Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Twisted hexaazatrianthrylene: synthesis, optoelectronic properties and near-infrared electroluminescent heterojunctions thereof†
Diego
Cortizo-Lacalle
a,
Antonio
Pertegás
b,
Laura
Martínez-Sarti
b,
Manuel
Melle-Franco
c,
Henk J.
Bolink
*b and
Aurelio
Mateo-Alonso
*ad
aPOLYMAT, University of the Basque Country UPV/EHU, Avenida de Tolosa 72, E-20018 Donostia-San Sebastian, Spain. E-mail: amateo@polymat.eu
bInstituto de Ciencia Molecular, Universidad de Valencia, c/Catedrático J. Beltrán 2, 46980 Paterna, Spain. E-mail: henk.bolink@uv.es
cCentro ALGORITMI, 4710-057 Braga, Portugal
dIkerbasque, Basque Foundation for Science, Bilbao, Spain
First published on 4th August 2015
Abstract
The synthesis, optoelectronic properties and near-infrared electroluminescent heterojunctions of a twisted and soluble 7,8,15,16,23,24-hexaazatrianthrylene derivative are reported.
Introduction
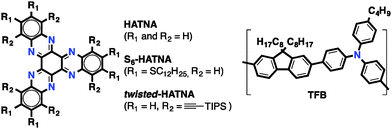
Near-infrared organic light-emitting diodes (NIR-OLEDs)1 are receiving a great deal of attention due to their potential applications in broad-band optical communications, night-vision technologies, and among others. In general, the emission in NIR-OLEDs originates from the radiative relaxation of excitons crossing the inherent low HOMO–LUMO gaps of NIR dyes and polymers.2 However, organic NIR-emitting materials are scarce because low HOMO–LUMO gaps favour non-emissive deactivation pathways,3 and also, many of the low HOMO–LUMO gap organics possess limited photo- and chemical stability.An alternative way to fabricate NIR-OLEDs without the use of NIR emitters consists of exploiting the exciplex-based emission that originates at organic heterojunctions4 between p-type and n-type materials. In this case, the emission is the result of the radiative decay of a charge pair exciton formed between the LUMO of the n-type material and the HOMO of the p-type material. Therefore, the emission of low energy photons can be achieved by a careful selection of stable organic semiconductors. For example, red-4b (670 nm) and NIR-emitting4c,5 (816 nm) exciplexes have been obtained from green 5,6,11,12,17,18-hexaazatrinaphtylene (HATNA) emitters (S6-HATNA and twisted-HATNA, respectively) – a family of electron-conducting N-containing starphenes – combined with hole-conducting poly(9,9′-dioctylfluorene-alt-N-(4-butylphenyl)-diphenylamine) (TFB) (Fig. 1).
In analogy to linear systems,2a,6 extending the conjugation of HATNA by condensing additional rings will provide materials with lower LUMO levels and thus, heterojunctions with deeper NIR luminescence. Yet, such extended starphenes are very insoluble7 and thus difficult to synthesise, purify and characterise because of their great tendency to aggregate through π–π and van der Waals interactions. This lack of solubility also hampers the preparation of blends with other p-type materials and consequently processing thereof by means of low-cost and large-area liquid deposition methods.
In this work, we report the synthesis of twisted 7,8,15,16,23,24-hexaazatrianthrylene (twisted-HATANT) (Scheme 1), an extended member of the N-containing starphene family. While, starphenes with an equal number of fused rings are virtually insoluble,7a,btwisted-HATANT shows a high solubility because of the distorted π-system and the six triisopropylsilyl (TIPS) substituents. The enhanced solubility of twisted-HATANT allows the preparation of blends with TFB and the deposition thereof by liquid methods. Furthermore, twisted-HATANT/TFB organic heterojunctions show NIR electroluminescence arising from the exciplex at substantially higher wavelengths (848 nm) than those previously reported4b,5 for HATNA heterojunctions.
