Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Properties modulation of organic semi-conductors based on a donor-spiro-acceptor (D-spiro-A) molecular design: new host materials for efficient sky-blue PhOLEDs†
Maxime
Romain
a,
Denis
Tondelier
b,
Olivier
Jeannin
a,
Bernard
Geffroy
*bc,
Joëlle
Rault-Berthelot
*a and
Cyril
Poriel
*a
aUMR CNRS 6226, Institut des Sciences Chimiques de Rennes, Université de Rennes 1, Campus de Beaulieu, 35042 Rennes cedex, France. E-mail: cyril.poriel@univ-rennes1.fr; joelle.rault-berthelot@univ-rennes1.fr; Tel: +33-2-2323-5977
bUMR CNRS 7647, LPCIM, École Polytechnique, 91128 Palaiseau, France. E-mail: bernard.geffroy@polytechnique.edu
cLICSEN/NIMBE UMR 3685, CEA Saclay, 91191 Gif Sur Yvette, France
First published on 4th August 2015
Abstract
Four high triplet organic semi-conductors based on the donor-spiro-acceptor design (D-spiro-A) have been synthesized. Their physicochemical and photophysical properties have been studied, compared and discussed in light of the nature of their respective donor/acceptor units. The four compounds have been used as host materials in efficient sky-blue (EQE > 10% at 10 mA cm−2) phosphorescent organic light emitting diodes.
In the last twenty years, many families of organic emissive materials have been developed for flat-panel displays and solid state lighting sources.1–3 The design of fluorescent organic semi-conductors for green and red emission has led to highly efficient organic light emitting diodes (OLEDs).4 However, the blue colour, despite fantastic recent progresses in the design of fluorescent materials5–12 and in the device architectures,13 remains less efficient and less stable than the other colours.4 In addition, in the last fifteen years, it has been demonstrated that highly efficient blue emission should not be obtained in OLED devices using pure organic singlet exciton emissive layers (EMLs) but should require more sophisticated EMLs in which a blue phosphor is doped in an organic host material to harvest both singlet and triplet excitons. Such devices, phosphorescent organic light emitting diodes (PhOLEDs), have therefore attracted fantastic interest.14–18 One of the weakest links of this technology remaining is the design of highly efficient host materials for blue phosphors. Indeed, the prerequisites for an ideal host for a blue dopant are (i) a high triplet energy (ET) to avoid reverse energy transfers from the guest back to the host, (ii) a high glass-transition temperature (Tg) and decomposition temperature (Td) for stability, (iii) matching HOMO–LUMO levels for injection of the charges and (iv) good and balanced charge transporting properties to insure efficient recombination in the dopant. For industrial production, long and sophisticated chemical syntheses of the host should be also clearly proscribed. Bipolar molecules incorporating hole and electron transporting units to adjust the energy levels of frontier orbitals appear to date to be the most promising candidates for PhOLED applications.2,16–22 Thus, when designing bipolar hosts, one should carefully consider the selection of the donor/acceptor pair and their relative positions within the dye. Indeed, the donor/acceptor combination often results in π-conjugation enlargement, which would accordingly reduce the singlet and triplet energies. Therefore, to obtain bipolar hosts with high ET, the π-conjugation and the electronic coupling between the donor and the acceptor units must be restricted. The choice of the linker and the relative position of the donor and acceptor units must then be carefully performed.
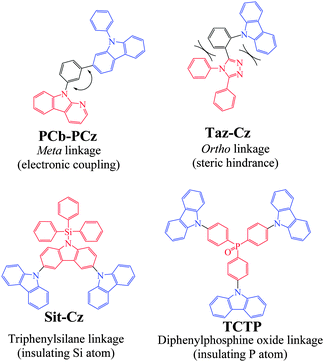
To date, many successful design strategies have been developed to gather all the above mentioned properties in a single host and particularly to disrupt the π-conjugation. Some relevant examples are presented in Chart 1.
First, it is known that electronic coupling through a meta linkage is inherently weaker than that through a para one5,23–27 and this strategy has often been used to connect a hole transporting and an electron transporting unit in a host material.21,24,28,29 Thus, the meta linkages efficiently disrupt the π-conjugation between a donor and an acceptor as for instance in 9-(3-(diphenylcarbazol-3-yl)phenyl)-α-carboline (PCb-PCz, Chart 1), in which the N-phenylcarbazole donor unit is separated from the pyridoindole acceptor unit by a meta-substituted phenyl unit (HOMO: −5.75 eV, LUMO: −2.31 eV, ET = 2.74 eV).21 A second approach widely developed in the literature consists of introducing steric congestion within the dye to hinder the planarization between two connected π-systems.30–33 This steric hindrance strategy is most of the time performed through the incorporation of a sterically hindered ortho linkage efficiently leading to π-conjugation restriction. For example, in Taz-Cz (Chart 1), the carbazole and 1,2,4-triazole are both oriented with a dihedral angle larger than 45° with the central benzene ring, efficiently disrupting the π-conjugation between the carbazole and the triazole (HOMO: −5.7 eV, LUMO: −2.3 eV, ET = 3.09 eV).31
Using an insulating heteroatom is also an efficient strategy to obtain high ET materials with a short π-conjugated pathway. Thus, silicium (silane),2,18,34,35 and a phosphoryl group (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O),2,18,20,29,36,37 may act as effective breaking points of the π-conjugation between the main core of the molecule and the outer groups linked to the heteroatom (phenyl, for example in the case of the efficient electron transporting diphenylphosphine oxide unit). Thus, the electronic structure of the bipolar molecules is almost identical to those of the corresponding central core, which is a crucial point to avoid the decrease of ET.20 For example, in 9′-triphenylsilanyl-9′H-[9,3′,6′,9′′]ter-carbazole (Sit-Cz), the direct connection of the silane to a carbazole unit through the nitrogen atom renders this carbazole electro-deficient (HOMO: −5.54 eV, LUMO: −2.3 eV, ET = 3.0 eV).38 Similarly, in the star-shaped molecule 4,4′,4′′-tri(N-carbazolyl)triphenylphosphine oxide (TCTP), the electron acceptor phosphine oxide unit is used both as the core and as the electron donor, with the carbazole moiety acting as the branch.39 The HOMO/LUMO levels respectively lie at −5.25 eV/−1.67 eV, and the disrupted conjugation via the phosphine oxide linkage preserves a high ET of 3.03 eV.
O),2,18,20,29,36,37 may act as effective breaking points of the π-conjugation between the main core of the molecule and the outer groups linked to the heteroatom (phenyl, for example in the case of the efficient electron transporting diphenylphosphine oxide unit). Thus, the electronic structure of the bipolar molecules is almost identical to those of the corresponding central core, which is a crucial point to avoid the decrease of ET.20 For example, in 9′-triphenylsilanyl-9′H-[9,3′,6′,9′′]ter-carbazole (Sit-Cz), the direct connection of the silane to a carbazole unit through the nitrogen atom renders this carbazole electro-deficient (HOMO: −5.54 eV, LUMO: −2.3 eV, ET = 3.0 eV).38 Similarly, in the star-shaped molecule 4,4′,4′′-tri(N-carbazolyl)triphenylphosphine oxide (TCTP), the electron acceptor phosphine oxide unit is used both as the core and as the electron donor, with the carbazole moiety acting as the branch.39 The HOMO/LUMO levels respectively lie at −5.25 eV/−1.67 eV, and the disrupted conjugation via the phosphine oxide linkage preserves a high ET of 3.03 eV.
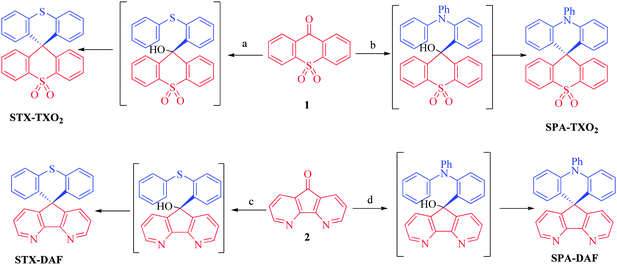
More recently, another promising and simple molecular design based on π-conjugation breaking induced by an insulating spiro bridge (called a D-spiro-A design) has been introduced in the literature. This design, which allows separation of the HOMO and LUMO levels, has a great potential in thermally activated delayed fluorescence (TADF)40,41 but remains rarely used to date in the field of host materials for blue phosphorescent dopants.35,42–44 Our attention for this design was to avoid the introduction of pendant hole or electron transporting units, strongly simplifying the molecular structure and hence the chemical synthesis. In the present work, we wish to report a structure–properties relationship study of new donor/acceptor molecules based on this D-spiro-A design. These semi-conductors present different electronic properties and have been used as hosts in sky-blue PhOLEDs leading in some cases to high efficiencies (EQE > 10% at 10 mA cm−2). The large differences observed in terms of PhOLED efficiencies highlight the importance of the donor/acceptor combination used in the molecular design of the host. Such a structure–properties relationship study may provide interesting insights for the development of future materials for optoelectronics. Thus, in this work, as a development to our preliminary note on the dye SPA-TXO2 (Scheme 1),45 we have investigated through a D-spiro-A design two hole transporting moieties, namely N-phenylacridine (PA)43 and thioxanthene (TX), and two electron transporting moieties, namely thioxanthene dioxide (TXO2) and diazafluorene (DAF) (Scheme 1). These four molecular fragments are briefly described below.
