Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Air-stable n-channel organic field-effect transistors based on a sulfur rich π-electron acceptor†
Agathe
Filatre-Furcate
*ab,
Toshiki
Higashino
*a,
Dominique
Lorcy
b and
Takehiko
Mori
a
aDepartment of Organic and Polymeric Materials, Tokyo Institute of Technology, O-okayama 2-12-1, Meguro-ku, 152-8552, Japan. E-mail: higashino.t.aa@m.titech.ac.jp; mori.t.ae@m.titech.ac.jp
bInstitut des Sciences Chimiques de Rennes, Université de Rennes 1, CNRS UMR 6226, Matière Condensée et Systèmes Electroactifs (MaCSE), campus de Beaulieu, Bât 10A, 35042 Rennes cedex, France. E-mail: agathe.filatre-furcate@univ-rennes1.fr
First published on 27th February 2015
Abstract
Thin-film and single-crystal n-channel organic field-effect transistors are built from the sulfur rich π-electron acceptor, (E)-3,3′-diethyl-5,5′-bithiazolidinylidene-2,4,2′,4′-tetrathione (DEBTTT). Different source and drain electrode materials are investigated: gold, the conducting charge transfer salt (tetrathiafulvalene)(tetracyanoquinodimethane), and carbon paste. Regardless of the nature of the electrodes, air-stable n-channel transistors have been obtained. Single crystals exhibit a higher performance than the thin-film transistors with a mobility of up to 0.22 cm2 V−1 s−1. These thin-film and single-crystal devices exhibit excellent long-term stability as demonstrated by the mobility measured during several weeks. The high mobility and air stability are ascribed to the characteristic three-dimensional S–S network coming from the thioketone sulfur atoms.
Introduction
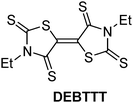
Over the last few decades, organic field-effect transistors (OFETs) have attracted a lot of attention owing to their potential application to flexible, and low-cost electronic devices.1–3 For that purpose, a variety of p-channel and n-channel organic semiconductors have been studied and over the years a significantly improved performance has been obtained.2,3 Among these OFETs, despite the increasing number of related studies, n-channel organic semiconductors have been less investigated than p-channel organic semiconductors.4–12 Thus, air-stable n-channel transistors exhibiting high electronic performance are rare in comparison with p-channel devices. To prepare air-stable n-channel organic transistors, a strategy is the use of electron acceptors with the lowest unoccupied molecular orbital (LUMO) energy lower than −4.0 eV.12 For instance, small molecules such as TCNQ (7,7,8,8-tetracyano-p-quinodimethane) and DM-DCNQI (2,5-dimethyl-N,N′-dicyano-p-quinonediimine), which are both planar and strong electron acceptors, give air-stable n-channel thin-film OFETs.7–9 Moreover, single crystals of TCNQ afford higher-performance n-channel devices than the thin-film transistors.7,8 The introduction of chalcogen atoms such as sulfur within the acceptor skeleton is another structural feature which leads to high-performance and air-stable n-channel OFETs.5 In fact, it is well known that the presence of sulfur atoms can enhance intermolecular interactions due to sulfur⋯sulfur contacts and thus can increase the effective dimensionality of the electronic structure and consequently the transport properties.13 Therefore, it is interesting to explore this category of molecules as candidates of new electron-transporting organic semiconductors. We have recently reported a sulfur-rich electron acceptor, (E)-3,3′-diethyl-5,5′-bithiazolidinylidene-2,4,2′,4′-tetrathione (DEBTTT); due to its electron accepting ability, (E1/21 = −0.05 V and E1/22 = −0.44 V vs. SCE), this acceptor forms a charge-transfer salt with decamethylferrocene exhibiting ferromagnetic interaction.14 This molecule was first obtained as a side product of nickel dithiolene complexes,15 and it can be simply prepared from the oxidation of the dithiolate ligand.14 The monoketone form of the half unit of this acceptor is known as 3-ethyl rhodanine, which is included in dyes such as merocyanine, and used in the prototypical OFET and organic photovoltaic cell.16,17 The DEBTTT molecule with its six sulfur atoms exhibits a planar geometry in its neutral and monoanionic states and undergoes two sequential and reversible monoelectronic reduction processes at less anodic potential than TCNQ (E1/21 = 0.18 V and E1/22 = −0.37 V vs. SCE),18 demonstrating a slightly lower accepting ability than TCNQ. From redox potential analysis, the LUMO energy of DEBTTT was estimated to be −4.4 eV, which is within the region of air-stable electron transport.19 Thus, DEBTTT presents favorable prerequisites for the elaboration of air-stable n-channel OFETs.12In this paper, we report the characteristics of DEBTTT thin-films and single-crystal n-channel OFETs, using different source and drain electrode materials such as gold, the conducting charge transfer salt (tetrathiafulvalene)(tetracyanoquinodimethane) ((TTF)(TCNQ)), and carbon paste. These transistors show excellent air and long-term stability owing to the fairly strong acceptor ability.