Results and discussion
Synthesis and structure
The synthesis of twisted-HATANT was achieved following the synthetic route set out in Scheme 1. Even if naphthalenediamine 1 has been previously reported,8 we have developed an alternative synthetic route. First, dibromothiadiazole 2 was prepared from the commercially available 1,2-naphthalenediamine in two steps following reported procedures.9 Then, 2 was transformed into acetylene-substituted thiodiazole 3 by Stille coupling. The reduction of the thiadiazole ring of 3 with LiAlH4 provided diamine 1.8 Finally, twisted-HATANT was obtained by cyclocondensation of 1 with 1,2,3,4,5,6-hexaketonecyclohexane in refluxing acetic acid. The yield of twisted-HATANT is low (3%) in comparison to the structurally equivalent twisted-HATNA5 (32%). This low yield is consistent with the lower nucleophilicity of diamine 1, a recent report describes the formation of the linear diketone10 as a side but major product, and the difficult purification, which reflect that both the synthesis and purification of twisted-HATANT are quite challenging.DFT calculations using a B3LYP-6-31g(d,p) Hamiltonian11 reveal a propeller-like structure for twisted-HATANT (Fig. 2), consistent with the previously reported crystal structure5 of twisted-HATNA. Most importantly, the calculations evidence a higher average twist angle between blades of 67° (65°, 68° and 69° for each blade) in comparison to the calculated twist angles of twisted-HATNA (64°) at the same level. The twist angle between blades is defined as the torsion angle between carbon A, B, C and D (Fig. 2). The higher level of distortion can be rationalised in terms of the more strained structure of HATANT due to the additional annulated ring.
Optical and electrochemical characterisation
The colour of twisted-HATANT is old brick red with a red photoluminescence. The absorption features of twisted-HATANT in CHCl3 (maxima at 319, 331, 401, 556, and 597 nm) correspond to those of previously reported5 for twisted-HATNA but as expected they appear to be substantially red-shifted, as much as 138 nm (Fig. 3 and Fig. S4, ESI†). The photoluminescence spectrum of twisted-HATANT in CHCl3 obtained by excitation at 400 nm reveals a featureless band centred at 636 nm with a shoulder at 687 nm that extends into the NIR up to 820 nm (Fig. 3). The photoluminescence of twisted-HATANT is also substantially red-shifted (132 nm) in comparison to twisted-HATNA5 (Fig. S4, ESI†).To shed light on the nature of the electronic transitions of twisted-HATANT, the lower energy singlet excited states were calculated using the program Gaussian 0911 using time-dependent density functional theory (TD-DFT B3LYP-CHCl3-6-31g(d,p)). From TD-DFT it is found that all excited states are singlets (transitions involving triplets were found to be optically inactive). The TD-DFT model computes the experimental band centred at 597 nm as a HOMO → LUMO transition at 715 nm. The experimental band centred at 556 is calculated as the sum of two electronic transitions: HOMO−1 → LUMO and HOMO−2 → LUMO at ∼680 nm. The experimental band at 401 nm is calculated as the sum of several transitions from HOMO−1 and HOMO−2 to LUMO+1 and LUMO+2 that range from 528 to 537 nm. This arises from the fact that HOMO−1/HOMO−2 and LUMO+1/LUMO+2 couples are, respectively, nearly degenerated with ΔE < 8 meV.
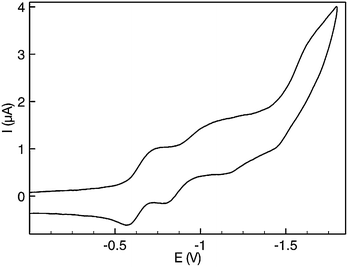
Cyclic voltammograms of twisted-HATANT (0.1 M nBu4PF6 in CH2Cl2) exhibit two reduction waves at E1/2 = −0.66 and −0.95 V (Fig. 4), which were assigned to the radical-anion and the dianion in analogy to phenazine derivatives.12 When the scans were performed close to the reduction of the solvent-supported electrolyte an additional irreversible curve was observed with peak potential Epc = −1.64 V from which an additional process evolved with peak potential Epa = −1.16 V on the reverse scan. On the oxidative scan, no redox processes were observed within the solvent-supported electrolyte window.