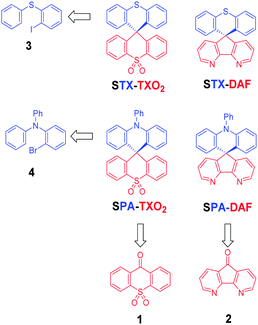 | ||
| Scheme 1 Retrosynthetic analysis of the four dyes investigated in this work: STX-TXO2, STX-DAF, SPA-TXO2 and SPA-DAF. | ||
In PA, two phenyl rings of a triphenylamine unit are connected by a methylene unit forming a hexagon. Structurally, the presence of this hexagon renders the N-phenylacridine unit more similar to a triphenylamine than to a N-phenylcarbazole unit.46 The strong electron rich nature of the PA unit has been previously exploited in TADF,40,41,47 in non-doped blue OLEDs,48 and in host materials for blue PhOLEDs.35,42,43,49,50
TX is the structural analogue of xanthene, possessing an intracyclic sulphur atom instead of an oxygen atom. Recently, Yam et al. have reported various spiro-configured dyes based on the connection of a TX (and TXO2) unit with 2,7-bis(diphenylamine)fluorene leading to very promising donor materials in the construction of high performance organic photovoltaic devices.51
TXO2 is the oxidized analogue of TX. The presence of the sulfone in TXO2 leads to better electron injection and transport abilities due to the decrease of the LUMO level.12 Indeed, sulfones have been efficiently incorporated (i) in highly efficient blue emitting materials,52–54 (ii) in host materials for PhOLEDs,55–57 and (iii) in electron transporting materials.58 However, the use of the TXO2 fragment remains scarce and our group, in a preliminary note, has recently demonstrated its potential in the design of hosts for blue PhOLEDs.45 Thus, by a simple oxidation step, it is hence possible to switch from hole transporting properties in TX to electron transporting properties in TXO2, highlighting the versatility of these systems.
The electron acceptor DAF may be regarded as a bipyridine unit possessing then a similar molecular arrangement to that of the well-known bridged biphenyl, namely fluorene. The strong electron affinity of this unit59,60 could decrease the LUMO energy level, thereby improving the electron injection and transport properties of the materials. Up to now, DAF fragments have been investigated in many research fields, such as electron transporting materials,61 organic emitters,59,62 and sensors.63 The DAF fragment is also an interesting platform to coordinate various metals such as cadmium,64 zinc,64 rhenium,65 silver66 and iridium.67,68 However, to the best of our knowledge, the DAF unit remains rarely incorporated in host materials for PhOLEDs.60,61,69
Results and discussion
Synthesis
It is crucial for the future of organic optoelectronics to establish short and very efficient synthetic approaches of organic materials. Herein, all the dyes have been synthesised through a one-pot very efficient route using common and easily synthesizable intermediates. Thus, the two halogeno-aryls 3 and 4, judiciously coupled with the two ketones 1 and 2, provide STX-TXO2 (3 + 1), STX-DAF (3 + 2), SPA-TXO2 (4 + 1) and SPA-DAF (4 + 2) (Scheme 1).Regarding the TXO2 derivative (Scheme 2, top), a lithium–bromine exchange was first performed on either 2-bromo-N,N-diphenylaniline 4 or (2-iodophenyl)(phenyl)sulfane 3 followed by the trapping of the lithiated intermediate by ketone 9H-thioxanthen-9-one-10,10-dioxide 1 (obtained by oxidation of commercially available 9H-thioxanthen-9-one with hydrogen peroxide, see ESI†). An electrophilic intramolecular cyclization reaction of the resulting dioxothioxanthenols (not isolated) in acidic media (HCl in acetic acid) afforded SPA-TXO2 or STX-TXO2 in high yields (78 and 88% respectively over the three steps). This one-pot synthetic strategy is very simple, easy to perform, highly efficient for a gram scale synthesis and is versatile to many different donor and acceptor fragments.
An identical strategy has been developed to synthesise STX-DAF and SPA-DAF (Scheme 2, bottom) involving first the synthesis of the known diazafluorenone 2 (obtained by oxidation and ring contraction of phenanthroline in water in the presence of KMnO4 and KOH, see ESI†).70 Reaction of 2 with the corresponding lithiated intermediates derived from 3 or 4 was then performed leading to the corresponding diazafluorenol derivatives. However the intramolecular cyclization reaction under similar conditions as those exposed above (HCl/CH3CO2H, Scheme 2) did not occur, highlighting the very different reactivity of diazafluorenols vs. dioxothioxanthenols. The X-ray structure of the triphenylamine diazafluorenol shows short distances (ca. 2 Å, see Fig. S2 in the ESI†) between a hydrogen atom of the hydroxide unit of one molecule and a nitrogen atom of the diazafluorene unit of a second molecule. These short distances signal the presence of intermolecular interactions that may explain the stabilization of diazafluorenol and hence its weak reactivity. In addition, the nitrogen atoms of the diazafluorene unit might be protonated during the cyclisation step, decreasing again its reactivity. Using more drastic conditions and 3 equivalents of methane sulfonic acid at high temperature (180 °C in 1,2-dichlorobenzene), the target compounds STX-DAF (yield: 39%) and SPA-DAF (yield: 47%) were finally isolated. It should be noted that in the course of this work, the synthesis of SPA-DAF as an intermediate building block has been reported by Adachi and coworkers using Eaton's reagent to cyclise the diazafluorenol derivative.40 However, the properties of SPA-DAF were not reported in this work.
1H NMR studies
1H NMR spectroscopy is an interesting tool to evaluate the strength of electron withdrawing/donating moieties on the environment. Thus, the effect of the incorporation of heteroatoms in the spirobifluorene-like molecules described herein can be interestingly visualized through NMR spectroscopy. The complete assignments of all signals have been performed by 2D NMR spectroscopy experiments (HMBC, HMQC, 1H/1H COSY, see ESI†).For STX-TXO2 and SPA-TXO2, one can note that the hydrogen atoms (Ha–d) of the TXO2 fragment (Fig. 1a and b, red lines) are centred around 7.35 ppm except for Ha, which appears to be deshielded due to the proximity of the sulfone unit. Considering the chemical shift of benzene (7.35 ppm in CD2Cl2)71 one can conclude that TXO2 is a weak electron withdrawing unit. On the opposite side, the signals for Hf–h of the TX unit and Hl–o of the PA core appear to be shielded, highlighting their electron donating nature. We can note that the incorporation of the nitrogen atom within the PA unit leads to stronger shielding than that observed for sulphur in TX, highlighting the stronger electron donating nature of the former. This will be confirmed by electrochemistry (see below). It should be mentioned that the resonances of the pendant phenyl ring of PA (Hi–k) are found at low field (δ 7.58/7.8 ppm) meaning that the free doublet of the nitrogen atom is conjugated with the acridine and not with the phenyl unit.
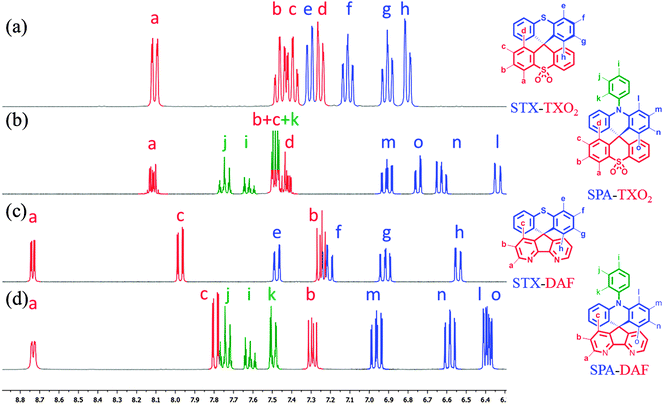 | ||
| Fig. 1 Portion of the 1H NMR spectra of (a) STX-TXO2, (b) SPA-TXO2, (c) STX-DAF and (d) SPA-DAF in CD2Cl2. | ||
Similar effects have been detected for STX-DAF and SPA-DAF with in addition two very low field resonances recorded at 8 and 8.65 ppm (respectively assigned to Hc in the γ position and to Ha in the β position of the nitrogen atom of DAF). This highlights the strong electron withdrawing nature of the DAF fragment.
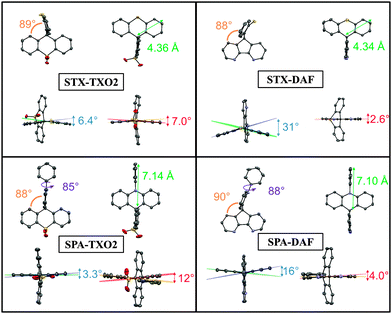
Structural properties
The four compounds have been crystallized by vapour diffusion of pentane in CDCl3 solution in order to confirm their molecular structures by X-ray crystallography and to study the structural characteristics (see X-ray data in the ESI†). STX-TXO2 and SPA-TXO2 both crystallized in a triclinic system. The STX-TXO2 asymmetric unit contains two different molecules and one molecule of CDCl3 whereas that of SPA-TXO2 contains only one molecule without the solvent. STX-DAF crystallizes in an orthorhombic system with only one molecule per unit and SPA-DAF crystallizes with two molecules of solvent in a monoclinic system.The molecular radius of each molecule (distance from the spiro carbon atom to the farthest carbon atom, green arrow in Fig. 2) has been evaluated at 7.14 and 7.10 Å for SPA-TXO2 and SPA-DAF respectively, and at 4.36 and 4.34 Å for STX-TXO2 and STX-DAF respectively. For the four molecules, this radius is imposed by the donor groups (TX or DAF) which are the longest units.