Results and discussion
Fabrication of devices
Thin films of DEBTTT have been deposited by vacuum evaporation on a tetratetracontane (TTC)-modified SiO2/Si substrate; TTC behaves as an excellent passivation layer.20,21 The top-contact source and drain electrodes are fabricated by evaporating gold or (TTF)(TCNQ) through a shadow mask leading to a channel of 100 μm length and 1 mm width. Concerning the top-contact single crystal devices, polystyrene (PS) was used as a passivation layer and carbon paste as a source-drain electrode. The transistor characteristics were measured under vacuum (10−4 Pa) and under an ambient atmosphere (see ESI† for details). The mobility was evaluated in the saturated region.Thin film properties
The solid state morphology and crystallinity of the thin films have been investigated by ultraviolet-visible spectroscopy (UV/vis) (ESI†), X-ray diffraction (XRD), and atomic force microscopy (AFM). The XRD pattern, (Fig. 1a), shows diffraction peaks which can be attributed to the polycrystalline passivation layer as well as to the organic semiconducting film (peak at d = 11.6 Å). Based on the crystal structure,14 this d-spacing, at d = 11.6 Å, corresponds to the c sinα sinβ distance of the crystal lattice, indicating that the crystallographic ab plane is aligned parallel to the substrate as shown in Fig. 1b. The molecules are almost perpendicular to the substrate (tilt angles: 85.5° and 88.4°).Accordingly, the stacking direction of the molecules is almost parallel to the substrate which is generally favorable for charge transport in transistors. Indeed, it has been reported in a naphthalene diimide series that there is a correlation between mobility and molecular arrangement and that the perpendicular orientation is the most favorable for charge transport in organic transistors as it minimizes the misfit between the domain boundaries.22 The low-angle peaks come from the passivation layer. TTC is known to show five different packing modes: monoclinic (M001 and M101), triclinic (T), orthorhombic I (OI) and orthorhombic II (OII) systems.23 The observed XRD peaks are ascribed to the orthorhombic I (OI) system, where the TTC molecules stand perpendicular to the SiO2 surface.
AFM images of the evaporated thin films of DEBTTT (Fig. 2) show homogeneous microcrystalline features. This crystallinity could be due to the ability of DEBTTT to establish strong intramolecular and intermolecular interactions14 and to the influence of the TTC passivation layer because it has been previously reported that the crystallinity of a wide variety of organic semiconductors increases when deposited on TTC.20,21
Intermolecular interactions
Overlap integral calculations have been performed in order to determine the strength of the interactions between neighbouring molecules in the solid state. The transfer integrals (Fig. 3) for the adjacent molecules between the π-type LUMOs14 are determined based on AM1 molecular orbital calculations (see ESI†).24The transfer integral a along the column is significantly smaller than those for the diagonal interactions b1, b2 and p between the adjacent columns within the ab plane. It finds its origin in the steric effect of the ethyl group which induces a rotation of the molecules relative to each other in the stack (Fig. 3c), and reduces the intracolumnar interactions. Another reason for this small intracolumnar interaction is the nature of the LUMO which is not largely populated on the ring sulfur atoms. Contrariwise, there are significant contributions in the LUMO on the four exocyclic sulfur atoms involved in short S⋯S contacts associated with these strong diagonal interactions b1, b2 and p. As shown in Fig. 3c, the thioketone sulfur atoms afford many S⋯S contacts shorter than the van der Waals distance not only in the stacking direction but also in the other two (b and c) directions. It is noteworthy that this compound does not show a simple one-dimensional intermolecular interaction coming from the π-stacks. The three-dimensional interactions mediated by the thioketone S⋯S contacts are responsible for the highly crystalline nature and the robust electron transport.
