica tubes. Samples were heated (700 °C for x = 0, 650 °C for all other samples) two or three times with intermediate grinding to obtain single phase products. Oxygen insertion to form materials of the type Fe1−xMgxSb2O4+y was studied by heating samples in oxygen in a Netzsch STA 449 F1 Jupiter thermogravimetric analyser; larger samples were obtained by heating Fe1−xMgxSb2O4 in air at 400 °C for 10 min.Powder X-ray diffraction (PXD) data were collected on a Bruker D8 diffractometer in transmission mode using Cu-Kα1 radiation selected using a primary beam germanium monochromator. PND data were collected on the high resolution D2B diffractometer at ILL, Grenoble, France. PXD data from the samples examined were used to calibrate the neutron wavelength and 1.5923 Å was adopted for all work reported here. Rietveld refinement of PXD and PND data used the General Structure Analysis System (GSAS)21 in conjunction with the Graphical User Interface, EXPGUI.22 Magnetic refinements for reduced and oxidised materials used Fe2+ and Fe3+ magnetic form factors, respectively. Magnetic susceptibility data were measured with a MPMS Quantum Design XL instrument (applied field 500 Oe) for samples during warming after both field cooled (FC) and zero field cooled (ZFC) cooling to 5 K. Scanning electron microscope (SEM) images were recorded from unpolished sintered pellets using a JEOL 6060 microscope and an acceleration voltage of 20 kV. 57Fe Mössbauer spectra were recorded in constant acceleration mode using a ca. 25 mCi 57Co source and a liquid helium closed-cycle cryorefrigerator. All the spectra were computer fitted with those recorded from Mg0.50Fe0.50Sb2O4 and Mg0.25Fe0.75Sb2O4 at 16 K being fitted using the method of Kundig.23 All the chemical isomer shift data are quoted relative to metallic iron at room temperature.
Results and discussion
Structural characterisation of Fe1−xMgxSb2O4 (x = 0.25, 0.50, 0.75)
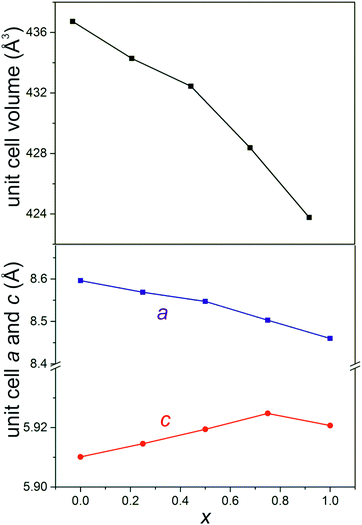
SEM images showed that materials of composition Fe1−xMgxSb2O4 (x = 0.25, 0.50, 0.75) had acicular crystals (Fig. 3) and energy-dispersive X-ray spectroscopy confirmed the cation ratios to be as expected for the desired compositions. FeSb2O4 and MgSb2O4 showed more uniform crystal shapes.PXD showed that materials with composition Fe1−xMgxSb2O4 (x = 0.25, 0.50, 0.75) were single phase with the tetragonal schafarzikite structure but with anisotropic broadening of some diffraction peaks consistent with the needle-like morphology revealed by SEM with the unique axis being along [001]. The parameters from the Rietveld refinement of the PXD data recorded from the Fe1−xMgxSb2O4 materials at 300 K are collected in Table 1 and the refinement plot for Fe0.50Mg0.50Sb2O4 is shown in Fig. 4. In Table 1, O1 corresponds to the apical site and O2 is equatorial. The fractional coordinates reported in Table 1 are close to those expected for a situation in which increasing amounts of magnesium substitute for iron on the octahedral sites. As expected from the crystal morphologies observed from SEM, preferred orientation was not apparent for FeSb2O4 and MgSb2O4; however, for the mixed iron–magnesium phases, a significant preferred orientation correction was necessary and was modelled using the March–Dollase correction in GSAS.21 Although the displacement parameters for Sb and O1 are normally anisotropic for this structure, the PXD data were insensitive to this and a common isotropic displacement parameter was adopted for all ions. The variations of unit cell parameters and volume with composition in Fe1−xMgxSb2O4 are shown in Fig. 5. The results show that increasing magnesium content causes the unit cell volume to decrease in accordance with the smaller ionic radius of 6-coordinate Mg2+ (0.72 Å) compared with Fe2+ (0.78 Å).24Fig. 5 demonstrates that the overall contraction relates to a decrease in the a and b unit cell parameters, whereas c shows less change but actually increases as Mg is substituted for Fe in FeSb2O4. At first sight, this seems unusual since both apical and equatorial Fe(Mg)–O bond distances decrease similarly: for x = 0 and x = 1, for example, both decrease by ∼2.5%. We attribute this slight expansion in c to the unfavourable increase in Fe(Mg)−Fe(Mg) repulsions across the common edge of adjacent octahedra that would result if the decrease in bond lengths caused a simple contraction in c. Instead, the octahedra accommodate the shortened equatorial bonds by reducing the O2–Fe(Mg)–O2 bond angle, where the two O2 atoms form a common edge between two adjacent octahedra. In this way, the c parameter, and hence the dependent cation repulsions along the chains, are maintained. For example, the angles are 90.5° and 87.9° for x = 0 and x = 1, respectively.