Energy levels
The HOMO–LUMO gap of twisted-HATANT (1.9 eV) was estimated from the absorption onset. The electrochemical LUMO of twisted-HATANT (−3.8 eV) was estimated from the potential onsets of the first reduction waves. The theoretical estimation, B3LYP-CHCL3-6-31g(d,p), of the HOMO–LUMO gap (2.1 eV) and the LUMO (−3.4 eV) are in good agreement with the HOMO–LUMO gap and the LUMO estimated experimentally. In the simulations, both HOMO and LUMO are delocalised along the HATANT core. Nevertheless the HOMO coefficients are larger in the points (the naphthyl residue) of twisted-HATANT, while larger coefficients are found in the central ring for the LUMO (Fig. 5).Electroluminescence
OLEDs incorporating a blend of twisted-HATANT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TFB (1
TFB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
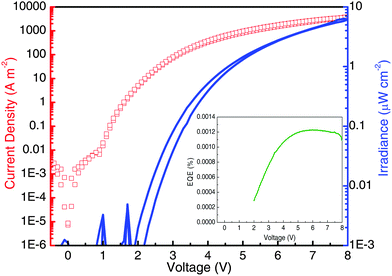
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were fabricated with the device structure shown in Fig. 6. On an ITO electrode, a PEDOT:PSS layer (70 nm) followed by a twisted-HATANT:TFB layer (95 nm) were deposited via spin coating. On top of which, a Ba cathode (5 nm) and a silver contact (70 nm) were evaporated, consecutively. The electroluminescence spectrum and the current and radiance versus voltage characteristics of the OLEDs were recorded and are shown in Fig. 7 and 8. The electroluminescence spectrum displays a structureless emission band centred at 848 nm (1.46 eV). This is consistent with the emission of an exciplex state estimated at 1.5 eV from the individual HOMO of TFB4c and LUMO of twisted-HATANT. Importantly, no emission from twisted-HATANT or TFB was detected. The LEDs show a turn on voltage of about 2 V and an irradiance of 6.3 μW cm−2 at a current density of 100 A m−2 with an external quantum efficiency (EQE) of 0.0012% (inset Fig. 8). Although these values seem to be low, they are within the expectations13 for LEDs with emission at similar and lower energies and in agreement with the energy gap law.3
1) were fabricated with the device structure shown in Fig. 6. On an ITO electrode, a PEDOT:PSS layer (70 nm) followed by a twisted-HATANT:TFB layer (95 nm) were deposited via spin coating. On top of which, a Ba cathode (5 nm) and a silver contact (70 nm) were evaporated, consecutively. The electroluminescence spectrum and the current and radiance versus voltage characteristics of the OLEDs were recorded and are shown in Fig. 7 and 8. The electroluminescence spectrum displays a structureless emission band centred at 848 nm (1.46 eV). This is consistent with the emission of an exciplex state estimated at 1.5 eV from the individual HOMO of TFB4c and LUMO of twisted-HATANT. Importantly, no emission from twisted-HATANT or TFB was detected. The LEDs show a turn on voltage of about 2 V and an irradiance of 6.3 μW cm−2 at a current density of 100 A m−2 with an external quantum efficiency (EQE) of 0.0012% (inset Fig. 8). Although these values seem to be low, they are within the expectations13 for LEDs with emission at similar and lower energies and in agreement with the energy gap law.3
Experimental
Synthetic procedures
Reagents for synthesis were, if not otherwise specified, purchased from Aldrich, Fluka or Acros. Commercial chemicals and solvents were used as received. Anhydrous chloroform from Sigma-Aldrich was used as purchased. Anhydrous THF was dried using an Innovative Pure Solve solvent purification system. Column chromatography was carried out using Silica gel 60 (40–60 μm) from Scharlab. Analytical thin layer chromatography (TLC) was carried out using aluminum sheets (20 × 20 cm) pre-coated with silica gel RP-18W 60 F254 from Merck. UV-active compounds were detected using a UV-lamp from CAMAG at wavelength λ = 254 or 366 nm. NMR spectra were recorded on a Bruker Avance 400 spectrometer at 298 K using partially deuterated solvents as internal standards. Matrix Assisted Laser Desorption Ionization (coupled to a Time-Of-Flight analyzer) experiments (MALDI-TOF) were recorded on a Bruker REF LEX spectrometer in POLYMAT by Dr Antonio Veloso. Absorption and emission spectra were recorded on a Perkin-Elmer Lambda 950 spectrometer and a LS55 Perkin-Elmer Fluorescence spectrometer, respectively. Electrochemical measurements were carried out on a Princeton Applied Research Parstat 2273 in a 3-electrode single compartment cell with a Pt disc working electrode (∅ = 0.5 mm), a platinum wire counter electrode (∅ = 0.5 mm) and a silver wire pseudoreference electrode. The cell and the electrodes were custom made. The reduction potentials were referred to SCE using ferrocene (Fc) as internal reference (EFc(SCE)1/2 = +0.48 V) after the measurements.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was carried out. The main fractions were precipitated, the precipitate was filtered and a bright pink solid was obtained (104 mg, 48%). 1H NMR (CDCl3): 8.60–8.54 (q, 2H), 7.61–7.53 (q, 2H) and 1.32–1.23 (m, 42H). 1H-NMR is consistent with previous reports.8a