Transistor characteristics
Transfer and output characteristics of the thin film and single crystal transistors are, respectively, shown in Fig. 4 and 5. From these characteristics, the maximum and average of the apparent mobilities, μmax and μav, are estimated as well as the on-off ratio ION/IOFF and the threshold voltage VTh. The device performances are summarized in Table 1. Among the thin-film transistors, the transistors using (TTF)(TCNQ) electrodes exhibit higher performances than those with the gold electrodes (Table 1). The high temperature used for evaporating gold electrodes (T > 500 °C) is known to generate slight damage on the organic semiconductor film in some cases. The better performance of the (TTF)(TCNQ) transistors is partially due to the comparatively low-temperature evaporation of the (TTF)(TCNQ) electrodes (130 °C). The differences in the electrode work function also influence the performance of the devices (see below). The transistors are operated even in air and the performance is not much reduced (Table 1). The (TTF)(TCNQ)-based transistors tend to afford smaller VTh than the Au-based transistors.| Electrodes | Conditions | Measurements | μ av [μmax] (cm2 V−1 s−1) | V Th (V) | I ON/IOFF | |
|---|---|---|---|---|---|---|
| Thin film | Au | Pristine | Under vacuum | 5.9 × 10−3 [1.5 × 10−2] | 12 | 6 × 103 |
| In air | 4.6 × 10−3 [1.2 × 10−2] | 31 | 4 × 103 | |||
| After ten weeks under vacuum | Under vacuum | 6.5 × 10−3 [1.6 × 10−2] | 47 | 2 × 104 | ||
| In air | 6.6 × 10−3 [1.4 × 10−2] | 59 | 2 × 103 | |||
| After additional four weeks in air | Under vacuum | 8.1 × 10−3 [1.8 × 10−2] | 47 | 7 × 103 | ||
| In air | 6.1 × 10−3 [1.7 × 10−2] | 69 | 2 × 104 | |||
| (TTF)(TCNQ) | Pristine | Under vacuum | 8.6 × 10−3 [1.4 × 10−2] | 12 | 4 × 104 | |
| In air | 6.7 × 10−3 [8.2 × 10−3] | 21 | 2 × 104 | |||
| After ten weeks under vacuum | Under vacuum | 8.5 × 10−3 [1.4 × 10−2] | 38 | 6 × 104 | ||
| In air | 7.5 × 10−3 [1.4 × 10−2] | 50 | 7 × 103 | |||
| After additional four weeks in air | Under vacuum | 9.7 × 10−3 [1.8 × 10−2] | 63 | 6 × 104 | ||
| In air | 6.9 × 10−3 [1.4 × 10−2] | 66 | 6 × 104 | |||
| Single crystal | Carbon paste | Pristine | Under vacuum | 0.11 [0.21] | −4.5 | 7 × 102 |
| In air | 0.14 [0.22] | 3.1 | 2 × 102 | |||
| After six weeks under vacuum | Under vacuum | 0.10 [0.16] | −5.8 | 6 × 101 | ||
| In air | 0.11 [0.19] | −3.3 | 6 × 101 | |||
| After additional four weeks in air | Under vacuum | 0.12 [0.21] | 2.9 | 9 × 101 | ||
| In air | 0.11 [0.17] | 6.2 | 2 × 104 |
Moreover, the transistors based on single crystals of DEBTTT exhibit higher performance than the thin-film devices with a mobility of up to 0.22 cm2 V−1 s−1 (Fig. 5 and Table 1). The single crystal device also achieves a good performance and low threshold voltage even in air. These results are due to the fact that with crystals, complications resulting from grain boundaries and film morphology are excluded. In addition, the three-dimensional S–S network is expected to block the attack of gaseous water and oxygen, and is related to the extreme robustness of the electron transport against air.
After several-week storage, the mobilities of the thin-film transistors are practically not changed (Table 1), though the threshold voltages are shifted (Fig. 4). In the single-crystal transistors, the threshold shift is very small (Fig. 5), and the average mobility is not modified. In addition, we have carried out one hundred cycles of measurements in air and the performance is practically unchanged (Fig. 5c and Fig. S3, ESI†). Thus these n-channel transistors exhibit excellent long-term stability.