| FeSb2O4 | Fe0.75Mg0.25Sb2O4 | Fe0.50Mg0.50Sb2O4 | Fe0.25Mg0.75Sb2O4 | MgSb2O4 | ||
|---|---|---|---|---|---|---|
| a (Å) | 8.59621(7) | 8.5689(1) | 8.5472(2) | 8.5031(1) | 8.4602(1) | |
| c (Å) | 5.91014(5) | 5.91455(7) | 5.91942(9) | 5.92477(7) | 5.92069(9) | |
| Volume (Å3) | 436.729(9) | 434.28(2) | 432.44(2) | 428.38(2) | 423.77(2) | |
| Sb | x | 0.1763(2) | 0.1773(3) | 0.1765(3) | 0.1762(3) | 0.1753(3) |
| y | 0.1660(2) | 0.1654(3) | 0.1656(3) | 0.1648(3) | 0.1648(3) | |
| O1 | x | 0.6793(9) | 0.686(1) | 0.682(1) | 0.678(1) | 0.680(1) |
| y | 0.1793(9) | 0.186(1) | 0.182(1) | 0.178(1) | 0.180(1) | |
| O2 | x | 0.100(1) | 0.093(6) | 0.100(2) | 0.101(2) | 0.100(2) |
| y | 0.644(1) | 0.632(2) | 0.628(2) | 0.633(2) | 0.635(2) | |
| U iso (Å2) | 0.0199(4) | 0.0121(7) | 0.0126(6) | 0.0076(6) | 0.0095(6) | |
| Fe/Mg occupancy | — | 0.73(1)/0.27(1) | 0.57(1)/0.43(1) | 0.29(1)/0.71(1) | — | |
| χ 2 | 2.763 | 1.490 | 1.292 | 1.884 | 1.963 | |
| R wp | 0.047 | 0.049 | 0.048 | 0.064 | 0.077 | |
| R F 2 | 0.041 | 0.078 | 0.063 | 0.052 | 0.060 | |
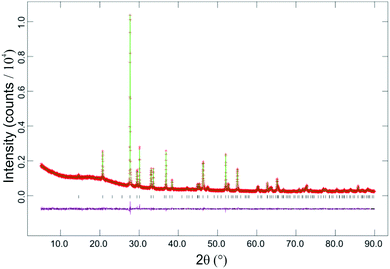 | ||
| Fig. 4 Plots for Rietveld structure refinement against PXD data recorded at 300 K from Fe0.50Mg0.50Sb2O4. Observed data are red crosses, calculated green line and difference plot is magenta. | ||
Oxygen uptake in Fe1−xMgxSb2O4 (x = 0.25, 0.50, 0.75)
The materials of composition Fe1−xMgxSb2O4 (x = 0.25, 0.50, 0.75) were found to incorporate oxygen into the empty channels on low temperature oxidation in oxygen or air, in similar fashion to that which we recently reported for other schafarzikite-related materials.20 Thermogravimetric analysis of samples containing both Fe and Mg under flowing oxygen is shown in Fig. 6. The compounds showed topotactic uptake of oxygen in the approximate temperature range 300–500 °C before further oxidation at higher temperatures resulted in decomposition to form Sb2O4, MgSb2O6 and FeSbO4. The properties of the materials formed at the low temperature plateaus were determined from samples prepared by heating the Fe1−xMgxSb2O4 phases at 400 °C for 10 min. under flowing oxygen. PXD confirmed the products to have retained the schafarzikite-related structure (Fig. 7) and that Fe0.25Mg0.75Sb2O4+y, for which the value of y is smallest (see Fig. 6), is most crystalline (smallest peak widths) and gave the smallest shifts in peak positions consistent with the sample having the lowest oxygen uptake. In contrast, the iron-rich materials Fe1−xMgxSb2O4 (x = 0.25, 0.50) gave samples which showed greater shifts in peak position and a lower degree of crystallinity. Rietveld refinement of the PXD data showed the materials to consist of two (or possibly more) schafarzikite-related phases whose unit cell sizes suggested that the phases had different levels of oxygen absorption. One phase was always closely related to the initial, unoxidised material, and suggested that for these low temperature oxidations, kinetic factors limited the potential to oxidise the centres of large particles. Attempts to produce single phase materials of composition Fe1−xMgxSb2O4+y by varying the heating conditions were unsuccessful. The observed increase in mass following heating of Fe1−xMgxSb2O4 at 400 °C for 10 min. exceeded that expected from simple oxidation of Fe2+ to Fe3+ (Table 2). It is seen, for example, that for x = 0.25 a mass increase of 2.3(1)% occurred which corresponds to a final oxygen content of 4.51(3), whereas oxidation of all Fe2+ to Fe3+ would provide a value of only 4.375. The amount of oxygen absorbed decreases as x increases, i.e. as the Fe2+ concentration decreases. This has previously been discussed in detail20 and the enhanced oxygen uptake occurs because of the simultaneous oxidation of both Fe2+ to Fe3+ and Sb3+ to Sb5+.| x | Mass increase (%) | y | Expected y for 100% oxidation of Fe2+ to Fe3+ |
|---|---|---|---|
| 0.25 | 2.3(1) | 0.51(3) | 0.375 |
| 0.50 | 1.8(1) | 0.40(2) | 0.25 |
| 0.75 | 0.98(7) | 0.21(1) | 0.125 |
