Spin-state switches in molecular materials chemistry
Shinya
Hayami
a,
Stephen M.
Holmes
b and
Malcolm A.
Halcrow
*c
aDepartment of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan. E-mail: hayami@sci.kumamoto-u.ac.jp
bDepartment of Chemistry and Biochemistry and Center for Nanoscience, University of Missouri-St. Louis, St. Louis, Missouri 63121, USA. E-mail: holmesst@umsl.edu
cSchool of Chemistry, University of Leeds, Woodhouse Lane, Leeds, UK LS2 9JT. E-mail: m.a.halcrow@leeds.ac.uk
 Shinya Hayami |
 Stephen M. Holmes |
 Malcolm A. Halcrow |
Spin-transition compounds are one of the most versatile types of molecular switch.1–3 They undergo an electronic rearrangement under a physical stimulus from a high-spin state, with at least two unpaired electrons per switching centre, to a low-spin state where at least some of those electrons have become paired. Such spin-transitions inevitably lead to a change in magnetic moment which, in favourable cases, can correspond to “on–off” switchable diamagnetism/paramagnetism.4 In many cases the electronic rearrangement also changes the colour of a compound,4 while bulk properties such as conductivity,5,6 fluorescence,7 magnetic ordering,8 dielectric constant9 and mechanical properties10 are also affected by spin-state switching in the solid state. The stimulus is most commonly a change in temperature. However, spin-transitions in bulk materials can also be induced by high pressures (of the order of GPa), or by light irradiation. Low temperatures are usually required in the latter case, to prevent the sample from simply relaxing back to its electronic ground (spin) state.
The most widespread class of spin-state switch is the spin-crossover compounds, where a transition metal ion switches between its crystal field high-spin and low-spin states without any other electron redistribution. While there are occasional exceptions,11 spin-crossover chemistry is mostly limited to 3d metal ions, whose weaker ligand fields are more suited to accessing high-spin configurations. The spin-crossover phenomenon is most heavily studied in iron(II) chemistry,12 and is also well-known in iron(III)13 and cobalt(II)14 compounds. Complexes of other 3d metal ions, notably recently manganese(III),15 can also exhibit the effect, however. Iron(II) complexes are particularly favoured because their low-spin state is diamagnetic (S = 0), leading to on–off switchable paramagnetism as before. Iron(II) compounds also tend to undergo larger structural changes between their spin states than compounds of metal ions, leading to the more cooperative transitions in the solid state that are desirable for application purposes.16
Spin-crossover chemistry was first developed in the mid-1960s, when observations from 30 years previously were confirmed and explained.17 Work from that time was mostly aimed at discovering new spin-crossover compounds, and elucidating the solid state chemistry and physics of spin-state switching in solid materials.18 While their potential for applications in molecular devices was recognised early, application studies of spin-crossover were hampered because the temperature and form of a spin-crossover transition varies markedly between compounds, or even in different samples of the same compound.16 A genuine molecular switch requires an abrupt, hysteretic switching event around room temperature, which is still very difficult to design into a material de novo. However that problem was addressed in 1993, when the first spin-crossover material that is bistable at room temperature was produced, by a rational modification of an iron(II)/triazole coordination polymer.19 The same class of coordination polymer is still widely used in nanoscience and molecular devices involving spin-state switches,20 and only a handful of other compounds have since been discovered with comparably favourable switching characteristics.