In all molecules, the donor and the acceptor fragments are almost orthogonal with a twist angle (value in orange in Fig. 2) varying from 88° for both STX-DAF and SPA-TXO2, 89° for STX-TXO2 to 90° for STX-DAF. This orthogonality between the donor and the acceptor moiety is at the origin of the absence of significant π-conjugation between them. Additionally, in the PA derivatives, the angle between the mean plane of the acridine units (indicated in purple in Fig. 2) and that of the attached phenyl is 85 and 88° in SPA-TXO2 and SPA-DAF respectively. This large angle indicates a second π-conjugation interruption at the nitrogen atom between the acridine unit and the phenyl group. As discussed below, these π-conjugation breaks are essential to keep the high ET (see below).
For each aromatic unit, a torsional angle has been defined as the dihedral angle between the two external benzene rings of each unit (values in blue for the donor units and in red for the acceptor units in Fig. 2).
For the donor units, the TX torsion angles are impressively different depending on the acceptor core. Thus, a small angle of 6.4° is recorded for STX-TXO2 and a very large angle of 31° is recorded for STX-DAF. Similarly, the torsion angles measured in the PA units are 5 times larger for SPA-DAF (16°) than for SPA-TXO2 (3.3°). Thus, the donor fragments in SPA-DAF and STX-DAF are strongly more distorted than in their counterparts SPA-TXO2 and STX-TXO2. The presence of the DAF unit within the dyes leads hence to a very distorted donor with large dihedral angles. This feature may be assigned to the packing forces induced by the presence of DAF. In addition and regardless of the acceptor, the PA units are always less distorted than the TX units surely due to the presence in PA of a pendant phenyl ring which provides a certain degree of rigidity.
For the acceptor units, the TXO2 torsion angle is 7° for STX-TXO2 and 12° for SPA-TXO2 whereas this torsion angle is very weak in the case of the DAF unit: 2.6° for STX-DAF, and 4.0° for SPA-DAF. Thus, the very rigid DAF unit only allows very weak deformations due the presence of the C–C bond in the α position of the nitrogen atoms. Such conformational locking is very similar to that observed for the fluorene unit. Oppositely, the presence of the sulphur atom linking the two phenyl groups in the TXO2 unit allows some deviations from planarity.
Thermal properties
Before any possible OLED applications and in order to confirm the interest of the present D-spiro-A design, the four compounds have been studied by thermogravimetric analysis (TGA) (Fig. 3) and differential scanning calorimetry (DSC) (see ESI†).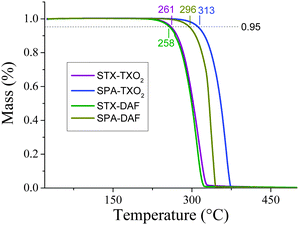 | ||
| Fig. 3 TGA curves of STX-TXO2 (violet line), STX-DAF (green line), SPA-TXO2 (blue line) and SPA-DAF (yellow-green line). | ||
The decomposition temperature, Td, is defined as the temperature corresponding to 5% of the mass loss.72 Herein, these temperatures are recorded as 261/258 °C for STX-TXO2/STX-DAF and as 313/296 °C for SPA-TXO2/SPA-DAF respectively (Fig. 3). As a complete mass loss then occurs, we believe that this mass loss is attributed to a sublimation process as previously observed for other π-conjugated systems.24 Thus, the presence of the PA unit within the dye leads to a material with a higher Td (by ca. 40/50°) than that obtained with the TX unit. DSC studies reveal no phase transition between room temperature and the decomposition of the molecules (see ESI†). The rigid molecular structure of the two spiro-annulated fragments is at the origin of the interesting thermal properties of the four molecules.
Electrochemical properties
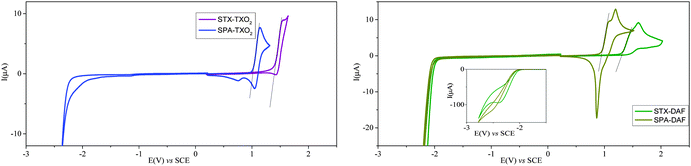
The electrochemical properties have been investigated by cyclic voltammetry (CV) in CH2Cl2 in oxidation and reduction (Fig. 4 and Table 1, potentials given vs. SCE). In oxidation, the two TX derivatives present an irreversible oxidation with a maximum at 1.55 V for STX-TXO2 (violet line) and at 1.48 V for STX-DAF (green line). From their respective onset oxidation potentials (1.42/1.34 V), their HOMO energy levels were determined as −5.79/−5.74 eV for STX-TXO2/STX-DAF.| Electrochemical propertiesa | Theoretical calculations | Thermal properties | ||||||
|---|---|---|---|---|---|---|---|---|
| E ox (V) | E oxonset (V)/HOMO (eV) | E red (V) | E redonset (V)/LUMO (eV) | ΔEel (eV) | HOMO/LUMO | ΔEtheo (eV) | T d (°C) | |
| a All potentials are given vs. SCE. | ||||||||
| STX-TXO2 | 1.55 | 1.39/−5.79 | — | −2.40/−2.00 | 3.79 | −6.03/−1.59 | 4.44 | 261 |
| SPA-TXO2 | 1.14 | 1.02/−5.42 | — | −2.40/−2.00 | 3.43 | −5.57/−1.44 | 4.13 | 313 |
| STX-DAF | 1.48/1.60 | 1.30/−5.70 | −2.41 | −2.06/−2.34 | 3.36 | −5.87/−1.87 | 4.00 | 258 |
| SPA-DAF | 1.11/1.20 | 0.95/−5.35 | ∼−2.5 | −2.09/−2.31 | 3.04 | −5.49/−1.72 | 3.77 | 296 |
In the PA series, the two derivatives are oxidized at even lower anodic potentials and present one reversible oxidation wave with a maximum at 1.14 V for SPA-TXO2 and two very close oxidation waves with maxima at 1.11/1.20 V for SPA-DAF. For SPA-DAF, recording CV up to the first oxidation wave only shows an irreversible oxidation wave indicating a high reactivity of the SPA-DAF˙+ species at the timescale of the CV (see Fig. S1 in the ESI†). However, when reaching the second oxidation wave, an adsorption-like reduction peak is observed showing that the species formed during the two oxidation processes are strongly adsorbed at the electrode surface. From their onset potentials measured at 1.03/0.95 V for SPA-TXO2/SPA-DAF respectively, the HOMO levels were calculated as −5.42 eV for SPA-TXO2 and −5.35 eV for SPA-DAF.
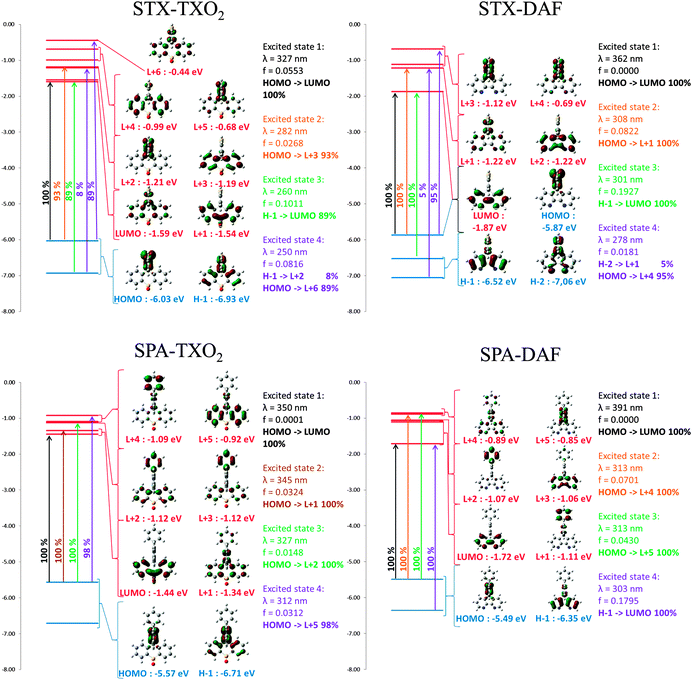
In light of the electronic distribution of the HOMO (DFT calculations performed at the Gaussian 09 B3LYP/6-311+G(d,p) level of theory, Fig. 6), the first oxidations are assigned to the oxidation of the electron rich fragment, namely the TX unit in STX-TXO2/STX-DAF and to the PA core in SPA-TXO2/SPA-DAF.
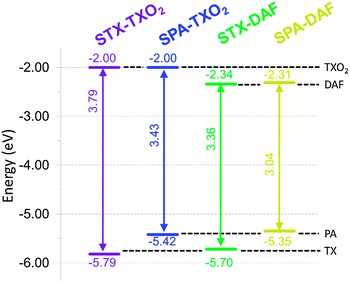
In reduction (Fig. 4), the TXO2 derivatives are reduced at potential values close to that of the electrolytic medium reduction and their onset potentials are detected at ca. −2.4 V, with the LUMO levels lying hence around −2.0 eV. The DAF derivatives are reduced at less negative potentials than those of the TXO2 derivatives and an irreversible reduction wave is observed (see inset in Fig. 4, right) with a maximum recorded at −2.42/−2.5 V for STX-DAF/SPA-DAF respectively. Thus, the LUMO levels of STX-DAF and SPA-DAF have been evaluated at −2.34/−2.31 eV, being ca. 0.3 eV lower than the LUMO levels of the TXO2 derivatives (−2.0 eV). This feature clearly indicates the stronger electron accepting capability of DAF compared to that of TXO2 and its potential to decrease the LUMO level. Thus, SPA-DAF, possessing the strongest donor/acceptor combination, displays an electrochemical gap ΔEelec of 3.04 eV, which is strongly more contracted than that of STX-TXO2 (3.79 eV), which possesses the weakest donor/acceptor combination (Fig. 5). STX-DAF and SPA-TXO2 possess therefore intermediate values of 3.36 and 3.43 eV respectively. For comparison purposes, structurally related 9,9′-spirobifluorene (SBF)72,73 possessing two spiro-connected fluorene units possesses a wide ΔEelec of 4.05 eV (HOMO: −5.94 eV, LUMO: −1.89 eV),33 which is widened by more than 1 eV compared to that of SPA-DAF. Thus, compared to SBF, we can note that the TX unit leads to dyes with higher HOMO levels (−5.94 eV for SBFvs. −5.70/−5.79 eV for STX-DAF/STX-TXO2) and that the TXO2 unit leads to dyes with slightly lower LUMO levels (−1.89 eV for SBFvs. −2.0 eV for both STX-TXO2 and SPA-TXO2). The effect of these fragments on the molecular orbital energy levels are hence relatively weak compared to those of fluorene. Oppositely, the PA/DAF units lead to a strong increase/decrease of the HOMO/LUMO energy levels compared to SBF. Thus, the PA/DAF combination seems to be the more attractive leading to the smallest gap, 3.04 eV, in the series whereas the TX/TXO2 combination leads to the widest gap in the series, 3.79 eV, highlighting not only the efficiency of the present design to tune the electronic properties of spiro-connected compounds but also the importance of the chosen donor/acceptor combination.