Magnetic properties of Fe1−xMgxSb2O4 (x = 0.25, 0.50, 0.75)
The magnetic susceptibility measurements recorded between 5 and 300 K for Fe1−xMgxSb2O4 (x = 0.25, 0.50, 0.75) are compared with FeSb2O4 (x = 0) in Fig. 8. The latter compound shows an antiferromagnetic transition at ∼45 K as previously reported.3,4 The magnetic order has previously been reported to be complex with predominantly A-type order but with a minor C-type contribution;3 however, for the sample prepared in this study a significant ferromagnetic contribution is seen, which suggests a canted ground state. For x = 0.25 and 0.50, a low temperature divergence of FC and ZFC data at low temperatures is also indicative of canted antiferromagnetic order, whereas the magnesium-rich Fe0.25Mg0.75Sb2O4 appears paramagnetic. The inverse susceptibility data (Fig. 8) all obeyed the Curie–Weiss law and the Weiss constants, effective magnetic moments and magnetic ordering temperatures (TN) are collated in Table 3. The values of TN correspond to the low temperature maximum in the ZFC data (x = 0.25) and the onset of significant divergence between ZFC and FC data (x = 0.50). The negative Weiss constants are indicative of predominantly antiferromagnetic exchange and the canted configuration for x ≤ 0.50 is similar to that observed in FeSb2−xPbxO4.11 The effective magnetic moments determined for all four materials are seen to be significantly larger than the spin-only value for high-spin Fe2+ ions, 4.9 μB. However, they are similar to those expected for octahedral Fe2+ ions, where the 5T2g ground state results in a significant orbital contribution to the moment, and agree well, for example, with the range reported for Fe2+ substituted into MgO: 5.5–5.7 μB.25 It should be noted that the coordination around Fe is distorted in the compounds under investigation, so that comparisons can only be approximate. The magnetic ordering temperatures decrease with increasing magnesium content, consistent with the Fe2+–Fe2+ magnetic exchange interactions being weakened by the presence of non-magnetic Mg2+ ions. The change in the Weiss constant also reflects the weakening of magnetic exchange as x increases but |θ| is always significantly larger than the ordering temperatures. This has previously been observed in related materials12 and is commonly observed for antiferromagnetic order when the mean field of each magnetic sublattice is not solely determined by the other sublattice.| x | θ/K | μ eff per Fe/μB | T N/K |
|---|---|---|---|
| 0.0 | −131(2) | 6.3(1) | 45(5) |
| 0.25 | −92(2) | 5.9(1) | 45(5) |
| 0.50 | −60(2) | 5.8(1) | 20(2) |
| 0.75 | −25(1) | 5.5(1) | — |
In order to explore the nature of the magnetic order in Fe1−xMgxSb2O4, PND data were collected at 300 and 4 K for Fe0.5Mg0.5Sb2O4. The observed and calculated profiles for 300 K data are shown in Fig. 9(a) and, at first sight, the data collected at 4 K (Fig. 9(b)) appeared similar; the figure shows only the low angle range since we are primarily concerned with the effects of magnetic order. However, it is seen (Fig. 9(b)) that refinement using a single nuclear phase consistent with the 300 K structure produced anomalies at low angle which suggested magnetic order. The scattering is, in fact, fitted well (Fig. 9(c)) by including an A-type magnetic phase shown in Fig. 2(a). The refined magnetic moment of 2.9(1) μB is significantly lower than the spin-only value of 4 μB; although covalence reduces the moment, the large orbital contribution will increase it. For example, in LiFePO4, the Fe2+ moment is reported to be 4.15(3) μB from PND.26 The reduction in magnetic moment therefore suggests incomplete magnetic order, presumably resulting from the weaker overall exchange caused by the Mg substitution. In fact, the moment suggests approximately 20–30% paramagnetic Fe2+ ions and it may be relevant that a random arrangement of Fe and Mg would result in 25% of Fe2+ ions having Mg2+ for both its nearest neighbours which may result in the disappearance of magnetic order for this site.