More recently, other types of molecular spin-state switch have also come to prominence. One important class involves charge transfer-induced spin-transitions (CTIST), where a high/low spin-transition is coupled to an intramolecular electron transfer event. There are two important classes of CTIST switching. First are heterometallic metal/cyanide assemblies.21 The most common examples contain iron and cobalt centres, which can undergo a thermally-induced valence tautomerism (eqn (1); HS = high-spin, LS = low-spin):
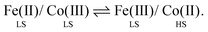 | (1) |
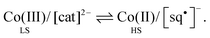 | (2) |
A third type of spin-state switch does not involve a spin-state change at individual metal ions, but instead reflects large changes to magnetic coupling interactions between radical spins in a molecule or material. Again, two main classes can be identified under this heading. First are some six-coordinate copper(II) complexes of nitroxyl radical ligands, which undergo an unusual re-alignment of their Jahn–Teller distortions around their coordination sphere upon cooling.25 The associated re-orientation of the {dx2−y2}1 magnetic orbital strongly affects magnetic superexchange between the metal and ligand spins, leading to a significant change in the magnetic moment of the material at the transition temperature.26 Lastly, “on–off” switchable paramagnetism is also found in certain classes of conjugated radical, which can undergo reversible π-dimerisation upon heating or cooling.27 This results in strong antiferromagnetic pairing of the two radical centres, so the material becomes diamagnetic below the transition temperature; it also leads to a significant colour change.28 Particularly large structural rearrangements are required to accommodate such a dimerisation in the solid state. As a result, magnetic transitions caused by radical dimerisations often exhibit thermal hysteresis.27
Despite their underlying differences, these various types of spin-state switches exhibit very similar physical characteristics. They all lead to a change in magnetic moment, from a weakly paramagnetic (or diamagnetic) form at low temperature to a strongly paramagnetic state at high temperature. This reflects the thermodynamics of spin-transitions, which are driven by the higher electronic and vibrational entropy associated with open shell, high-spin molecules.† All the forms of spin-state switching can occur in solution or solid phases, and can lead to materials exhibiting hysteretic switching in the solid state. Most of them have also been shown to occur under pressure (which usually stabilises the low-spin form), or under laser irradiation at low temperature. Hence, all these types of compound have potential for use as switching centres in materials chemistry.
The past ten years has also seen great development in the nanoscience of spin-state switches. Nanoparticles and thin films of spin-crossover materials or CTIST switches down to 30–50 nm in size can be routinely prepared, and usually function almost as well as the bulk materials.29 At smaller length scales the spin-transitions become more gradual and incomplete, as the (inactive) surfaces begin to dominate over (active) switching centres in the bulk of the nanostructures. Interestingly, however, in nanoparticles smaller than 5 nm, the spin-transition can recover some cooperativity. That was attributed to the influence of the relatively hard particle surface, which contains most of the atoms in a particle at such small scales.30 Monolayer and sub-monolayer films of spin-crossover centres can also be prepared, usually by vacuum deposition methods.29 Individual high-spin and low-spin molecules can be distinguished on gold surfaces by STM techniques, although many studies report that such molecules cannot be switched between their spin states under an STM tip below a particular surface coverage.31
The use of spin-transitions to modulate other properties of a material has also seen recent advances. Particular success has been obtained with fluorescent compounds, where cycling through a spin transition leads to changes in emission colour.7 Interestingly, all these studies have used pendant fluorophores, remote from the spin-crossover ion, as the emissive centres, rather than fluorescent ligands directly bound to the metal ion. Using spin-crossover to modulate the conductivity of molecular semiconductors has also shown some promise, in that the spin-transition can induce a discontinuity in the ρ vs. T curve in such compounds.5 Moreover, even insulating spin-crossover materials usually exhibit a change in their resistivity associated with the spin transition.6 “On–off” switching from insulating to metallic behaviour hasn't yet been achieved, however.
Using thermal spin-crossover to induce bulk magnetic ordering in a molecular material is also an on-going challenge, because of the low temperatures that would be required.8 However, on–off switching of single-chain magnetism under irradiation has been achieved, activated by light-induced low → high-spin switching of iron sites in coordination polymer materials.32 The same effect has since been taken to its logical conclusion, by demonstrating that a light-induced high-spin complex can itself act as a single ion magnet at very low temperatures. These samples are therefore tristable under those conditions (low-spin, and magnetically bistable high-spin).33
Spin-transition compounds have also found use in switchable soft materials. Mesogenic spin-crossover materials have been pursued for some time, although few examples are liquid crystalline over the entire temperature range of a spin-transition.34 More usual is for a small change in spin state population to be observed around a crystal → liquid-crystal phase transition above room temperature. This magnetic transition may be hysteretic, and it can also be “reverse” in character.† Some micellar assemblies35 and gels36 of spin-crossover molecules have also been prepared, which undergo unexpectedly cooperative spin-state switching, reflecting interaction of the switchable molecules with the semi-rigid supramolecular architectures.
