Axial chiral aggregation-induced emission luminogens with aggregation-annihilated circular dichroism effect†
Abstract
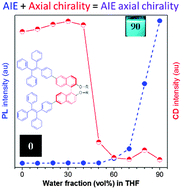
Axial chiral aggregation-induced emission (AIE) luminogens of (R)-3,3′-BTPE-BINA, (R)-6,6′-BTPE-BINA and (S)-6,6′-BTPE-BINA were synthesized for the first time by covalently attaching the AIE-active tetraphenylethene (TPE) units to the axial chiral binaphthol (BINOL) moieties at their 3,3′- or 6,6′-positions. It was found that the circular dichroism (CD) value when TPE was attached to BINOL at its 3,3′-positions was much larger than that found after its attachment at 6,6′-positions. The resultant AIE-active luminogens (AIEgens) show high quantum yields (up to 42.4%) in their aggregated states. Interestingly, these AIEgens exhibit an abnormal aggregation-annihilation CD (AACD) phenomenon. The decrease in the twisted angle between the two naphthalene rings upon aggregation was rationalized as the cause of this unique effect.

 Please wait while we load your content...
Please wait while we load your content...