1) was carried out. The main fractions were precipitated, the precipitate was filtered and a bright pink solid was obtained (104 mg, 48%). 1H NMR (CDCl3): 8.60–8.54 (q, 2H), 7.61–7.53 (q, 2H) and 1.32–1.23 (m, 42H). 1H-NMR is consistent with previous reports.8a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was carried out. The product was isolated as dark powder (10 mg, 3%). 1H NMR (CDCl3): 8.86–8.79 (q, 6H), 7.80–7.73 (q, 6H) and 1.23–1.18 (m, 126H); 13C NMR (CDCl3): 143.62, 140.90, 135.74, 128.56, 127.94, 122.97, 109.57, 102.64, 19.13 and 11.88; EM (MALDI-TOF) (m/z): calculated for C102H138N6Si6: 1615.96; found: 1616.99 [M + H]+ and 1638.98 [M + Na]+; UV-vis (log
1) was carried out. The product was isolated as dark powder (10 mg, 3%). 1H NMR (CDCl3): 8.86–8.79 (q, 6H), 7.80–7.73 (q, 6H) and 1.23–1.18 (m, 126H); 13C NMR (CDCl3): 143.62, 140.90, 135.74, 128.56, 127.94, 122.97, 109.57, 102.64, 19.13 and 11.88; EM (MALDI-TOF) (m/z): calculated for C102H138N6Si6: 1615.96; found: 1616.99 [M + H]+ and 1638.98 [M + Na]+; UV-vis (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 401 (5.06), 419 (4.96), 556 (3.95), 597 (3.88).
ε): 401 (5.06), 419 (4.96), 556 (3.95), 597 (3.88).
OLED fabrication
The sandwiched OLEDs were prepared as follows: the pre-patterned ITO glass plates were extensively cleaned, using water–soap, water and 2-propanol baths and UV-ozone treatment, just before the deposition of the thin films. Prior to the deposition of the active layer, an 80 nm layer of poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS, CLEVIOS™ P VP AI 4083, aqueous dispersion, 1.3–1.7% solid content, Heraeus) was deposited. Subsequently, the twisted-HATANT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TFB blend was deposited by spin-coating using 10 mg mL−1 (1
TFB blend was deposited by spin-coating using 10 mg mL−1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio) of chlorobenzene solution, which yields a 90 nm thick layer. The film thickness was determined using an Ambios XP1 profilometer. After spinning the organic layers, the samples were transferred to an inert atmosphere glovebox (<0.1 ppm O2 and H2O, MBraun). Barium (5 nm) and silver (70 nm) were thermally evaporated using a shadow mask under a vacuum (<1 × 10−6 mbar, 1 bar ≈ 100
1 mass ratio) of chlorobenzene solution, which yields a 90 nm thick layer. The film thickness was determined using an Ambios XP1 profilometer. After spinning the organic layers, the samples were transferred to an inert atmosphere glovebox (<0.1 ppm O2 and H2O, MBraun). Barium (5 nm) and silver (70 nm) were thermally evaporated using a shadow mask under a vacuum (<1 × 10−6 mbar, 1 bar ≈ 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa) using an Edwards Auto500 evaporator integrated into an inert atmosphere glovebox. I–V–L measurements were obtained by applying a voltage scan over the device and monitoring the current flow (using a Keithley 2400 sourcemeter) and simultaneously the current generated by a Si-photodiode (Hamamatsu S1336-8BK) using a Keithley 6485 picoamperometer calibrated using a Minolta LS100 luminance meter. The electroluminescence spectra were recorded using an Avantes AvaSpec-2048 Fiber Optic Spectrometer.
000 Pa) using an Edwards Auto500 evaporator integrated into an inert atmosphere glovebox. I–V–L measurements were obtained by applying a voltage scan over the device and monitoring the current flow (using a Keithley 2400 sourcemeter) and simultaneously the current generated by a Si-photodiode (Hamamatsu S1336-8BK) using a Keithley 6485 picoamperometer calibrated using a Minolta LS100 luminance meter. The electroluminescence spectra were recorded using an Avantes AvaSpec-2048 Fiber Optic Spectrometer.