The multi-functional properties of spin-state switches have proven particularly useful for the production of prototype devices. The first such application was a thermochromic display device produced by Kahn et al. in the 1990s.37 More recently, thermoelectrical devices based on switchable conductivity38 and optical devices modulated by spin-crossover effectors39 have been proposed. Mechanical motion of a material induced by the structural changes associated with spin-crossover is also an exciting prospect for actuator applications.10
Lastly, spin-state switching in solution is also gaining increasing attention. On one hand, guest-induced switching in a supramolecular spin-crossover complex is an exciting prospect for colorimetric sensor applications.40 On the other, the “on–off” switchable paramagnetism of spin-crossover complexes is gaining interest for switchable MRI contrast agents.41 There is clear potential to use the same principles to produce chemo-responsive materials based on spin-state switches.42
Conclusions
This special issue, dedicated to molecular spin-switches in materials chemistry, aims to highlight these exciting advances. Many of the areas in the preceding paragraphs are represented in the issue, including discovery chemistry and crystal engineering, nanoparticle and thin film nanoscience, multifunctional dielectric and fluorescent materials, switchable hybrid and composite materials, and supramolecular assemblies of spin-crossover components. This breadth of coverage confirms the great potential for functional materials based on spin-state switches, and points to exciting new developments in the near future.Notes and references
-
Spin Crossover in Transition Metal Compounds I-III, ed. P. Gütlich and H. A. Goodwin, Top. Curr. Chem., 2004, vol. 233–235 Search PubMed
.
-
Spin-Crossover Materials – Properties and Applications, ed. M. A. Halcrow, John Wiley & Sons, Chichester, 2013, p. 568 Search PubMed
.
- A. Bousseksou, G. Molnár, L. Salmon and W. Nicolazzi, Chem. Soc. Rev., 2011, 40, 3313 RSC
; P. Gütlich, Eur. J. Inorg. Chem., 2013, 581 CrossRef CAS PubMed
; S. Brooker, Chem. Soc. Rev., 2015, 44, 2880 RSC
.
- O. Kahn, J. Kröber and C. Jay, Adv. Mater., 1992, 4, 718 CrossRef CAS PubMed
.
- H. Phan, S. M. Benjamin, E. Steven, J. S. Brooks and M. Shatruk, Angew. Chem., Int. Ed., 2015, 54, 823 CrossRef CAS PubMed
.
- M. Matsuda and H. Tajima, Chem. Lett., 2007, 36, 700 CrossRef CAS
; A. Rotaru, I. A. Gural'skiy, G. Molnár, L. Salmon, P. Demont and A. Bousseksou, Chem. Commun., 2012, 48, 4163 RSC
.
- See e.g. Y. Garcia, F. Robert, A. D. Naik, G. Zhou, B. Tinant, K. Robeyns, S. Michotte and L. Piraux, J. Am. Chem. Soc., 2011, 133, 15850 CrossRef CAS PubMed
; C.-F. Wang, R.-F. Li, X.-Y. Chen, R.-J. Wei, L.-S. Zheng and J. Tao, Angew. Chem., Int. Ed., 2015, 54, 1574 CrossRef PubMed
.
- See e.g. M. Clemente-León, E. Coronado, M. López-Jordà, C. Desplanches, S. Asthana, H. Wang and J.-F. Létard, Chem. Sci., 2011, 2, 1121 RSC
; J. A. Rodríguez-Velamazán, O. Fabelo, C. M. Beavers, E. Natividad, M. Evangelisti and O. Roubeau, Chem. – Eur. J., 2014, 20, 7956 CrossRef PubMed
.
- S. Bonhommeau, T. Guillon, L. M. L. Daku, P. Demont, J. S. Costa, J.-F. Létard, G. Molnár and A. Bousseksou, Angew. Chem., Int. Ed., 2006, 45, 1625 CrossRef CAS PubMed
.
- M. D. Manrique-Juarez, S. Rat, L. Salmon, G. Molnár, C. M. Quintero, L. Nicu, H. J. Shepherd and A. Bousseksou, Coord. Chem. Rev., 2015 DOI:10.1016/j.ccr.2015.04.005
.
- N. Bridonneau, J. Long, J.-L. Cantin, J. von Bardeleben, S. Pillet, E.-E. Bendeif, D. Aravena, E. Ruiz and V. Marvaud, Chem. Commun., 2015, 51, 8229 RSC
.