Compared to previously reported structurally related PA compounds bridged by spiroanthracenone (HOMO/LUMO: −5.50/−1.90 eV) or by two phenyls units (HOMO/LUMO: −5.42/−1.74 eV),42SPA-TXO2 and SPA-DAF (HOMO: −5.42 and −5.35 eV respectively) have (i) a very similar HOMO energy level, due to the presence of the PA unit, but (ii) a lower LUMO energy level (LUMO: −2 eV and −2.31 eV) due to the presence of either the TXO2 or the DAF unit instead of the anthracenone core. Thus, the present design allows selective tuning of the LUMO energy level without changing that of the HOMO.
Theoretical calculations
Geometry optimization of the four dyes in the singlet and triplet states was performed using density functional theory (DFT) at the Gaussian 09 B3LYP/6-311+G(d,p) level of theory. All the results are reported in Table 1 and in Fig. 6. The electronic distribution and the energy levels of the HOMOs and LUMOs (and the corresponding energy gaps, ΔEtheo) have been determined on optimized geometries. The same tendency is observed between the HOMO/LUMO values determined by CV and those obtained by theoretical calculations. The HOMO levels (calculated at −5.49 and −5.57 eV) of SPA-DAF and SPA-TXO2 are exclusively centred on the PA units (Fig. 6) in accordance with an electron transfer centred on this unit as suggested above in the electrochemical part. One can note that there is no electron density on the pendant N-phenyl group meaning that the electronic properties of the PA unit are independent of this pendant phenyl. The HOMOs of STX-TXO2 and STX-DAF are mainly localized on the TX unit, leading to deeper HOMO energy levels (−6.03 and −5.87 eV) than those of the PA derivatives.The calculated LUMO levels of STX-DAF and SPA-DAF are lower in energy (−1.87 and −1.72 eV respectively) than those of STX-TXO2 and SPA-TXO2 (−1.59 and −1.44 eV) clearly showing the stronger electron accepting nature of the DAF unit compared to that of the TXO2 unit. For the two DAF derivatives, the LUMO level is localized on the DAF fragment (in accordance with a first electrochemical reduction centred on this fragment, see above) and is separated by more than 0.6 eV from the LUMO+1 level. However, for the TXO2 derivatives, the situation is slightly different. Indeed, the LUMO of SPA-TXO2 is exclusively spread out on the TXO2 core whereas that of STX-TXO2 seems to be not only spread out on the TXO2 core but also on the central TX unit. For both TXO2 derivatives, the gap between the LUMO and the LUMO+1 is less than 0.1 eV (0.05/0.1 eV for STX-TXO2/SPA-TXO2) indicating some possible mixing of the LUMO and LUMO+1 orbitals. In conclusion the electronic separation between the donor (HOMO) and the acceptor unit (LUMO), the key point in host design, is clearly more efficient for the DAF derivatives than for the TXO2 derivatives. The calculated energy gaps, ΔEtheo, follow the same trend as the electrochemical ones with the lowest gap calculated for SPA-DAF (3.77 eV) and the highest recorded for STX-TXO2 (4.44 eV).
Absorption spectroscopy
The UV-visible absorption spectra of the four D-spiro-A dyes, recorded in cyclohexane, are presented in Fig. 7 and their main characteristics are summarized in Table 2. The four compounds present different absorption spectra with three or four absorption bands with low absorption coefficients (between 5 × 103 to 20 × 103 L mol−1 cm−1) in the range 260–350 nm. This is fully consistent with the presence of small and weakly conjugated units.| λ abs (nm) [103ε (L mol−1 cm−1)] | ΔEopt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (eV) b (eV) |
λ
em![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (nm) b (nm) |
λ
em![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (nm) c (nm) |
Δνb (cm−1/eV) | Φ (%) [λexc (nm)] |
E
T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (eV) d (eV) |
ΔS − Te (eV) | |
|---|---|---|---|---|---|---|---|---|
| a λ max. b In cyclohexane. c In a thin film. d In frozen methylcyclohexane/2-methylpentane (1/1) (77 K). e ΔS − T = 1239.84/λem(cyclohexane) − ET. | ||||||||
| STX-TXO2 | 309 (5.1); 281a (6.6); 274 (6.4) | 3.73 | 338 | 362 | 2780/0.34 | 0.8 [308] | 3.06 | 0.61 |
| SPA-TXO2 | 323 (5.1); 297 (5.9); 280 (7.2); 273a (7.6) | 3.54 | 350 | 371 | 2390/0.30 | 4.1 [323] | 3.08 | 0.46 |
| STX-DAF | 319 (12.1); 307a (12.9) | 3.79 | 348 | 394 | 2610/0.32 | 0.1 [319] | 3.03 | 0.53 |
| SPA-DAF | 330 (sh); 319a (20.4); 306 (16.4); 271 (11.8) | 3.64 | 388 | 420 | 5570/0.69 | 0.1 [319] | 2.98 | 0.22 |
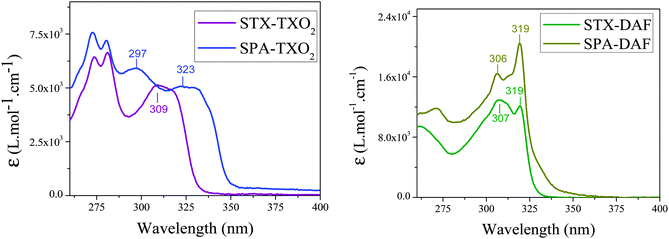
The absorption spectra of the two TXO2 derivatives (Fig. 7, left) are similar in the high energy range (maxima at 274/281 nm for STX-TXO2 and at 273/280 nm for SPA-TXO2) indicating that these two absorption bands may be associated to the TXO2 fragment. A series of previously reported TXO2 derivatives spiro-linked to 2,7-triphenylamine-fluorene or to 2,7-N-phenylcarbazole fluorene also present absorption bands of the TXO2 units close to 300 nm.51
In the lowest energy range, the two spectra are nevertheless different with one broad band centred at 309 nm for STX-TXO2 and two broad bands centred at 297 and 323 nm for SPA-TXO2. The band at 309 nm may therefore be associated to TX and the two bands at 297 and 323 nm to PA. The absorption of TX has been previously reported and possesses a maximum centred at 266 nm in ethanol.74 Thus, there is a red-shift of 43 nm between the absorption of TX and that of the spiro-bridged TX recorded herein for STX-TXO2. A similar red-shift of 32 nm has been reported for spiro-connected TX (bridged with 2,7-triphenylamine-fluorene, λmax: 298 nm in CH2Cl2).51 This red shift is due to the different influence of a methylene bridge and of a spiro-aromatic bridge on the TX core. Interestingly, the absorption spectra of structurally related compounds built on the spiro-connection of PA units with anthracenone41 present the same profile as that presented herein for SPA-TXO2 with two absorption bands with maxima at 300/320 nm.41
The two DAF derivatives (Fig. 7, right) present similar absorption spectra at lower energy, with the absorption being however broader for SPA-DAF than for STX-DAF. The two main absorption bands centred at 306/307 and 319 nm may be assigned to the absorption of the DAF core in accordance with the literature data.64,66,69,75 In addition, we have shown above that the band at 309 nm in STX-TXO2 was assigned to the TX absorption. Thus, in STX-DAF, there is a clear superimposition of the absorptions of TX and DAF cores leading to a large band at 307 nm, being relatively more intense than that at 319 nm. Similarly, in the case of SPA-DAF, there is an overlap between the PA and DAF fragments, with the shoulder observed above 327 nm being surely due to the absorption of the PA core. Finally, it should be stressed that higher absorption coefficients are obtained for SPA-DAF than for STX-DAF (SPA-DAF: ε319 nm = 20.4 × 103 L mol−1 cm−1 and ε306 nm = 16.4 × 103 L mol−1 cm−1, STX-DAF: ε319 nm = 12.1 × 103 L mol−1 cm−1 and ε307 nm = 12.9 × 103 L mol−1 cm−1).