57Fe Mössbauer spectra were recorded at 300 and 16 K for samples with x = 0.25, 0.50, 0.75. Spectral parameters for original and oxidised samples of composition Fe1−xMgxSb2O4 (x = 0.50, 0.75) at 16 K are contained in Table 4, those from Fe0.75Mg0.25Sb2O4 at 300 and 16 K are collected in Table 5.
| δ ± 0.02 (mm s−1) | Δ ± 0.02 (mm s−1) | B hf ± 0.5 (T) | θ (°) | ψ (°) | η | Area ± 3% | |
|---|---|---|---|---|---|---|---|
| a η is the asymmetry parameter for the electric field gradient at the nucleus; θ and ψ are the polar and azimuthal angles that specify the direction of the magnetic field with respect to the coordinates of the electric field gradient. | |||||||
| Fe0.50Mg0.50Sb2O4 | 1.26 | 2.70 | 12.1 | 38.7 | 0 | 0.25 | 22 |
| 1.23 | 2.94 | 78 | |||||
| Fe0.50Mg0.50Sb2O4+y | 0.46 | 1.46 | 20 | ||||
| 0.52 | −0.06 | 46.7 | 16 | ||||
| 0.56 | −0.12 | 42.4 | 64 | ||||
| Fe0.25Mg0.75Sb2O4 | 1.21 | 2.89 | 100 | ||||
| Fe0.25Mg0.75Sb2O4+y | 1.21 | 2.80 | 31 | ||||
| 0.41 | 0.46 | 69 | |||||
| T | δ ± 0.02 (mm s−1) | Δ or e2qQ/2 ± 0.02 (mm s−1) | B hf ± 0.5 (T) | θ (°) | ψ (°) | η | Area ± 3% |
|---|---|---|---|---|---|---|---|
| a η is the asymmetry parameter for the electric field gradient at the nucleus; θ and ψ are the polar and azimuthal angles that specify the direction of the magnetic field with respect to the coordinates of the electric field gradient. | |||||||
| 300 K | 1.08 | 2.09 | |||||
| 16 K | 1.24 | 2.85 | 17.0 | 63 | 0 | 0.25 | 35 |
| 1.24 | 2.85 | 13.7 | 54 | 0 | 0.25 | 38 | |
| 1.36 | 2.85 | 5.0 | 0.1 | 0 | 0.25 | 27 | |
The 57Fe Mossbauer spectrum recorded from Fe0.25Mg0.75Sb2O4 at 16 K (Fig. 10) shows a quadrupole split absorption characteristic of paramagnetic Fe2+. The absence of magnetic hyperfine interaction at 16 K contrasts with the spectra recorded19 from FeSb2O4 and suggests that the high concentration of Mg2+ on the cation sites along the c-axis precludes significant magnetic interactions between the Fe2+ ions because of their increased separation. The result is consistent with magnetisation data which showed no magnetic transition between 5 and 300 K (see above).
For x = 0.5, the 57Fe Mossbauer spectrum recorded at 16 K (Fig. 10 and Table 4) showed a major quadrupole split absorption corresponding to paramagnetic Fe2+ ions and a minor area component corresponding to magnetically ordered Fe2+ ions which was best fitted according to the method of Kundig.23 The material thus shows mixed magnetic phases. Magnetisation measurements indicated a transition to a magnetically ordered state at 20 K (see above and Table 3) but the transition is not clearly defined. Clearly this sample is mainly paramagnetic at 16 K, and ordering probably occurs over a substantial temperature range caused by the statistical distribution of Fe2+ and Mg2+ cations. It should be recalled that the PND data suggest some disorder even at 4 K in this material.