Conclusions
We have reported the synthesis and characterisation of twisted-HATANT, an extended and distorted member of the N-containing starphene family. In contrast to starphenes with an equal number of rings,7a,btwisted-HATANT is highly soluble in organic solvents, which allowed a complete characterisation (including absorption, emission, and cyclic voltammetry) and the estimation of its electronic properties. Calculations carried out at the DFT level illustrate the distorted nature of twisted-HATANT and also provide a detailed perspective on the nature of the observed optoelectronic properties. Furthermore, the enhanced solubility of twisted-HATANT allowed the preparation of blends with TFB and the deposition thereof by spin coating. OLEDs fabricated from such blends show NIR electroluminescence that originates from the exciplex at the twisted-HATANT/TFB heterojunction. The electroluminescence appears at substantially higher wavelengths (centred at 848 nm) than for previously reported4b,5 heterojunctions with HATNA derivatives and is consistent with the extended conjugation of the HATANT core. Overall this work illustrates that electron-deficient N-containing starphenes can be considered a general platform to prepare and fine-tune the properties of exciplex-based NIR-OLEDs.Acknowledgements
We are grateful to the Basque Science Foundation for Science (Ikerbasque), POLYMAT, the University of the Basque Country (SGIker), Deutsche Forschungsgemeinschaft (AU 373/3-1 and MA 5215/4-1), Gobierno de España (Ministerio de Economía y Competitividad, MAT2012-35826 and MAT2014-55200), Gobierno Vasco (BERC program), Diputación Foral de Guipuzcoa, the Fundação para a Ciência e a Tecnologia, ON2 (NORTE-07-0162-FEDER-000086), the Generalitat Valenciana (Prometeo/2012/053) and the European Union (ERA-Chemistry, Career Integration Grant No. 618247, and FEDER).Notes and references
- Z. I. Wang, Near-Infrared Organic Materials and Emerging Applications, CRC Press, 2013 Search PubMed.
- (a) R. Garcia, M. Melle-Franco and A. Mateo-Alonso, Chem. Commun., 2015, 51, 8037–8040 RSC; (b) L. Yao, S. Zhang, R. Wang, W. Li, F. Shen, B. Yang and Y. Ma, Angew. Chem., Int. Ed., 2014, 53, 2119–2123 CrossRef CAS PubMed; (c) B. Stender, S. F. Völker, C. Lambert and J. Pflaum, Adv. Mater., 2013, 25, 2943–2947 CrossRef CAS PubMed; (d) M. Chen, E. Perzon, M. R. Andersson, S. Marcinkevicius, S. K. M. Jönsson, M. Fahlman and M. Berggren, Appl. Phys. Lett., 2004, 84, 3570–3572 CrossRef CAS PubMed.
- R. Englman and J. Jortner, Mol. Phys., 1970, 18, 145–164 CrossRef CAS PubMed.
- (a) Y.-s. Huang, S. Westenhoff, I. Avilov, P. Sreearunothai, J. M. Hodgkiss, C. Deleener, R. H. Friend and D. Beljonne, Nat. Mater., 2008, 7, 483–489 CrossRef CAS PubMed; (b) J. Clark, R. Archer, T. Redding, C. Foden, J. Tant, Y. Geerts, R. H. Friend and C. Silva, J. Appl. Phys., 2008, 103, 124510 CrossRef PubMed; (c) G. Tregnago, C. Fléchon, S. Choudhary, C. Gozalvez, A. Mateo-Alonso and F. Cacialli, Appl. Phys. Lett., 2014, 105, 143304 CrossRef PubMed; (d) T.-W. Ng, M.-F. Lo, M.-K. Fung, W.-J. Zhang and C.-S. Lee, Adv. Mater., 2014, 26, 5569–5574 CrossRef CAS PubMed.
- S. Choudhary, C. Gozalvez, A. Higelin, I. Krossing, M. Melle-Franco and A. Mateo-Alonso, Chem. – Eur. J., 2014, 20, 1525–1528 CrossRef CAS PubMed.