The calculated absorption spectra from TD-DFT (Fig. 6) show that, except for STX-TXO2, the HOMO/LUMO transitions are theoretically forbidden (oscillator strength: 0.000). This is a crucial point, which finds its origin in the spatial separation of the HOMO and LUMO levels leading to through space forbidden transitions. The calculated energy of this HOMO/LUMO transition varies from 327 nm for STX-TXO2 to 391 nm for SPA-DAF when going from weak donor/acceptor units (TX and TXO2) to strong ones (PA and DAF). In STX-TXO2, as the electronic densities of the HOMO and the LUMO allow some orbital overlap, the oscillator strength of the HOMO/LUMO transition is slightly higher (0.0553) and the transition is weakly allowed. Thus, in the case of the DAF derivatives SPA-DAF and STX-DAF, the theoretical HOMO/LUMO transitions (391 and 362 nm respectively) are not detectable experimentally in their corresponding absorption spectra. Similarly, no band at 350 nm is observed for SPA-TXO2. Finally, the HOMO/LUMO theoretical transition calculated at 327 nm for STX-TXO2 would be overlapped with the other absorptions bands.
Regarding the other transitions, one can note that a high oscillator strength (f > 0.17) transition is observed for both DAF derivatives. Indeed for both STX-DAF and SPA-DAF, HOMO−1/LUMO transitions between the DAF units are detected at 301 nm and 303 nm respectively. These oscillator strengths are in accordance with the high ε value recorded for these molecules (see above). For TXO2 derivatives, the situation is different. Indeed, for SPA-TXO2, all the oscillator strengths are very low (f < 0.033), with the more intense transitions being observed at 345 nm between the HOMO and LUMO+1 (f = 0.0324) and at 312 nm between the HOMO and LUMO+5 (f = 0.0312). The transitions all involve PA units. In the case of STX-TXO2, all the oscillator strengths are also very low except for a HOMO−1/LUMO transition (f = 0.1011) with both orbitals involving the TX and TXO2 molecular fragments.
Optical gaps, ΔEopt, have been evaluated from the onset of the last absorption band, varying from 3.54 eV to 3.79 eV (STX-TXO2: 3.73 eV, SPA-TXO2: 3.54 eV, STX-DAF: 3.79 eV and SPA-DAF: 3.64 eV). Thus, one can note that TXO2 derivatives present a ΔEopt in accordance with the electrochemical gap ΔEel, which is not the case for DAF derivatives (Table 1). Indeed, STX-DAF and SPA-DAF possess a ΔEopt strongly wider than their corresponding ΔEel (see Tables 1 and 2). Indeed and as exposed above, all molecules possess a theoretical forbidden through space HOMO/LUMO transition, calculated at a higher energy for TXO2 derivatives (327 and 350 nm) than for DAF derivatives (362 and 391 nm). ΔEopt should hence be determined with this absorption band. Thus, in the case of TXO2 derivatives, this hypothetical transition would be overlapped in the tail of the large absorption band at 323 nm (SPA-TXO2) and 309 nm (STX-TXO2) whereas in the case of DAF derivatives this band would be found at a lower energy (362 and 391 nm). Since ΔEopt does not correspond for DAF derivatives to the energy difference between the HOMO and LUMO, this feature explains the difference observed between ΔEopt and ΔEel. As DAF derivatives present an almost identical ΔEopt (3.79 and 3.64 eV), for both compounds, the transition involved is surely a HOMO−1/LUMO transition implying only the DAF units (Fig. 6).
Emission spectroscopy
TX derivatives present structureless emission spectra with maxima recorded at 338 nm for STX-TXO2 and at 348 nm for STX-DAF (Fig. 8, left). The literature reports the emission of 4,5-diazaspirobifluorene66 at 350 nm (in THF) in accordance with that of STX-DAF, highlighting the weak influence of the spiro-connected unit (fluorene in diazaspirobifluorene and thioxanthene in STX-DAF) on the DAF core. The emissions of the PA derivatives also appear structureless and are red-shifted compared to those of the TX derivatives (λmax = 348/350 nm for SPA-TXO2, and λmax = 388 nm for SPA-DAF). Thus, one can observe a red-shift of the emission maxima (and hence a gap contraction) as the donor and acceptor strength increases. It is important to mention that the emission of all compounds fits well with the energy of the HOMO/LUMO transition calculated by TD-DFT (STX-TXO2: 327 nm, STX-DAF: 350 nm, SPA-TXO2: 362 nm, and SPA-DAF: 391 nm, see above). This clearly confirms the above mentioned assignment of a through space electronic transfer depending on the electrochemical HOMO/LUMO gap. The emission of the four dyes is hence due to a photoinduced intramolecular charge transfer (ICT) between the donor and the acceptor in the excited state (see the below solvatochromic experiments). The greater the donor–acceptor strength, the more contracted the gap and the more red-shifted the emission. Another signature of a through space electronic transfer is the very low quantum yield (QY) translating the very weak probability of this transition. In the present case, the QYs are indeed very low varying from 0.1% for the two DAF derivatives to 0.8/4.1% for SPA-TXO2/STX-TXO2 respectively, in perfect accordance with our above mentioned conclusions. In addition, it should be noted that another emission of low intensity is detected at high energy (ca. 330/350 nm) for SPA-DAF, which can be tentatively assigned to an emission induced by the fragments (PA or DAF) alone corresponding to a locally excited state or to an emission from a higher excited state. Indeed, for comparison purposes, the emission of triphenylamine is reported at 358 nm (in THF)76 and that of N-phenyl-carbazole at 347 nm (in THF)76 in accordance with the tiny band detected for SPA-DAF.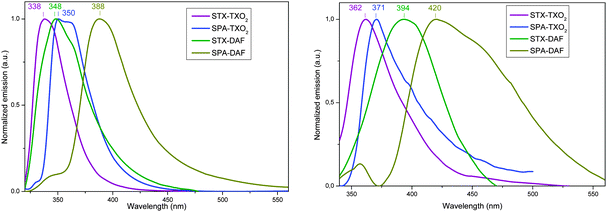 | ||
| Fig. 8 Emission spectra of the four dyes in cyclohexane (left) (concentration ∼ 10−3 M) and in the solid state (right) (thin film, deposited on a sapphire plate from 50 μL of a 10 g L−1 solution). | ||
Regarding the solid state fluorescence properties (Fig. 8, right), the emission spectra appear broader and red-shifted (from 21 to 46 nm) compared to the solution ones. This red-shift is assigned not only to the different dielectric constants between the two environments (liquid vs. solid) but also to the existence of intermolecular interactions in the solid state which may appear surprising due to the 3D geometry induced by the spiro carbon.
Solvatochromic experiments allow a deeper understanding of the photophysical properties of the dyes through the determination of the polarity of the excited states (Table 3 and Fig. 9). First, as the absorption spectra do not differ much as a function of the polarity, the characteristics of the ground and Franck–Condon excited states are very similar (see the ESI†). Oppositely, the fluorescence spectra show an intense solvatochromic effect. Indeed, the emission maxima of all the fluorophores are red-shifted with the increased polarity of the solvent (from cyclohexane to acetonitrile, see Fig. 9). This shift is of 58, 93, 110 and 111 nm for STX-TXO2, STX-DAF, SPA-TXO2 and SPA-DAF respectively. The large bathochromic shift of the fluorescence emission is the consequence of the stabilization of the intramolecular charge transfer (ICT) excited state relative to the ground state, leading to an energy gap contraction. This is caused by dipole–dipole interactions between the dyes and polar solvent molecules and hence there is a photoinduced ICT between the donor and the acceptor. Δμ values of 13.4 D (STX-TXO2), 32.6 D (SPA-TXO2), 14.3 D (STX-DAF) and 29.2 D (SPA-DAF) have been evaluated using the Lippert–Mataga formalism (the dipole moments at the ground state obtained through DFT calculations were: 4.8, 7.5, 3.1 and 6.2 D for STX-TXO2, SPA-TXO2, STX-DAF and SPA-DAF, see details of the calculations in the ESI†). We note that the PA derivatives (SPA-TXO2, Δμ: 32.6 D and SPA-DAF, Δμ: 29.2 D) lead to Δμ values twice as large as those calculated for the TX derivatives (STX-TXO2, Δμ: 13.4 D and STX-DAF, Δμ: 14.3 D). This reflects the stronger electron donating ability of PA compared to TX and hence the stronger ICT induced.
| λ abs (nm)/λem (nm)/Φ (%) [λexc (nm)] | Δμ/μ(S1)/μ(S0)a (D) | |||||
|---|---|---|---|---|---|---|
| Cyclohexane | Toluene | Chloroform | Ethyl acetate | Acetonitrile | ||
| a μ(S0) has been obtained through DFT calculations, and Δμ has been obtained using the Lippert–Mataga formalism, Δμ = μ(S1) − μ(S0). | ||||||
| STX-TXO2 | 309/338/0.8 [309] | 311/348/0.7 [309] | 312/354/0.7 [312] | 310/353/0.9 [309] | 310/396/1.1 [310] | 13.4/18.2/4.8 |
| SPA-TXO2 | 324/350/4.1 [323] | 326/382/2.3 [325] | 328/421/1.2 [328] | 326/410/1.7 [326] | 300/460/1.4 [323] | 32.6/40.1/7.5 |
| STX-DAF | 319/348/0.1 [319] | 322/391/0.1 [321] | 322/398/0.1 [322] | 320/395/0.1 [319] | 320/441/0.1 [319] | 14.3/17.4/3.1 |
| SPA-DAF | 319/388/0.1 [319] | 321/429/0.1 [321] | 323/440/0.2 [332] | 320/465/0.1 [319] | 321/499/0.5 [320] | 29.2/35.4/6.2 |
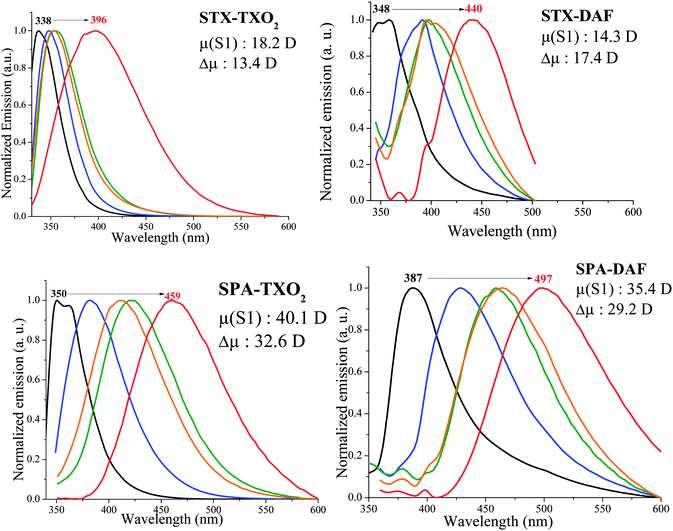 | ||
| Fig. 9 Normalized PL spectra of the four dyes in cyclohexane (black line), toluene (blue line), chloroform (green line), ethyl acetate (orange line) and acetonitrile (red line). | ||
Phosphorescence of the four dyes was studied at 77 K in frozen methylcyclohexane/2-methylpentane (1/1) (Fig. 10). TXO2 derivatives present a first phosphorescent transition at 405/402 nm corresponding to an ET of 3.06 and 3.08 eV for STX-TXO2 and SPA-TXO2 respectively. For the DAF derivatives, a first phosphorescence contribution is observed at 409 nm (ET: 3.03 eV) for STX-DAF and at 416 nm (ET: 2.98 eV) for SPA-DAF. In light of these results, the spin density of the triplet state of the four molecules is probably located on their corresponding acceptor unit. This is a key point to be considered for the future design of structurally related host materials with a D-spiro-A architecture. The four compounds present hence a very high ET (3.03 ± 0.05 eV), enabling their use as a host for blue dopants.