- M. A. Halcrow, Polyhedron, 2007, 26, 3523 CrossRef CAS PubMed
.
- M. Nihei, T. Shiga, Y. Maeda and H. Oshio, Coord. Chem. Rev., 2007, 251, 2606 CrossRef CAS PubMed
.
- S. Hayami, Y. Komatsu, T. Shimizu, H. Kamihata and Y. H. Lee, Coord. Chem. Rev., 2011, 255, 1981 CrossRef CAS PubMed
.
- B. Gildea, M. M. Harris, L. C. Gavin, C. A. Murray, Y. Ortin, H. Müller-Bunz, C. J. Harding, Y. Lan, A. K. Powell and G. G. Morgan, Inorg. Chem., 2014, 53, 6022 CrossRef CAS PubMed
and references therein.
- M. A. Halcrow, Chem. Soc. Rev., 2011, 40, 4119 RSC
.
- A. H. Ewald, R. L. Martin, G. Ross and A. H. White, Proc. R. Soc. London, Ser. A, 1964, 280, 235 CrossRef CAS
.
- P. Gütlich, A. Hauser and H. Spiering, Angew. Chem., Int. Ed., 1994, 33, 2024 CrossRef PubMed
.
- J. Kröber, E. Codjovi, O. Kahn, F. Grolière and C. Jay, J. Am. Chem. Soc., 1993, 115, 9810 CrossRef
.
- O. Roubeau, Chem. – Eur. J., 2012, 18, 15230 CrossRef CAS PubMed
.
-
K. R. Dunbar, C. Achim and M. Shatruk, in Spin Crossover Materials: Properties and Applications, ed. M. A. Halcrow, John Wiley & Sons, Chichester, 2013, ch. 6, pp. 171–202 Search PubMed
.
- E. S. Koumousi, I.-R. Jeon, Q. Gao, P. Dechambenoit, D. N. Woodruff, P. Merzeau, L. Buisson, X. Jia, D. Li, F. Volatron, C. Mathonière and R. Clérac, J. Am. Chem. Soc., 2014, 136, 15461 CrossRef CAS PubMed
; Y.-Z. Zhang, P. Ferko, D. Siretanu, R. Ababei, N. P. Rath, M. J. Shaw, R. Clérac, C. Mathonière and S. M. Holmes, J. Am. Chem. Soc., 2014, 136, 16854 CrossRef PubMed
.
- T. Tezgerevska, K. G. Alley and C. Boskovic, Coord. Chem. Rev., 2014, 268, 23 CrossRef CAS PubMed
.
- C. G. Pierpont and C. W. Lange, Prog. Inorg. Chem., 1994, 41, 331 CrossRef CAS PubMed
.
- S. L. Veber, E. A. Suturina, M. V. Fedin, K. N. Boldyrev, K. Y. Maryunina, R. Z. Sagdeev, V. I. Ovcharenko, N. P. Gritsan and E. G. Bagryanskaya, Inorg. Chem., 2015, 54, 3446 CrossRef CAS PubMed
and references therein.
-
V. Ovcharenko and E. G. Bagryanskaya, in Spin Crossover Materials: Properties and Applications, ed. M. A. Halcrow, John Wiley & Sons, Chichester, 2013, ch. 9, pp. 239–280 Search PubMed
.
-
J. M. Rawson and J. J. Hayward, in Spin Crossover Materials: Properties and Applications, ed. M. A. Halcrow, John Wiley & Sons, Chichester, 2013, ch. 8, pp. 225–237 Search PubMed
.
- J. M. Rawson, A. Alberola and A. Whalley, J. Mater. Chem., 2006, 16, 2560 RSC
; K. E. Preuss, Polyhedron, 2014, 79, 1 CrossRef CAS PubMed
.
- M. Cavallini, Phys. Chem. Chem. Phys., 2012, 14, 11867 RSC
; H. J. Shepherd, G. Molnár, W. Nicolazzi, L. Salmon and A. Bousseksou, Eur. J. Inorg. Chem., 2013, 653 CrossRef CAS PubMed
.
- H. Peng, S. Tricard, G. Félix, G. Molnár, W. Nicolazzi, L. Salmon and A. Bousseksou, Angew. Chem., Int. Ed., 2014, 53, 10894 CrossRef CAS PubMed
.