When we investigate the energy levels of the acceptor and the electrode materials, considerable difference exists between the electron affinity of the acceptor (4.4 eV) and the gold work function (5.1 eV) (Fig. 6).25 By contrast, the Fermi level of (TTF)(TCNQ) is located more close to the acceptor energy level, because the (TTF)(TCNQ) energy level is located in between the highest occupied molecular orbital level of TTF (4.78 eV) and the lowest unoccupied molecular orbital level of TCNQ (4.62 eV).26–29 The resulting reduced Schottky barrier is another origin of the better performance of the (TTF)(TCNQ)-based transistors. In addition, the Fermi level of carbon (4.8 eV) indicates that carbon is also a good candidate of source-drain electrodes in high-performance n-channel transistors based on DEBTTT.30
Conclusions
In conclusion, we have investigated organic transistors based on DEBTTT. It is noticed that the transistors show sufficiently stable performance even in air. A higher performance has been obtained for (TTF)(TCNQ) electrodes than for the ordinary gold ones, partly owing to the reduction of the heat damage in the electrode evaporation process. The thin-film transistor of DEBTTT shows a better performance than the TCNQ-based thin-film transistors.5a In particular, the single-crystal transistors exhibit mobility as high as 0.22 cm2 V−1 s−1. In contrast to the ordinary organic transistors which exhibit a highly two-dimensional layered structure, the extensive three-dimensional S–S interactions are characteristics of the present material. This may be related to the extensive air stability of this material.Acknowledgements
We thank Rennes Métropole and the Agence Nationale de la Recherche, France (ANR project no. 12-BS07-0032) for financial support to AFF internship. The authors are grateful to Tokyo Institute of Technology Center for Advanced Materials Analysis for XRD measurement and Prof. Kakimoto for AFM measurements. This work was partly supported by a Grant-in Aid for Scientific Research (B) (no. 23350061) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.Notes and references
- (a) C. D. Dimitrakopoulos and P. R. L. Malenfant, Adv. Mater., 2002, 14, 99 CrossRef CAS; (b) Z. Bao and J. Locklin, Organic Field-Effect Transistors, CRC Press, New-York, 2007 Search PubMed.
- C. Wang, H. Dong, W. Hu, Y. Liu and D. Zhu, Chem. Rev., 2012, 112, 2208 CrossRef CAS PubMed.
- A. R. Murphy and J. M. J. Fréchet, Chem. Rev., 2007, 107, 1066 CrossRef CAS PubMed.
- C. R. Newman, C. D. Frisbie, D. A. da Silva Filho, J.-L. Brédas, P. C. Ewbank and K. R. Mann, Chem. Mater., 2004, 16, 4436 CrossRef CAS.
- (a) Y. Wen and Y. Liu, Adv. Mater., 2010, 22, 1331 CrossRef CAS PubMed; (b) Y. Yamashita, Chem. Lett., 2009, 38, 870 CrossRef CAS; (c) Y. Qiao, Y. Guo, C. Yu, F. Zhang, W. Xu, Y. Liu and D. Zhu, J. Am. Chem. Soc., 2012, 134, 4084 CrossRef CAS PubMed; (d) X. Gao, C. Di, Y. Hu, X. Yang, H. Fan, F. Zhang, Y. Liu, H. Li and D. Zhu, J. Am. Chem. Soc., 2010, 132, 3697 CrossRef CAS PubMed; (e) Y. Hu, X. Gao, C. Di, X. Yang, F. Zhang, Y. Liu, H. Li and D. Zhu, Chem. Mater., 2011, 23, 1204 CrossRef CAS; (f) L. Tan, Y. Guo, G. Zhang, Y. Yang, D. Zhang, G. Yu, W. Xu and Y. Liu, J. Mater. Chem., 2011, 21, 18042 RSC; (g) F. Zhang, Y. Hu, T. Schuettfort, C. Di, X. Gao, C. R. McNeill, L. Thomsen, S. C. B. Mannsfeld, W. Yuan, H. Sirringhaus and D. Zhu, J. Am. Chem. Soc., 2013, 135, 2338 CrossRef CAS PubMed; (h) X. Gao and Y. Hu, J. Mater. Chem. C, 2014, 2, 3099 RSC.