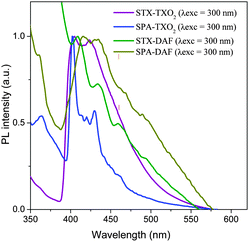 | ||
| Fig. 10 Emission spectra of the four dyes recorded at 77 K in frozen methylcyclohexane/2-methylpentane (1/1). | ||
Phosphorescent OLEDs
Sky-blue (FIrpic) PhOLEDs were fabricated and characterised using the four host materials. The device configuration was ITO/CuPc (10 nm)/NPB (40 nm)/TCTA (10 nm)/EML: FIrpic (20 nm)/TPBi (40 nm)/LiF (1.2 nm)/Al (100 nm). ITO is used as the anode, CuPc (copper phthalocyanine) is the hole injecting layer, NPB (N,N′-di(1-naphthyl)-N,N′-diphenyl-[1,10-biphenyl]-4,4′-diamine) is the hole transporting layer, TCTA (4,4′,4′′-tris(carbazol-9-yl)-triphenylamine) is the electron/exciton blocking layer, TPBI (1,3,5-tris(1-phenyl-1H-benzimidazol-2-yl)benzene) is both the electron transporting layer and the hole blocking layer and a thin film of lithium fluoride covered with aluminium is the cathode. The device architecture and the relative energy levels of the successive layers can be found in our previous work.32,33The four dyes were used as hosts for the sky-blue dopant FIrpic (ET: 2.62 eV).‡![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77 Different FIrpic concentrations were tested and the best performances were obtained with a FIrpic 20% doped EML. Those performances are reported in Fig. 11 and the main device characteristics are summarized in Table 4. The more efficient blue PhOLEDs were based on SPA-DAF, STX-DAF and SPA-TXO2, whereas devices using STX-TXO2 as the host were clearly less efficient.
77 Different FIrpic concentrations were tested and the best performances were obtained with a FIrpic 20% doped EML. Those performances are reported in Fig. 11 and the main device characteristics are summarized in Table 4. The more efficient blue PhOLEDs were based on SPA-DAF, STX-DAF and SPA-TXO2, whereas devices using STX-TXO2 as the host were clearly less efficient.
| Host | V on (V) | CE (cd A−1) | PE (lm W−1) | EQE (%) | CIE (x;y) | L max (cd m−2) (Jb) | ||
|---|---|---|---|---|---|---|---|---|
| L = 1a | J = 1b | J = 10b | J = 1b | J = 10b | J = 10b | J = 10 | ||
| a cd m−2, b mA cm−2. | ||||||||
| STX-TXO2 | 6.7 | 3.4 | 4.0 | 1.2 | 1.2 | 1.9 | 0.21; 0.41 | 1300 (70) |
| SPA-TXO2 | 3.1 | 17.1 | 27.7 | 12.3 | 14.7 | 10.6 | 0.17; 0.42 | 9200 (90) |
| STX-DAF | 3.6 | 22.5 | 22.0 | 13.7 | 10.4 | 8.0 | 0.17; 0.42 | 4300 (60) |
| SPA-DAF | 2.9 | 21.7 | 24.3 | 16.4 | 14.0 | 10.2 | 0.19; 0.43 | 6100 (70) |
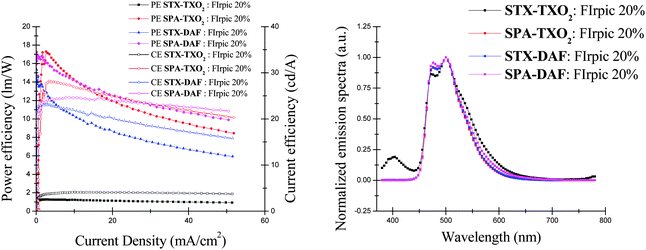
In the TXO2 family, PhOLEDs using SPA-TXO2 as the host reach a high EQE of 10.6% (at 10 mA cm−2), whereas those using STX-TXO2 as the host are much less efficient displaying a very low EQE of 1.9%. The corresponding luminous (CE) and power (PE) efficiencies are recorded at 27.7 cd A−1 and at 14.7 lm W−1 for SPA-TXO2 and at 4 cd A−1 and at 1.2 lm W−1 for STX-TXO2. The devices incorporating DAF derivatives do not display such differences as both display an interesting performance at 10 mA cm−2 with an EQE of 8% (CE = 22 cd A−1 and PE = 10.4 lm W−1) and 10.2% (CE = 24.3 cd A−1 and PE = 14 lm W−1) for STX-DAF and SPA-DAF respectively.
From these blue PhOLED performances, several conclusions can be drawn. First, it is clear that the incorporation of PA units within the structure is strongly correlated to the high performance of the PhOLED. Thus, PhOLED performances based on the PA units are always higher than those based on the TX unit due to the higher HOMO energy levels measured for the former. In addition, STX-TXO2, with the lowest HOMO and the highest LUMO levels in the series, appears to be a very bad host material highlighting how an unsuitable combination can dramatically decrease the efficiency of a device. The potential of the DAF core is more difficult to evaluate. Indeed, the association of the DAF and TXO2 fragments with the PA unit leads to high performance devices with very similar efficiencies (Table 4, EQE of 10.6% for SPA-TXO2 and of 10.2% for SPA-DAF at 10 mA cm−2). However, the association of the DAF and TX fragments in STX-DAF provides a PhOLED with an EQE four times higher than that of STX-TXO2 (8% vs. 1.9% respectively). The strong decrease of the LUMO level of STX-DAF compared to that of STX-TXO2, leading to more efficient electron injection, may be involved in this impressive difference in efficiency. However, this effect is not found for SPA-DAFvs.SPA-TXO2 and more experiments would be therefore necessary to fully unravel this peculiar behaviour. We believe nevertheless that the potential of the TX unit is clearly not as high as that of the PA unit.
Except for STX-TXO2 for which the performance is very low both in terms of the EQE (1.9%) and in terms of Von (6.7 V), the D-spiro-A design seems to be promising for hosting blue phosphors. Indeed, the present device performances are higher than those reported for non-bipolar structurally related hosts such as the parent molecule SBF (EQE: 6.5% at 10 mA cm−2).33 As the device architecture of previous work with SBF is the same as that presented herein (except for the host material), the different performance can only be attributed to the nature of the host and more particularly to the adjustments of the HOMO/LUMO energy levels. Similarly, 4-substituted SBF hosts, recently developed by our group such as 4-phenyl-SBF (EQE: 6%),33 4-5-pyrimidinyl-SBF (5%)78 or 4-pyridinyl-SBF32 (3.9 to 5.1%), all lead to a lower blue PhOLED performance. Moreover, the present devices using SPA-DAF, SPA-TXO2 and STX-DAF as hosts emit light at a lower Von (2.9 to 3.1 V for L = 1 cd m−2) than the above mentioned 4-phenyl-SBF,33 4-5-pyrimidinyl-SBF78 and 4-pyridinyl-SBF32 for which Von is higher than 4 V. SPA-DAF and SPA-TXO2 based blue PhOLEDs even surpass the performance of those based on the known and efficient host N,N-dicarbazolyl-3,5-benzene (mCP, ET: 2.9 eV) previously reported in the literature with exactly the same device architecture (EQE = 8.6% at 10 mA cm−2, Von = 3.2 V).45 The better performance of the present hosts is due to their bipolar character, which allows a good adjustment of their HOMO/LUMO energy levels.
Interestingly, comparing the results obtained at 100 cd m−2 to those recorded at higher luminance (1000 cd m−2, see the ESI†) shows an increase of the EQE for the three devices with SPA-TXO2/STX-DAF and SPA-DAF as the host from 5.8/8.1 and 8.7% to 10.8/8.3 and 10.2% respectively. These results show the stability and the efficiency of the devices even at high luminance.