- A. Pronschinske, R. C. Bruce, G. Lewis, Y. Chen, A. Calzolari, M. Buongiorno-Nardelli, D. A. Shultz, W. You and D. B. Dougherty, Chem. Commun., 2013, 49, 10446 RSC
and references therein.
- S. Ohkoshi, K. Imoto, Y. Tsunobuchi, S. Takano and H. Tokoro, Nat. Chem., 2011, 3, 564 CrossRef CAS PubMed
; R. Ababei, C. Pichon, O. Roubeau, Y.-G. Li, N. Bréfuel, L. Buisson, P. Guionneau, C. Mathonière and R. Clérac, J. Am. Chem. Soc., 2013, 135, 14840 CrossRef PubMed
.
- X. Feng, C. Mathonière, I.-R. Jeon, M. Rouzières, A. Ozarowski, M. L. Aubrey, M. I. Gonzalez, R. Clérac and J. R. Long, J. Am. Chem. Soc., 2013, 135, 15880 CrossRef CAS PubMed
; C. Mathonière, H.-J. Lin, D. Siretanu, R. Clérac and J. M. Smith, J. Am. Chem. Soc., 2013, 135, 19083 CrossRef PubMed
.
- A. B. Gaspar and M. Seredyuk, Coord. Chem. Rev., 2014, 268, 41 CrossRef CAS PubMed
.
- C. Gandolfi, C. Moitzi, P. Schurtenberger, G. G. Morgan and M. Albrecht, J. Am. Chem. Soc., 2008, 130, 14434 CrossRef CAS PubMed
.
- K. Kuroiwa, H. Kikuchi and N. Kimizuka, Chem. Commun., 2010, 46, 1229 RSC
; P. Grondin, O. Roubeau, M. Castro, H. Saadaoui, A. Colin and R. Clérac, Langmuir, 2010, 26, 5184 CrossRef CAS PubMed
.
- O. Kahn and C. Jay Martinez, Science, 1998, 279, 44 CrossRef CAS
.
- J. Dugay, M. Gimenez-Marques, T. Kozlova, H. W. Zandbergen, E. Coronado and H. S. J. van der Zant, Adv. Mater., 2015, 27, 1288 CrossRef CAS PubMed
; D. Ghosh, P. Parida and S. K. Pati, Appl. Phys. Lett., 2015, 106, 193105 CrossRef PubMed
.
- G. Molnár, I. A. Gural'skiy, L. Salmon, W. Nicolazzi, C. Quintero, A. Akou, K. Abdul-kader, F. Felix, T. Mahfoud, C. Bergaud, C. Bartual-Murgui, C. Thibault, C. Vieu and A. Bousseksou, Proc. SPIE, 2012, 8425, 842513 CrossRef CAS PubMed
; M. Matsuda, K. Kiyoshima, R. Uchida, N. Kinoshita and H. Tajima, Thin Solid Films, 2013, 531, 451 CrossRef PubMed
.
- See e.g. Z. Ni, A. M. McDaniel and M. P. Shores, Chem. Sci., 2010, 1, 615 RSC
; M. C. Young, E. Liew and R. J. Hooley, Chem. Commun., 2014, 50, 5043 RSC
.
- See e.g. I.-R. Jeon, J. G. Park, C. R. Haney and T. D. Harris, Chem. Sci., 2014, 5, 2461 RSC
; M. Dommaschk, M. Peters, F. Gutzeit, C. Schütt, C. Näther, F. D. Sönnichsen, S. Tiwari, C. Riedel, S. Boretius and R. Herges, J. Am. Chem. Soc., 2015, 137, 7552 CrossRef CAS PubMed
.
- O. S. Wenger, Chem. Rev., 2013, 113, 3686 CrossRef CAS PubMed
.
- S. Hayami, M. R. Karim and Y. H. Lee, Eur. J. Inorg. Chem., 2013, 683 CrossRef CAS PubMed
.
Footnote |
| † “Reverse” low → high-spin transitions on cooling are very rare, and are usually driven by an increase in disorder in another part of the material to offset the unfavourable entropy of the reverse spin-state change.43 |
| This journal is © The Royal Society of Chemistry 2015 |