- T. Mori, Chem. Lett., 2011, 40, 428 CrossRef CAS.
- (a) E. Menard, V. Podzorov, S.-H. Hur, A. Gaur, M. E. Gershenson and J. A. Rogers, Adv. Mater., 2004, 16, 2097 CrossRef CAS; (b) A. R. Brown, D. M. de Leeuw, E. J. Lous and E. E. Havinga, Synth. Met., 1994, 66, 257 CrossRef CAS; (c) M. Yamagishi, Y. Tominari, T. Uemura and J. Takeya, Appl. Phys. Lett., 2009, 94, 053305 CrossRef PubMed.
- T. Takahashi, S. Tamura, Y. Akiyama, T. Kadoya, T. Kawamoto and T. Mori, Appl. Phys. Express, 2012, 5, 061601 CrossRef.
- H. Wada, K. Shibata, Y. Bando and T. Mori, J. Mater. Chem., 2008, 18, 4165 RSC.
- D. de Caro, K. Jacob, H. Hahioui, C. Faulmann, L. Valade, T. Kadoya, T. Mori, J. Fraxedas and L. Viau, New J. Chem., 2011, 35, 1315 RSC.
- (a) S. Handa, E. Miyazaki, K. Takimiya and Y. Kunugi, J. Am. Chem. Soc., 2007, 129, 11684 CrossRef CAS PubMed; (b) Z. Liang, Q. Tang, J. Xu and Q. Miao, Adv. Mater., 2011, 23, 1535 CrossRef CAS PubMed; (c) A. Lv, S. R. Puniredd, J. Zhang, Z. Li, H. Zhu, W. Jiang, H. Dong, Y. He, L. Jiang, Y. Li, W. Pisula, Q. Meng, W. Hu and Z. Wang, Adv. Mater., 2012, 24, 2626 CrossRef CAS PubMed; (d) T. Kono, D. Kumaki, J. Nishida, S. Tokito and Y. Yamashita, Chem. Commun., 2010, 46, 3265 RSC; (e) D. Song, H. Wang, F. Zhu, J. Yang, H. Tian, Y. Geng and D. Yan, Adv. Mater., 2008, 20, 2142 CrossRef CAS.
- H. Usta, A. Facchetti and T. J. Marks, Acc. Chem. Res., 2011, 44, 501 CrossRef CAS PubMed.
- W. Jiang, Y. Li and Z. Wang, Chem. Soc. Rev., 2013, 42, 6113 RSC.
- Y. Le Gal, N. Bellec, F. Barrière, R. Clérac, M. Fourmigué, V. Dorcet, T. Roisnel and D. Lorcy, Dalton Trans., 2013, 42, 16672 RSC.
- M. C. Aragoni, M. Arca, F. A. Devillanova, F. Isaia, V. Lippolis, A. Mancini, L. Pala, A. M. Z. Slawin and J. D. Woolins, Inorg. Chem., 2005, 44, 9610 CrossRef CAS PubMed.
- K. Kudo, M. Yamashita and T. Moriizumi, Jpn. J. Appl. Phys., 1984, 23, 130 CrossRef CAS.
- D. L. Morel, E. L. Stogryn, A. K. Ghosh, T. Feng, P. E. Purwin, R. F. Shaw, C. Fishman, G. R. Bird and A. P. Piechowski, J. Phys. Chem., 1984, 88, 923 CrossRef CAS.
- M. L. Kaplan, R. C. Haddon, F. B. Bramwell, F. Wudl, J. H. Marshall, D. O. Cowan and S. Gronowitz, J. Phys. Chem., 1980, 84, 427 CrossRef CAS.
- M. L. Tang, A. D. Reicharrdt, P. Wei and Z. Bao, J. Am. Chem. Soc., 2009, 131, 5264 CrossRef CAS PubMed.
- (a) M. Kraus, S. Richler, A. Opitz, W. Brütting, S. Haas, T. Hasegawa, A. Hinderhofer and F. Schreiber, J. Appl. Phys., 2010, 107, 094503 CrossRef PubMed; (b) M. Kraus, S. Haug, W. Brütting and A. Opitz, Org. Electron., 2011, 12, 731 CrossRef CAS PubMed.
