Energy level tuning of blue emitting and electron transporting vinylene bis(vinyl quinolinyl)benzene derivatives: synthesis, characterisation, thin film characterisation and performance in OLEDs†
Abstract
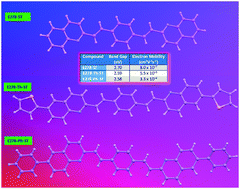
A number of thermally stable, conjugated, blue-emitting vinylene bis(vinyl quinolinyl)benzene derivatives were prepared and three of them were characterised by single crystal X-ray crystallography. They exhibit blue to bluish-green emission (fluorescence and electroluminescence) depending on the substituents. Their effectiveness as electron transporters in red and green organic light emitting diodes (OLEDs) has been explored. The phenyl and naphthyl substituted compounds were found to be superior to Alq3 in OLEDs as electron transporters. The electron mobility of the parent molecule, phenyl, thienyl and naphthyl substituted compounds were determined to be 8.0 × 10−7, 3.3 × 10−6, 5.5 × 10−6 and 8.0 × 10−6 cm2 V−1 s−1 respectively. Lifetime measurements were carried out for the red and green fluorescent devices and compared with Alq3 as an electron transporter. Some vinylene bis(vinyl quinolinyl)benzene derivatives show significantly longer lifetimes than analogous devices made with Alq3 as the electron transport layer. Purple to dark blue emitting devices were achieved from two of the derivatives.
- This article is part of the themed collection: Highlighting materials research in the UK for optical, magnetic and electronic devices


 Please wait while we load your content...
Please wait while we load your content...