All these features clearly highlight the efficiency of the D-spiro-A design to host the sky-blue phosphor FIrpic. Except for the device using STX-TXO2 as the host, the EL spectra of the three other devices are identical (Fig. 11, right), exclusively showing the emission of the blue dopant at 473 and 500 nm close to the photoluminescence of the pure FIrpic film (475/500 nm) with no parasite emission.77 The absence of other high energy emissions demonstrates an efficient energy transfer from the host to the guest. On the opposite side, the EL spectrum of the device using STX-TXO2 as the host presents in addition to the emission band of the dopant (FIrpic) another less intense emission centred at 400 nm in the range of the non-doped device emission (Fig. 11). This satellite emission and the lower efficiency of the device signifies a less efficient energy transfer from STX-TXO2 to FIrpic.
Conclusion
The present work reports the synthesis, the physicochemical and photophysical properties and the application in sky-blue PhOLEDs of four high triplet organic semi-conductors based on the D-spiro-A design. This promising chemical design is based on π-conjugation disruption induced by an insulating spiro bridge between a hole transporting unit, that is, a phenylacridine (PA) or thioxanthene (TX) moiety, and an electron transporting unit, that is, a dioxothioxanthene (TXO2) or diazafluorene (DAF) moiety. This molecular design leads to (i) a spatial separation of the HOMO and LUMO retaining a high ET, (ii) HOMO/LUMO levels of the constituting building blocks adapted to efficient charge injections and (iii) good physical properties, which are a key feature for device stability and performance. These host materials can be easily synthesized through a very short, efficient and highly adaptable synthetic strategy. In addition, we have shown that the properties of the dyes can be easily modulated depending on the strength of the donor/acceptor combination used, allowing adjustment of the HOMO/LUMO levels without disturbing the ET. These molecules have been incorporated as hosts in sky-blue PhOLEDs with EQEs at 10 mA cm−2 ranging from 2% to more than 10%. The best performance was obtained with SPA-DAF and SPA-TXO2, highlighting the importance of the chosen donor–acceptor combination on the device performance. We believe that the D-spiro-A design is very promising to elaborate efficient host materials for blue PhOLED applications.Acknowledgements
We wish to thank the CDIFX (Rennes) for X-ray diffraction data, the C.R.M.P.O for mass analysis, GENCI for allocation of computing time under project c2015085032 (Dr F. Barrière, Rennes), the Institut des Sciences Analytiques (Villeurbanne) for TGA, and the Service de Microanalyse-CNRS (Gif sur Yvette) for CHN analyses. MR, JRB and CP warmly thank the Région Bretagne and the ADEME for a studentship (MR), Dr B. Laffite (ADEME), the CNRS and the ANR (Projects HOME-OLED no. 11-BS07-020-01 and MEN IN BLUE no. 14-CE05-0024-01) for financial support.References
- B. Geffroy, P. Le Roy and C. Prat, Polym. Int., 2006, 55, 572–582 CrossRef CAS PubMed
.
- H. Sasabe and J. Kido, Chem. Mater., 2011, 23, 621–630 CrossRef CAS
.
- Y.-S. Tyan, J. Photonics Energy, 2011, 1, 011009 CrossRef PubMed
.
-
R. Mertens, The OLED Handbook, A guide to OLED Technology, Industry & Market, Ron Mertens, 2012 Search PubMed
.
- M. Romain, D. Tondelier, J.-C. Vanel, B. Geffroy, O. Jeannin, J. Rault-Berthelot, R. Métivier and C. Poriel, Angew. Chem., Int. Ed., 2013, 52, 14147–14151 CrossRef CAS PubMed
.
- N. Cocherel, C. Poriel, L. Vignau, J.-F. Bergamini and J. Rault-Berthelot, Org. Lett., 2010, 12, 452–455 CrossRef CAS PubMed
.
- C. Poriel, N. Cocherel, J. Rault-Berthelot, L. Vignau and O. Jeannin, Chem. – Eur. J., 2011, 17, 12631–12645 CrossRef CAS PubMed
.
- D. Thirion, M. Romain, J. Rault-Berthelot and C. Poriel, J. Mater. Chem., 2012, 22, 7149–7157 RSC
.
- X. Yang, X. Xu and G. Zhou, J. Mater. Chem. C, 2015, 3, 913–944 RSC
.
- H. Liang, X. Wang, X. Zhang, Z. Liu, Z. Ge, X. Ouyang and S. Wang, New J. Chem., 2014, 38, 4696–4701 RSC
.
- C. Liu, Q. Fu, Y. Zou, C. Yang, D. Ma and J. Qin, Chem. Mater., 2014, 26, 3074–3083 CrossRef CAS
.
- L. Yao, S. Sun, S. Xue, S. Zhang, X. Wu, H. Zhang, Y. Pan, C. Gu, F. Li and Y. Ma, J. Phys. Chem. C, 2013, 117, 14189–14196 CAS
.
- J.-H. Jou, S. Kumar, A. Agrawal, T.-H. Li and S. Sahoo, J. Mater. Chem. C, 2015, 3, 2974–3002 RSC
.
- M. A. Baldo, D. F. O'Brien, M. E. Thompson and S. R. Forrest, Phys. Rev. B, 1999, 60, 14422 CrossRef CAS
.
- M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikob, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151–154 CrossRef CAS
.
- L. Xiao, Z. Chen, B. Qu, J. Luo, S. Kong, Q. Cong and J. Kido, Adv. Mater., 2011, 23, 926–952 CrossRef CAS PubMed
.
- Y. Tao, C. Yang and J. Qin, Chem. Soc. Rev., 2011, 40, 2943–2970 RSC
.
- K. S. Yook and J. Y. L. Lee, Adv. Mater., 2012, 24, 3169–3190 CrossRef CAS PubMed
.
- E. Mondal, W.-Y. Hung, Y.-H. Chen, M.-H. Cheng and K.-T. Wong, Chem. – Eur. J., 2013, 19, 10563–10572 CrossRef CAS
.
- C. Han, Z. Zhang, H. Xu, J. Li, G. Xie, R. Chen, Y. Zhao and W. Huang, Angew. Chem., Int. Ed., 2012, 51, 10104–10108 CrossRef CAS PubMed
.
- C. W. Lee and Y. J. Lee, Chem. Mater., 2014, 26, 1616–1621 CrossRef CAS
.
- M. Romain, D. Tondelier, B. Geffroy, O. Jeannin, E. Jacques, J. Rault-Berthelot and C. Poriel, Chem. – Eur. J., 2015, 21, 9426–9439 CrossRef CAS PubMed
.
- C. Poriel, R. Métivier, J. Rault-Berthelot, D. Thirion, F. Barrière and O. Jeannin, Chem. Commun., 2011, 47, 11703–11705 RSC
.
- M. Romain, S. Thiery, A. Shirinskaya, C. Declairieux, D. Tondelier, B. Geffroy, O. Jeannin, J. Rault-Berthelot, R. Métivier and C. Poriel, Angew. Chem., Int. Ed., 2015, 54, 1176–1180 CrossRef CAS PubMed
.
- N. Fomina, S. E. Bradforth and T. E. Hogen-Esch, Macromolecules, 2009, 42, 6440–6447 CrossRef CAS
.
- S. Y. Hong, D. Y. Kim, C. Y. Kim and R. Hoffmann, Macromolecules, 2001, 34, 6474–6481 CrossRef CAS
.
- S. Karabunarliev, M. Baumgarten, N. Tyutyulkov and K. Müllen, J. Phys. Chem., 1994, 98, 11892–11901 CrossRef CAS
.
- B. Pan, B. Wang, Y. Wang, P. Xu, L. Wang, J. Chen and D. Ma, J. Mater. Chem. C, 2014, 2, 2466–2469 RSC
.
- S. Gong, Y.-L. Chang, K. Wu, R. White, Z.-H. Lu, D. Song and C. Yang, Chem. Mater., 2014, 26, 1463–1470 CrossRef CAS
.
- C. Fan, L. Zhu, T. Liu, B. Jiang, D. Ma, J. Qin and C. Yang, Angew. Chem., Int. Ed., 2014, 53, 2147–2151 CrossRef CAS PubMed
.
- M.-k. Leung, Y.-H. Hsieh, T.-Y. Kuo, P.-T. Chou, J.-H. Lee, T.-L. Chiu and H.-J. Chen, Org. Lett., 2013, 15, 4694–4697 CrossRef CAS PubMed
.
- S. Thiery, D. Tondelier, C. Declairieux, B. Geffroy, O. Jeannin, R. Métivier, J. Rault-Berthelot and C. Poriel, J. Phys. Chem. C, 2015, 119, 5790–5805 CAS
.
- S. Thiery, D. Tondelier, C. Declairieux, G. Seo, B. Geffroy, O. Jeannin, J. Rault-Berthelot, R. Métivier and C. Poriel, J. Mater. Chem. C, 2014, 2, 4156–4166 RSC
.
- M.-k. Leung, W.-H. Yang, C.-N. Chuang, J.-H. Lee, C.-F. Lin, M.-K. Wei and Y.-H. Liu, Org. Lett., 2012, 14, 4986–4989 CrossRef CAS PubMed
.
- C. Fan, Y. Chen, Z. Liu, Z. Jiang, C. Zhong, D. Ma, J. Qin and C. Yang, J. Mater. Chem. C, 2013, 1, 463–469 RSC
.
- J. Zhao, G.-H. Xie, C.-R. Yin, L.-H. Xie, C.-M. Han, R.-F. Chen, H. Xu, M.-D. Yi, Z.-P. Deng, S.-F. Chen, Y. Zhao, S.-Y. Liu and W. Huang, Chem. Mater., 2011, 23, 5331–5339 CrossRef CAS
.
- F. May, M. Al-Helwi, B. Baumeier, W. Kowalsky, E. Fuchs, C. Lennartz and D. Andrienko, J. Am. Chem. Soc., 2012, 134, 13818–13822 CrossRef CAS PubMed
.
- J.-K. Bin, N.-S. Cho and J.-I. Hong, Adv. Mater., 2012, 24, 2911–2915 CrossRef CAS PubMed
.
- J. Ding, Q. Wang, L. Zhao, D. Ma, L. Wang, X. Jing and F. Wang, J. Mater. Chem., 2010, 20, 8126–8133 RSC
.
- H. Ohkuma, T. Nakagawa, K. Shizu, T. Yasuda and C. Adachi, Chem. Lett., 2014, 43, 1017–1019 CrossRef CAS
.
- K. Nasu, T. Nakagawa, H. Nomura, C.-J. Lin, C.-H. Cheng, M.-R. Tseng, T. Yasuda and C. Adachi, Chem. Commun., 2013, 49, 10385–10387 RSC
.
- C.-J. Lin, H.-L. Huang, M.-R. Tseng and C.-H. Cheng, J. Disp. Technol., 2009, 5, 236–240 CrossRef CAS
.
- Y.-X. Zhang, L. Zhang, L.-S. Cui, C.-h. Gao, H. Chen, Q. Li, Z.-Q. Jiang and L.-S. Liao, Org. Lett., 2014, 16, 3748–3751 CrossRef CAS PubMed
.
- L. Ding, S.-C. Dong, Z.-Q. Jiang, H. Chen and L.-S. Liao, Adv. Funct. Mater., 2015, 25, 645–650 CrossRef CAS PubMed
.
- M. Romain, D. Tondelier, B. Geffroy, A. Shirinskaya, O. Jeannin, J. Rault-Berthelot and C. Poriel, Chem. Commun., 2015, 51, 1313–1315 RSC
.
- B.-C. Wang, H.-R. Liao, J.-C. Chang, L. Chen and J.-T. Yeh, J. Lumin., 2007, 127, 333–342 CrossRef PubMed
.
- G. Méhes, H. Nomura, Q. Zhang, T. Nakagawa and C. Adachi, Angew. Chem., Int. Ed., 2012, 51, 11311–11315 CrossRef PubMed
.
- Z. Jiang, Z. Liu, C. Yang, C. Zhong, J. Qin, G. Yu and Y. Liu, Adv. Funct. Mater., 2009, 19, 3987–3995 CrossRef CAS PubMed
.
- T. Liu, H. Sun, C. Fan, D. Ma, C. Zhong and C. Yang, Org. Electron., 2014, 15, 3568–3576 CrossRef CAS PubMed
.
- H. Chen, Z.-Q. Jiang, C.-H. Gao, M.-F. Xu, S.-C. Dong, L.-S. Cui, S.-J. Ji and L.-S. Liao, Chem. – Eur. J., 2013, 19, 11791–11797 CrossRef CAS PubMed
.
- C.-Y. Chan, Y.-C. Wong, M.-Y. Chan, S.-H. Cheung, S.-K. So and V. W.-W. Yam, Chem. Mater., 2014, 26, 6585–6594 CrossRef CAS
.
- Q. Zhang, J. Li, K. Shizu, S. Huang, S. Hirata, H. Miyazaki and C. Adachi, J. Am. Chem. Soc., 2012, 134, 14706–14709 CrossRef CAS PubMed
.
- H. Li, A. S. Batsanov, K. C. Moss, H. L. Vaughan, F. B. Dias, K. T. r. Kamtekar, M. R. Bryce and A. P. Monkman, Chem. Commun., 2010, 46, 4812–4814 RSC
.
- Y. Li, Z. Wang, X. Li, G. Xie, D. Chen, Y.-F. Wang, C.-C. Lo, A. Lien, J. Peng, Y. Cao and S.-J. Su, Chem. Mater., 2015, 27, 1100–1109 CrossRef CAS
.
- S.-J. Kim, J. Leroy, C. Zuniga, Y. Zhang, L. Zhu, J. S. Sears, S. Barlow, J.-L. Brédas, S. R. Marder and B. Kippelen, Org. Electron., 2011, 12, 1314–1318 CrossRef CAS PubMed
.
- H. Sasabe, Y. Seino, M. Kimura and J. Kido, J. Mater. Chem., 2012, 24, 1404–1406 CrossRef CAS
.
- F.-M. Hsu, C.-H. Chien, Y.-J. Hsieh, C.-H. Wu, C.-F. Shu, S.-W. Liu and C.-T. Chen, J. Mater. Chem., 2009, 19, 8002–8008 RSC
.
- S. O. Jeon, T. Earmme and S. A. Jenekhe, J. Mater. Chem. C, 2014, 2, 10129–10137 RSC
.
- K.-T. Wong, R.-T. Chen, F.-C. Fang, C.-c. Wu and Y.-T. Lin, Org. Lett., 2005, 7, 1979–1982 CrossRef CAS PubMed
.
- W.-Y. Hung, T.-C. Wang, H.-C. Chiu, H.-F. Chen and K.-T. Wong, Phys. Chem. Chem. Phys., 2010, 12, 10685–10687 RSC
.
- H.-F. Chen, T.-C. Wang, W.-Y. Hung, H.-C. Chiu, C. Yun and K.-T. Wong, J. Mater. Chem., 2012, 22, 9658–9664 RSC
.
- C.-C. Chi, C.-L. Chiang, S.-W. Liu, H. Yueh, C.-T. Chen and C.-T. Chen, J. Mater. Chem., 2009, 19, 5561–5571 RSC
.
- W.-J. Li, B. Liu, Y. Qian, L.-H. Xie, J. Wang, S.-B. Li and W. Huang, Polym. Chem., 2013, 4, 1796–1802 RSC
.
- M. Hong, K. Zhang, Y.-Z. Li and J. Zhu, Polyhedron, 2009, 28, 445–452 CrossRef CAS PubMed
.
- X. Li, H.-J. Chi, G.-H. Lu, G.-Y. Xiao, Y. Dong, D.-Y. Zhang, Z.-Q. Zhang and Z.-Z. Hu, Org. Electron., 2012, 13, 3138–3144 CrossRef CAS PubMed
.
- C.-C. Wang, C.-H. Yang, S.-M. Tseng, S.-Y. Lin, T.-Y. Wu, M.-R. Fuh, G.-H. Lee, K.-T. Wong, R.-T. Chen, Y.-M. Cheng and P.-T. Chou, Inorg. Chem., 2004, 43, 4781–4783 CrossRef CAS PubMed
.
- H.-F. Chen, K.-T. Wong, Y.-H. Liu, Y. Wang, Y.-M. Cheng, M.-W. Chung, P.-T. Chou and H.-C. Su, J. Mater. Chem., 2011, 21, 768–774 RSC
.
- H.-F. Chen, W.-Y. Hung, S.-W. Chen, T.-C. Wang, S.-W. Lin, S.-H. Chou, C.-T. Liao, H.-C. Su, H.-A. Pan, P.-T. Chou, Y.-H. Liu and K.-T. Wong, Inorg. Chem., 2012, 51, 12114–12121 CrossRef CAS PubMed
.
- C.-J. Zheng, J. Ye, M.-F. Lo, M.-K. Fing, X.-M. Ou, X.-H. Zhang and C.-S. Lee, Chem. Mater., 2012, 24, 643–650 CrossRef CAS
.
- M. J. Plater, S. Kemp and E. Lattmann, J. Chem. Soc., Perkin Trans. 1, 2000, 971–979 RSC
.
- G. R. Fulmer, A. J. M. Miller, N. H. Sherden, H. E. Gottlieb, A. Nudelman, B. M. Stoltz, J. E. Bercaw and K. I. Goldberg, Organometallics, 2010, 29, 2176–2179 CrossRef CAS
.
- T. P. I. Saragi, T. Spehr, A. Siebert, T. Fuhrmann-Lieker and J. Salbeck, Chem. Rev., 2007, 107, 1011–1065 CrossRef CAS PubMed
.
- C. Poriel, J.-J. Liang, J. Rault-Berthelot, F. Barrière, N. Cocherel, A. M. Z. Slawin, D. Horhant, M. Virboul, G. Alcaraz, N. Audebrand, L. Vignau, N. Huby, G. Wantz and L. Hirsch, Chem. – Eur. J., 2007, 13, 10055–10069 CrossRef CAS PubMed
.
- R. Badiello, E. M. Fielden and S. C. Lillicrap, Int. J. Radiat. Phys. Chem., 1973, 5, 173–181 CrossRef CAS
.
- R. P. Thummel, F. Lefoulon and R. Mahadevan, J. Org. Chem., 1985, 50, 3824–3828 CrossRef CAS
.
- C.-C. Lee, M.-k. Leung, P.-Y. Lee, T.-L. Chiu, J.-H. Lee, C. Liu and P.-T. Chou, Macromolecules, 2012, 45, 751–765 CrossRef CAS
.
- E. Baranoff and B. F. E. Curchod, Dalton Trans., 2015, 44, 8318–8329 RSC
.
- S. Thiery, C. Declairieux, D. Tondelier, G. Seo, B. Geffroy, O. Jeannin, R. Métivier, J. Rault-Berthelot and C. Poriel, Tetrahedron, 2014, 70, 6337–6351 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and methods and experimental details; synthesis and characterization of all compounds, absorption spectra in various solvents, DSC, a copy of the NMR spectra, and green device performance. CCDC 1046694–1046697 and 1046704. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5tc01812a |
| ‡ The four dyes have been also used as hosts for green Ir(ppy)3 and the device performances are gathered in the ESI.† |
| This journal is © The Royal Society of Chemistry 2015 |