For x = 0.25, the 57Fe Mössbauer data at 16 K (Fig. 10 and Table 5) were significantly different from the spectra recorded from compositions with lower iron contents. The spectrum was also best fitted according to the method of Kundig23 and was composed of a broad-lined magnetically split pattern similar to that recorded19 from FeSb2O4 at 30 K and reflecting similar combined magnetic hyperfine and electric quadrupole interactions of comparable strength. The result is consistent with magnetisation measurements which indicated magnetic ordering at ca. 45 K (see above, Table 3) and demonstrates that the higher iron content facilitates such magnetic order. Interestingly, the magnetic component in the spectrum was best fitted to three sextets. The parameters of the component with the largest magnetic hyperfine field are very similar to those recorded from FeSb2O4 at 30 K.19 The chemical isomer shift and quadrupole interaction of the component with magnetic hyperfine field Bhf = 13.7 T are also similar to those of FeSb2O4, and the smaller magnetic hyperfine field probably reflects the effect of magnesium weakening the magnetic interactions. The parameters of the third component (Bhf = 5 T) are very different, and also indicate weaker magnetic interactions. The spectrum recorded at 300 K (Fig. 11 and Table 5) showed a quadrupole split absorption characteristic of paramagnetic Fe2+, similar to that recorded from FeSb2O4 at 300 K.19
Magnetic properties of Fe1−xMgxSb2O4+y (x = 0.25, 0.50, 0.75)
PXD data from the oxidised samples revealed that each comprised a mixture of closely related phases with similar structure. It was assumed that the samples were inhomogeneous with respect to the distribution of the excess oxygen, y. Magnetic susceptibility data for the samples with x = 0.25, 0.50 and 0.75 are given in Fig. 12, which shows plots of susceptibility, χ, versus T and 1/χ versus T. Relevant magnetic data deduced from the plots are presented in Table 6. As for the original samples, magnetic transitions are observed for x = 0.25 and 0.50 but not for x = 0.75. For x = 0.25, the transition is clearly to an antiferromagnetic ground state but for x = 0.50, the appearance is similar to a simple ferromagnetic transition but with a very low resultant moment. It seemed likely that this transition actually resulted in a canted antiferromagnet which was subsequently confirmed using PND (see later). Whereas the data for x = 0.25 and 0.50 conform to the Curie–Weiss law, the sample with lowest Fe concentration (x = 0.75) showed slight curvature in the 1/χ–T plot. This probably reflects a small diamagnetic effect and the susceptibility could be modelled well using a modified Curie–Weiss equation that includes a temperature-independent component, α (χ = C/(T − θ) + α); the χ–T fit obtained is included in Fig. 12(c). Table 6 shows that all the magnetic moments are slightly higher than that expected for Fe3+ (spin-only moment 5.9 μB) even though no orbital contribution is expected for octahedral Fe3+ ions. The decrease in θ as x increases (Table 6) reflects the weakening of magnetic exchange energy as the Mg content increases. It is also interesting that the Weiss constants (θ) in Table 6, although confirming overall antiferromagnetic exchange above the ordering temperature, are significantly lower than those for the samples prior to oxidation (Table 3). This was thought likely to indicate the occurrence of different magnetic order compared with the unoxidised materials. The low temperature magnetic order was therefore examined for one sample (x = 0.50) using PND data collected at 4 K even though PXD data suggested that the samples contained some degree of inhomogeneity.| x | θ/K | μ eff per Fe/μB | T N/K |
|---|---|---|---|
| 0.25 | −69(2) | 6.4(1) | 56(5) |
| 0.50 | −37(2) | 6.7(1) | 29(4) |
| 0.75 | −29(2) | 6.4(2) | — |
Structure refinements against neutron data were, in fact, found to be satisfactory using a single phase, and fits were certainly very good in the low angle region used for magnetic structure evaluation. Refinements for the x = 0.50 sample at 300 and 4 K are shown in Fig. 13 and refined structural parameters are given in Table 7. For both data sets the Mg/Fe site occupancy remained as was weighed out within experimental error and was therefore constrained at this value. The Sb and O1 displacement parameters were strongly anisotropic, as is usually observed in this structure, and were refined accordingly. To improve the precision in the interstitial oxygen (O3) occupancy, the O3 and O2 isotropic displacement parameters were constrained to be equal. It is seen that the 300 K data can be fitted satisfactorily to a single phase as is shown for the low angle region in Fig. 13(a). The excess oxygen was located within the channels in sites previously reported20 and the refined O3 position is in excellent agreement with that reported for FeSb1.25Pb0.75O4.24, for which x = 0.540(5), y = 0.417(3), z = 0.254(7). Changes in the Mg/Fe coordination environment caused by oxidation were deduced from Mg/Fe−O bond distances and angles in Fe0.50Mg0.50Sb2O4 and Fe0.50Mg0.50Sb2O4+y (from PND data at 300 K), which are given in Table 8. Reduction in the bond distances can be attributed to the smaller Fe3+ ionic radius (compared with Fe2+)24 and the apical bond to O1 undergoes a larger contraction (3.1%) than the equatorial bond to O2 (1.4%). The O2−Mg/Fe−O2 angle where the two O2 atoms form a common edge is reduced from 88.9° to 86.6° by oxidation which is caused by stretching of the octahedra in the chains and relates to an increase in c from 5.9228(1) Å to 5.9577(2) Å. This increase is caused by the enhanced cation repulsions along the chains after oxidation.20