- (a) A. Opitz, M. Horlet, M. Kiwull, J. Wagner, M. Kraus and W. Brütting, Org. Electron., 2012, 13, 1614 CrossRef CAS PubMed; (b) M. Irimia-Vladu, E. D. Głowacki, P. A. Troshin, G. Schwabegger, L. Leonat, D. K. Susarova, O. Krystal, M. Ullah, Y. Kanbur, M. A. Bodea, V. F. Razumov, H. Sitter, S. Bauer and N. S. Sariciftci, Adv. Mater., 2012, 24, 375 CrossRef CAS PubMed; (c) E. D. Głowacki, L. Leonat, G. Voss, M.-A. Bodea, Z. Bozkurt, A. M. Ramil, M. Irimia-Vladu, S. Bauer and N. S. Sariciftci, AIP Adv., 2011, 1, 042132 CrossRef PubMed; (d) E. D. Głowacki, G. Voss, L. Leonat, M. Irimia-Vladu, S. Bauer and N. S. Sariciftci, Isr. J. Chem., 2012, 52, 540 CrossRef; (e) E. D. Głowacki, D. H. Apaydin, Z. Bozkurt, U. Monkowius, K. Demirak, E. Tordin, M. Himmelsbach, C. Schwarzinger, M. Burian, R. T. Lechner, N. Demitri, G. Vossa and N. S. Sariciftci, J. Mater. Chem. C, 2014, 2, 8089 RSC; (f) O. Pitayatanakul, T. Higashino, T. Kadoya, M. Tanaka, H. Kojima, M. Ashizawa, T. Kawamoto, H. Matsumoto, K. Ishikawa and T. Mori, J. Mater. Chem. C, 2014, 2, 9311 RSC; (g) E. D. Głowacki, H. Coskun, M. A. Blood-Forsythe, U. Monkowius, L. Leonat, M. Grzybowski, D. Gryko, M. S. White, A. Aspuru-Guzik and N. S. Sariciftci, Org. Electron., 2014, 15, 3521 CrossRef PubMed.
- T. Kakinuma, H. Kojima, M. Ashizawa, H. Matsumoto and T. Mori, J. Mater. Chem. C, 2013, 1, 5395 RSC.
- J.-P. Gorce, S. J. Spells, X.-B. Zeng and G. Ungar, J. Phys. Chem. B, 2004, 108, 3130 CrossRef CAS.
- (a) M. J. S. Dewar, E. G. Zoebisch, E. F. Healy and J. J. P. Stewart, J. Am. Chem. Soc., 1985, 107, 3902 CrossRef CAS; (b) T. Mori, A. Kobayashi, Y. Sasaki, H. Kobayashi, G. Saito and H. Inokuchi, Bull. Chem. Soc. Jpn., 1984, 57, 627 CrossRef CAS.
- H. Michelson, IBM J. Res. Dev., 1978, 22, 72 CrossRef.
- T. Kadoya, D. de Caro, K. Jacob, C. Faulmann, L. Valade and T. Mori, J. Mater. Chem., 2011, 21, 18421 RSC.
- A. S. Batsanov, M. R. Bryce, A. Chesney, J. A. K. Howard, D. E. John, A. J. Moore, C. L. Wood, H. Gershtenman, J. Y. Becker, V. Y. Khodorkovsky, A. Ellern, J. Bernstein, I. F. Perepichka, V. Rotello, M. Gray and A. O. Cuello, J. Mater. Chem., 2001, 11, 2181 RSC.
- Y. Takahashi, T. Hasegawa, Y. Abe, Y. Tokura and G. Saito, Appl. Phys. Lett., 2006, 88, 073504 CrossRef PubMed.
- S. Tamura, T. Kadoya, T. Kawamoto and T. Mori, Appl. Phys. Lett., 2013, 102, 063305 CrossRef PubMed.
- M. Shiraishi and M. Ata, Carbon, 2001, 39, 1913 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional information for overlap integral calculations, optical properties, device fabrication, and thin film properties. See DOI: 10.1039/c5tc00253b |
| This journal is © The Royal Society of Chemistry 2015 |