- (a) A. Mateo-Alonso, C. Ehli, K. H. Chen, D. M. Guldi and M. Prato, J. Phys. Chem. A, 2007, 111, 12669–12673 CrossRef CAS PubMed; (b) A. Mateo-Alonso, N. Kulisic, G. Valenti, M. Marcaccio, F. Paolucci and M. Prato, Chem. – Asian J., 2010, 5, 482–485 CrossRef CAS PubMed; (c) N. Kulisic, S. More and A. Mateo-Alonso, Chem. Commun., 2011, 47, 514–516 RSC; (d) S. More, R. Bhosale, S. Choudhary and A. Mateo-Alonso, Org. Lett., 2012, 14, 4170–4173 CrossRef CAS PubMed; (e) R. García, S. More, M. Melle-Franco and A. Mateo-Alonso, Org. Lett., 2014, 16, 6096–6099 CrossRef PubMed; (f) A. Mateo-Alonso, Chem. Soc. Rev., 2014, 43, 6311–6324 RSC; (g) P. Bodis, M. R. Panman, B. H. Bakker, A. Mateo-Alonso, M. Prato, W. J. Buma, A. M. Brouwer, E. R. Kay, D. A. Leigh and S. Woutersen, Acc. Chem. Res., 2009, 42, 1462–1469 CrossRef CAS PubMed; (h) S. More, R. Bhosale and A. Mateo-Alonso, Chem. – Eur. J., 2014, 20, 10626–10631 CrossRef CAS PubMed; (i) S. More, S. Choudhary, A. Higelin, I. Krossing, M. Melle-Franco and A. Mateo-Alonso, Chem. Commun., 2014, 50, 1976–1979 RSC; (j) A. B. Marco, D. Cortizo-Lacalle, C. Gozalvez, M. Olano, A. Atxabal, X. Sun, M. Melle-Franco, L. E. Hueso and A. Mateo-Alonso, Chem. Commun., 2015, 51, 10754–10757 RSC; (k) M. Grzelczak, N. Kulisic, M. Prato and A. Mateo-Alonso, Chem. Commun., 2010, 46, 9122–9124 RSC; (l) M. Grzelczak, N. Kulisic, M. Prato and A. Mateo-Alonso, Part. Part. Syst. Charact., 2014, 31, 121–125 CrossRef CAS PubMed.
- (a) E. Clar and A. Mullen, Tetrahedron, 1968, 24, 6719–6724 CrossRef CAS; (b) S. R. Marder, B. Kaafarani, S. Barlow, B. Kippelen, B. Domercq, Q. Zhang and T. Kondo, Charge-transport materials, methods of fabrication thereof, and methods of use thereof, WO Pat., 2005/123737 A2, 2005 Search PubMed; (c) E. Clar, The Aromatic Sextet, Wiley, London, 1972 Search PubMed.
- (a) A. L. Appleton, S. Miao, S. M. Brombosz, N. J. Berger, S. Barlow, S. R. Marder, B. M. Lawrence, K. I. Hardcastle and U. H. F. Bunz, Org. Lett., 2009, 11, 5222–5225 CrossRef CAS PubMed; (b) S. Miao, S. M. Brombosz, P. v. R. Schleyer, J. I. Wu, S. Barlow, S. R. Marder, K. I. Hardcastle and U. H. F. Bunz, J. Am. Chem. Soc., 2008, 130, 7339–7344 CrossRef CAS PubMed.
- P. Wei, L. Duan, D. Zhang, J. Qiao, L. Wang, R. Wang, G. Dong and Y. Qiu, J. Mater. Chem., 2008, 18, 806–818 RSC.
- C. Wang, J. Zhang, G. Long, N. Aratani, H. Yamada, Y. Zhao and Q. Zhang, Angew. Chem., Int. Ed., 2015, 54, 6292–6296 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford, CT, USA, 2009 Search PubMed.
- (a) A. M. Alonso, R. Horcajada, H. J. Groombridge, R. Mandalia, M. Motevalli, J. H. P. Utley and P. B. Wyatt, Chem. Commun., 2004, 412–413 RSC; (b) A. M. Alonso, R. Horcajada, H. J. Groombridge, R. Chudasama, M. Motevalli, J. H. P. Utley and P. B. Wyatt, Org. Biomol. Chem., 2005, 3, 2832–2841 RSC; (c) A. M. Alonso, R. Horcajada, M. Motevalli, J. H. P. Utley and P. B. Wyatt, Org. Biomol. Chem., 2005, 3, 2842–2847 RSC.
- (a) M. Sun, X. Jiang, W. Liu, T. Zhu, F. Huang and Y. Cao, Synth. Met., 2012, 162, 1406–1410 CrossRef CAS PubMed; (b) G. Qian, Z. Zhong, M. Luo, D. Yu, Z. Zhang, Z. Y. Wang and D. Ma, Adv. Mater., 2009, 21, 111–116 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5tc01754h |
| This journal is © The Royal Society of Chemistry 2015 |