| Atom | x | y | z | Site occupancy | 100 × Uiso/Å2 |
|---|---|---|---|---|---|
| a = 8.3895(4) (8.3766(4)) Å; c = 5.9577(2) (5.9459(2)) Å. Magnetic moment at 4 K: 2.24(5) μB. χ2 = 3.99 (6.10); Rwp = 0.049 (0.055); RF2 = 0.044 (0.049).a Anisotropic displacement parameters × 100/Å2. | |||||
| Mg/Fe | 0 | 0.5 | 0.25 | 0.5/0.5 | 1.64(7) |
| 0 | 0.5 | 0.25 | 0.5/0.5 | 1.04(6) | |
| Sb | 0.1648(6) | 0.1596(6) | 0 | 1.0 | |
| 0.1642(5) | 0.1587(5) | 0 | 1.0 | ||
| O1 | 0.6758(3) | 0.1758(3) | 0.25 | 1.0 | |
| 0.6742(3) | 0.1742(3) | 0.25 | 1.0 | ||
| O2 | 0.1027(4) | 0.6320(5) | 0 | 1.0 | 2.29(8) |
| 0.1017(4) | 0.6315(4) | 0 | 1.0 | 1.63(7) | |
| O3 | 0.551(3) | 0.415(3) | 0.245(4) | 0.098(4) | 2.29(8) |
| 0.558(2) | 0.420(2) | 0.251(3) | 0.107(3) | 1.63(7) | |
| Atom | U 11 | U 22 | U 33 | U 12 | U 13 | U 23 |
|---|---|---|---|---|---|---|
| Sb | 1.9(3) | 4.0(3) | 1.7(2) | 0.2(2) | 0 | 0 |
| 1.8(2) | 3.3(3) | 0.5(1) | 0.2(1) | 0 | 0 | |
| O1 | 5.3(2) | 5.3(2) | 1.1(1) | −3.1(2) | −0.8(2) | 0.8(2) |
| 3.6(1) | 3.6(1) | 0.5(1) | −2.0(2) | −0.4(1) | 0.4(1) | |
| Fe0.50Mg0.50Sb2O4 | Fe0.50Mg0.50Sb2O4+y | |
|---|---|---|
| Mg/Fe–O1 | 2.151(1) [×2] | 2.085(4) [×2] |
| Mg/Fe–O2 | 2.075(1) [×4] | 2.046(3) [×4] |
| O1–Mg/Fe–O2 | 83.58(4); 96.42(4) | 85.1(1); 94.9(1) |
| O2–Mg/Fe–O2 | 88.93(6); 92.50(6) | 86.6(2); 94.3(2) |
At 4 K, fitting using a non-magnetic phase shows additional peaks (Fig. 13(b)), but these are seen to be very different from those observed for the unoxidised material, Fig. 9(b). The peak at ∼11° is, in fact, characteristic of C-type magnetic order, Fig. 2(b), in which the moments are aligned along [001] with ferromagnetic order within a given chain. Including the corresponding magnetic unit cell resulted in the satisfactory fit shown in Fig. 13(c). In accordance with the mass increase on oxidation (Table 2), it was assumed that most Fe2+ had oxidised to Fe3+ and the Fe3+ magnetic form factor was adopted for the refinement. The magnetic moment per Fe3+ ion was determined to be 2.24(5) μB. The small peak seen at ∼19° corresponds to magnetic scattering from a very small (<1% by weight) contamination from Fe3O4, which is difficult to avoid in the synthesis of Fe-containing schafarzikite phases. The O3 site occupancies for the 300 and 4 K data agree well, not only with each other (indicating the compositions Mg0.50Fe0.50Sb2O4.39(2) and Mg0.50Fe0.50Sb2O4.43(1), respectively), but also with thermogravimetric data (Mg0.50Fe0.50Sb2O4.40(2)).
The magnetic structure is of interest for two main reasons:
• The magnetic ground state changes from A- to C-type on oxidation. The ferromagnetic ordering in the chains can be attributed to a reduction in the direct Fe–Fe antiferromagnetic exchange that results from the d-orbital contraction in Fe3+ and the increase in Fe–Fe separation along c.
• The moment is substantially lower than that expected for Fe3+ and is even lower than that found for the unoxidised sample (2.9(1) μB per Fe2+ ion) as discussed above. This reduction is indicative of ∼40–50% of the Fe3+ ions being magnetically disordered and paramagnetic. This aspect will be considered further during discussion of the results from Mössbauer spectroscopy.
The presence of C-type magnetic order in Fe0.50Mg0.50Sb2O4+y is consistent with the change in magnetic order observed in FeSb2−xPbxO4 where oxidation results from the substitution of Pb2+ for Sb3+, and may explain the higher magnetic moments calculated from the susceptibility data. High moments were also reported for some materials containing mixed transition metal ions (Mn/Co and Fe/Co) in this structure and this was attributed to the occurrence of small clusters of cations for which ferromagnetic order was retained above the long-range magnetic ordering temperature.12 In the present study, C-type order provides ferromagnetic order within each individual chain of octahedra, so small groups of Fe3+ cations would be expected to experience strong ferromagnetic exchange; the general disorder caused by the substitution of Mg2+ ions would weaken the antiferromagnetic exchange between the chains so some ferromagnetic clusters could exist to high temperatures and result in the slightly high magnetic moments determined from the susceptibility data for the oxidised samples, Table 6.
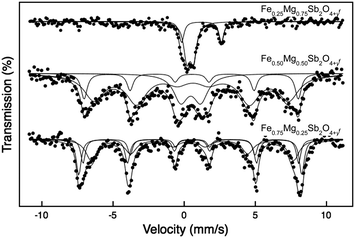
The 57Fe Mössbauer spectra recorded from Fe1−xMgxSb2O4+y at 16 K are shown in Fig. 14. The spectrum for x = 0.75 shows two quadrupole split absorptions with chemical isomer shifts characteristic of Fe2+ and Fe3+ (Table 4). The result shows that ca. 69% of the Fe2+ in Fe0.25Mg0.75Sb2O4 is oxidised to Fe3+ – presumably the remaining Fe2+ could be oxidised under different heating conditions. It is important to note that oxidation of Sb3+ to Sb5+ has been shown to occur20 simultaneously during such oxidations so that the oxygen content of the sample (y) is not directly related to the Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3+ ratio. The absence of magnetic hyperfine splitting illustrates the effective blocking by Mg2+ of magnetic interactions between Fe3+/Fe2+ ions for this composition.
Fe3+ ratio. The absence of magnetic hyperfine splitting illustrates the effective blocking by Mg2+ of magnetic interactions between Fe3+/Fe2+ ions for this composition.
The 57Fe Mössbauer spectrum recorded at 16 K Fe0.50Mg0.50Sb2O4+y (Fig. 14 and Table 4) is composed of broad lines which are best fitted to a doublet and two sextets. The chemical isomer shifts of all components are characteristic of Fe3+ and indicate complete oxidation, in contrast to the data from Fe0.25Mg0.75Sb2O4+y. It appears that the x = 0.50 material, with a higher iron content, has ca. 80% of the Fe3+ being magnetically ordered at 16 K. The different hyperfine fields for the magnetically ordered species relate to the local Fe/Mg distribution and will be discussed in detail in a subsequent publication. The presence of a significant amount of paramagnetic Fe3+ ions is therefore deduced from both PND and Mössbauer data, although the reduced magnetic moment from the PND data is suggestive of a higher percentage that does not contribute to the primary C-type order included in the refinement.
The spectrum recorded from Fe0.75Mg0.25Sb2O4+y at 300 K (Fig. 11 and Table 9) was best fitted to two quadrupole split absorptions whilst that recorded at 16 K (Fig. 14 and Table 9) was best fitted to three sextets. The chemical isomer shifts of all components are characteristic of Fe3+ and reflect the ease of oxidation of Fe2+ to Fe3+ in the Fe1−xMgxSb2O4 series. As for the x = 0.50 compound, the presence of Fe3+ species with different local environments, both structural and magnetic, can be related to the different local Fe/Mg distributions that are possible around a given Fe3+ ion.
| Temperature (K) | δ ± 0.02 (mm s−1) | Δ or e2qQ/2 ± 0.02 (mm s−1) | B hf ± 0.5 (T) | Area ± 3% |
|---|---|---|---|---|
| 300 | 0.38 | 0.97 | 33 | |
| 0.35 | 0.57 | 67 | ||
| 16 | 0.48 | −0.12 | 48.6 | 34 |
| 0.53 | −0.14 | 46.9 | 23 | |
| 0.45 | 0.26 | 43.8 | 43 | |
Conclusions
Three new phases of composition Fe1−xMgxSb2O4 (x = 0.25, 0.50, 0.75) have been synthesised and shown to adopt the schafarzikite structure. The materials with x = 0.25 and 0.50 are canted antiferromagnets with A-type magnetic order below TN, whereas for x = 0.75 paramagnetic behaviour occurs. The materials readily absorb oxygen between ca. 300 and 500 °C to an extent which exceeds that corresponding only to the simple oxidation of the Fe2+ ions. Although the oxidised materials are not strictly single phase, because of inhomogeneity of the excess oxygen concentration, their magnetic properties have been reliably determined using PND and magnetic susceptibility data. Importantly, the oxidised materials with x = 0.25 and x = 0.50 also undergo a magnetic ordering transition at low temperature but now the ground state is C-type. This confirms that for Sb-containing materials, the oxidation of Fe2+ to Fe3+ induces the presence of ferromagnetic order on the chains of octahedra (C-type order). The interpretation of the detailed features of the Mössbauer spectra recorded from magnetically ordered materials created by substitution of magnesium ions into the parent material FeSb2O4 are complex because of different local configurations of cations relating to the Fe/Mg statistical distribution.Acknowledgements
We thank the Engineering and Science Research Council for financial support of this research (EPSRC EP/L014114/1) and also acknowledge financial support from Spanish MINECO (Project MAT 2015-64110-C2-1-P). We are grateful to EPSRC, EU and ILL for the provision of PND facilities and to Dr Emma Suard for assistance in collecting the PND data. The X-ray diffractometers, TG analyser and magnetometer used in this research were obtained through Birmingham Science City: Creating and Characterising Next Generation Advanced Materials (West Midlands Centre for Advanced Materials Project 1), with support from Advantage West Midlands (AWM) and part funded by the European Regional Development Fund (ERDF). The Advanced Materials Facility is part of the Centre for Chemical and Materials Analysis in the School of Chemistry at the University of Birmingham. Data associated with the results shown in this paper are accessible from the University of Birmingham Archive: http://epapers.bham.ac.uk/2985/.References
- J. R. Gavarri and D. Weigel, J. Solid State Chem., 1975, 13, 252 CrossRef CAS.
- R. Fischer and F. Pertlik, Tschermaks Mineral. Petrogr. Mitt., 1975, 22, 236 CrossRef CAS.
- J. A. Gonzalo, D. E. Cox and G. Shirane, Phys. Rev., 1966, 147, 415 CrossRef CAS.
- R. Chater, J. R. Gavarri and A. W. Hewat, J. Solid State Chem., 1985, 60, 78 CrossRef CAS.
- E. Koyama, I. Nakai and K. Nagashima, Nippon Kagaku Kaishi, 1979, 6, 793 CrossRef.
- J. R. Gavarri, G. Calvarin and B. Chardon, J. Solid State Chem., 1983, 47, 132 CrossRef CAS.
- H. T. Witteveen, Solid State Commun., 1971, 9, 1971 CrossRef.
- S. Ståhl, Ark. Kemi, Mineral. Geol., 1943, 17B, 1 Search PubMed.
- M. T. Atanasova, A. M. Strydom, C. J. H. Schutte, L. C. Prinsloo and W. W. Focke, J. Mater. Sci., 2014, 49, 3497 CrossRef CAS.
- M. Abakumov, M. G. Rozova, E. V. Antipov, J. Hadermann, G. Van Tendeloo, M. Lobanov, M. Greenblatt, M. Croft, E. V. Tsiper, A. Llobet, K. A. Lokshin and Y. Zhao, Chem. Mater., 2005, 17, 1123 CrossRef.
- M. J. Whitaker, R. D. Bayliss, F. J. Berry and C. Greaves, J. Mater. Chem., 2011, 21, 14523 RSC.
- J. Cumby, B. P. de Laune and C. Greaves, J. Mater. Chem. C, 2016, 4, 201 RSC.
- E. O. Wollan and W. C. Koehler, Phys. Rev., 1955, 100, 545 CrossRef CAS.
- K. Caslin, R. K. Kremer, F. S. Ravazi, A. Shulz, A. Muñoz, F. Pertlik, J. Liu, M.-H. Whangbo and J. M. Law, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 014412 CrossRef.
- B. P. de Laune, F. J. Berry, J. F. Marco, S. L. Horswell and C. Greaves, J. Mater. Chem. C, 2016, 4, 5320 RSC.
- F. Varret, P. Imbert, A. Gerard and F. Hartmann-Boutron, Solid State Commun., 1968, 6, 889 CrossRef CAS.
- G. Pettit and M. R. Meder, Hyperfine Interact., 1978, 5, 323 CrossRef.
- M. Eibschutz and U. Ganiel, Solid State Commun., 1968, 6, 775 CrossRef.
- R. D. Bayliss, F. J. Berry, B. P. de Laune, C. Greaves, O. Helgason, J. F. Marco, M. F. Thomas, L. Vergara and M. J. Whitaker, J. Phys.: Condens. Matter, 2012, 24, 276001 CrossRef CAS PubMed.
- P. de Laune, G. J. Rees, M. J. Whitaker, H.-Y. Hah, C. E. Johnson, J. A. Johnson, D. E. Brown, M. G. Tucker, T. C. Hansen, F. J. Berry, J. V. Hanna and C. Greaves, Inorg. Chem., 2017, 56, 594 CrossRef PubMed.
- C. Larson and R. B. Von Dreele, General Structure Analysis System (GSAS), Los Alamos National Laboratory Report LAUR 86-748, 2004.
- H. Toby, J. Appl. Crystallogr., 2001, 34, 210 CrossRef.
- W. Kundig, Nucl. Instrum. Methods, 1967, 48, 219 CrossRef.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751 CrossRef.
- M. Valigi, F. Pepe and M. Schiavello, J. Chem. Soc., Faraday Trans., 1975, 71, 1631 RSC.
- G. Rousse, J. Rodriguez-Carvajal, S. Patoux and C. Masquelier, Chem. Mater., 2003, 15, 4082 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |