Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Formation of nanoscopic CaF2via a fluorolytic sol–gel process for antireflective coatings†
Alexander
Rehmer
,
Kerstin
Scheurell
and
Erhard
Kemnitz
*
Humboldt-Universität zu Berlin, Department of Chemistry, Brook-Taylor-Str. 2, 12489 Berlin, Germany. E-mail: erhard.kemnitz@chemie.hu-berlin.de; Fax: +49 30 2093 7277
First published on 22nd December 2014
Abstract
The synthesis of nanoscopic calcium fluoride was performed by the fluorolytic sol–gel process. Antireflective coatings of CaF2 were prepared from sols obtained by the reaction of CaCl2 with HF and subsequent dip coating. The addition of tetramethyl orthosilicate (TMOS) or tetraethyl orthosilicate (TEOS) after fluorination promotes the formation of transparent sols. The formation and crystallisation of CaF2 nanoparticles was studied by 19F liquid and solid state NMR spectroscopy, dynamic light scattering (DLS) and X-ray powder diffraction (XRD). The morphology of a CaF2-film was analysed by high resolution scanning electron microscopy (HR-SEM) and the mechanical stability of a CaF2-film was evaluated by the Crockmeter test using both felt and steel wool. The refractive index for a CaF2-film was measured by ellipsometry. The synthesis of CaF2 nanoparticles derived from CaCl2 is a good way to achieve porous antireflective coating layers.
Introduction
Calcium fluoride and other metal fluorides exhibit superior optical properties. Their optical transmittance ranges from low UV up to the high IR region. For example, calcium fluoride is a dielectric material with an optical window ranging from 0.13 to 10 μm (ref. 1) exhibiting a broad spectrum of optical applications like IR, UV, microscope, astronomical instrumentation and spectroscopy. Especially the use of calcium fluoride as ceramic material for laser applications is advantageous because not only the mechanical and optical properties compared with single crystals of calcium fluoride are better but it can be produced in large volumes.2–4 Furthermore, calcium fluoride and magnesium fluoride own a low refractive index (CaF2: n = 1.44 at 500 nm MgF2: n = 1.38 at 500 nm).1,5 Thus, calcium fluoride and magnesium fluoride are promising candidates for antireflective (AR) coatings. If the refractive index of the coating is lower than the refractive index of the substrate, the reflection of the light will decrease. The complete anti-reflection for single coatings depends on the mean geometric refractive index of the AR-layer with n12 = nsn0 and its thickness with n1d = λ/4, where n1, nS and n0 are the refractive indices of the coating, substrate and air, respectively, d the thickness of the film and λ the wavelength of the light.6 For example, common glass mainly built up by SiO2 (nS = 1.55 at 500 nm) usually is surrounded by the medium air (n0 = 1.0). Hence, the optimum refractive index is n1 = 1.22. For destructive interference, the antireflective coating is one quarter of the wavelength of light. In this case, the thickness of the AR-layer should be 102 nm. Thus, CaF2 is a good candidate to decrease the reflection of the incident light of glass. A comparison between CaF2 and MgF2 shows similar optical properties. Only the solubility in water reveals that CaF2 (16 mg L−1) is less soluble than MgF2 (76 mg L−1).7 This is a factor, which could be interesting for the chemical resistance of an AR-layer against outdoor conditions like rain or moisture. Due to its very low solubility, CaF2-films could be more weather-proof than MgF2-films.It is well known, that porous antireflective alkaline earth metal fluoride films can be prepared by spin coating from sols of calcium trifluoroacetate.8 That is, calcium trifluoroacetate sols in water/isopropanol are first prepared by reacting trifluoroacetic acid and calcium acetate. From the sols obtained this way a surface layer of calcium trifluorocacetate can be created by spin coating. The following annealing initiates the thermal decomposition of the trifluoroacetate resulting in a porous calcium fluoride layer as reported in J. Sol-Gel Sci. Technol.9 The disadvantage of this method is the thermal decomposition of the metal trifluoroacetates into toxic and corrosive reaction products like CF3COF, COF2 and HF. Another point is that a mixture of metal acetate and trifluoroacetic acid may yield metal oxide fluoride phases leading to an increase of the refractive index due to the oxygen content inside the film.
In this paper, we present an easy synthesis approach toward nanoscaled CaF2-sols via the fluorolytic sol–gel process to fabricate finally antireflective CaF2-films by dip-coating. For excellent AR-layers, it is necessary to start from a transparent sol with particle sizes of about 10 to 20 nm or smaller. The choice of the solvent is an important factor because only volatile solvents like e.g. methanol, ethanol, and isopropanol lead to homogeneous layers.
Here, we report a way to use the cost-efficient synthesis of calcium fluoride sols with commercially available calcium chloride precursors by reaction with anhydrous HF. The influence of TMOS to the calcium fluoride sol is investigated as well as the optical and mechanical properties of the calcium fluoride films obtained from thus prepared CaF2-sols.
Experimental
Precursor synthesis
All chemicals for the synthesis of calcium fluoride are commercially available and need no drying or further processing. The 19.84 M HF-solution was prepared by dissolving anhydrous HF in ethanol.A series of under-stoichiometric calcium fluoride sols with fluorine to calcium ratios F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca < 2 was prepared by dissolution of 1.33 g anhydrous CaCl2 (96% ABCR, 12 mmol) in ethanol (99.8%, ROTH, 0.4 M solution). Under vigorous stirring at ambient conditions, the required amount of HF-solution was added dropwise to the solution.
Ca < 2 was prepared by dissolution of 1.33 g anhydrous CaCl2 (96% ABCR, 12 mmol) in ethanol (99.8%, ROTH, 0.4 M solution). Under vigorous stirring at ambient conditions, the required amount of HF-solution was added dropwise to the solution.
The stoichiometric calcium fluoride sol (Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F = 1
F = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) for coating on glass slides (Borosilicate) was prepared by the reaction of 8.88 g of CaCl2 (80 mmol) with 8 mL of HF (160 mmol; 2 eq.) in 192 mL ethanol. After fluorination 0.59 mL of TMOS (5 mol% of Ca) respectively 0.88 mL of TEOS (5 mol% of Ca) was added. The influence of TMOS was studied by 19F NMR.
2) for coating on glass slides (Borosilicate) was prepared by the reaction of 8.88 g of CaCl2 (80 mmol) with 8 mL of HF (160 mmol; 2 eq.) in 192 mL ethanol. After fluorination 0.59 mL of TMOS (5 mol% of Ca) respectively 0.88 mL of TEOS (5 mol% of Ca) was added. The influence of TMOS was studied by 19F NMR.
Deposition of CaF2 coating films
A typical procedure for the deposition of calcium fluoride coating films was as follows. The freshly prepared calcium fluoride sols were deposited on borosilicate glass by dip-coating. Before the coating experiment the glass slides were cleaned in an alkaline solution (RBS®50) for 15 min. The substrates were rinsed with deionized water and dried by blowing compressed air. After the coating, the substrates were calcined in a vented air-oven (Barnstead Thermolyne type F48000) at 500 °C for 15 min followed by slow cooling down to room temperature.Materials characterization
The calcium fluoride sols were characterized by dynamic light scattering (DLS) and 19F liquid NMR spectroscopy. The DLS measurements were performed using a Zetasizer Nano ZS (Malvern instruments, Worcestershire, UK) using disposable PMMA cuvettes. Hydrodynamic diameters were calculated from the correlation functions by the Malvern Nanosizer Software. The viscosity was determined simultaneously to DLS measurements with a microviscometer from Anton Paar (AMVn, Graz, Austria) at 25 °C. The 19F NMR spectra of the sols with varying fluorine content were carried out using a Bruker AVANCE II 300 (liquid state NMR spectrometer with a Larmor frequency of 282.4 MHz). The 19F isotropic chemical shifts are given with respect to the CFCl3 standard.The Transmission Electron Microscope (TEM) analysis has been carried out using a Philips CM200 LaB6 microscope operating at 200 kV. A few drops of the solution containing the nanoparticles have been deposited on a carbon-coated copper grid and let them dry prior to the inspection.
The calcium fluoride xerogels were characterized by an X-ray powder diffractometer from Seifert (XRD 3003 TT) and by 19F solid state NMR spectroscopy. The static 19F solid state NMR spectra were recorded with a Bruker AVANCE 400 (solid state spectrometer, Larmor frequency of 376.4 MHz) with a 4 mm Bruker probe and the 19F MAS NMR spectra were recorded with a 2.5 mm Bruker probe. The 19F isotropic chemical shifts are given with respect to C6F6 as secondary standard with δiso = −166.6 ppm against CFCl3. Rotor-synchronized Hahn spin-echo experiments (rs-echo) were performed which react sensitive on homonuclear dipolar couplings. This means that fluorine species with an effective spin exchange (short spin-spin relaxation times T2, i.e. well-bridged species) are not or with decreased intensity detected after applying longer dipolar evolution times, whereas those with longer spin–spin relaxation times (not well-incorporated, terminal) are detected. The number of rotor periods before echo detection (L0) is given in the figure captions. The percentage of chloride in the xerogels was determined by elemental analysis.
The surfaces of calcium fluoride films were examined by high-resolution scanning electron microscopy (HR-SEM) from Zeiss (Supra 40). The refractive index n(500 nm) and thickness t of the CaF2-layers was determined by spectroscopic ellipsometry. The ellipsometric parameters psi and delta were measured with a SENpro instrument (Sentech, Berlin, Germany) with the software “SpectraRay3”. The calculated parameters psi and delta are determined with a Sellmeier model. The reflection and absorption of the CaF2-film was measured with an UV-Vis-spectrometer (Shimadzu UV-3100, Kyoto, Japan), in the range of 300–1400 nm.
The mechanic stability of the CaF2-films was tested by a crockmeter from Erichsen (scratchmarker 249) using felt and steel wool with a fineness of 0000. The stamp with a contact area of 4.5 cm2 was pressed on the sample with a force of 4 N.
Results and discussion
The fluorolytic sol–gel synthesis consists of the reaction between a suitable metal precursor and anhydrous hydrogen fluoride in a suitable organic solvent, preferentially methanol or ethanol.10 Several calcium precursors like Ca(OMe)2, Ca(OEt)2, Ca(OAc)2 and CaCl2 were investigated. In most of the cases, metal alkoxides like Si(OMe)4, Ti(OiPr)4 and Al(OiPr)3 or Mg(OMe)2, respectively, are used in the classical oxide sol–gel route.11–14 In case of the fluorolytic sol–gel synthesis, the synthesis of transparent CaF2 sols starting from calcium alkoxides is not recommended because of the insolubility of the calcium alkoxide in methanol and ethanol at room temperature. Thus, Ca(OMe)2 and Ca(OEt)2 always form crystalline white precipitates of their Ca(OR)2· 4 ROH solvates. By subsequent de-solvation fine crystalline powders with a three-dimensional polymeric structure of the CdI2 type are formed.15 Unfortunately, it is impossible to obtain transparent CaF2-sols under these circumstances; however, starting from calcium acetate suspended in an ethanol/acetic acid solution results in the formation of transparent CaF2-sols but the stability of these sols is poor. That means, after a few days the CaF2-sol is completely transformed to a gel. The reason for strong gelation tendency is esterification of acetic acid with the corresponding alcohol and the release of water. The more water is formed in the system the faster gelation occurs (eqn (1)).| AcOH + ROH ⇄ AcOR + H2O | (1) |
In spite of the gelation, coatings from these sols are possible for a short time only. The optical properties of such coatings are good but the mechanical properties are very poor due the high porosity in the film.16 Thus, calcium acetate as precursor for calcium fluoride coatings is not recommended. Therefore, we decided to use calcium chloride as precursor instead because of its good solubility in alcohol and the absence of an esterification reaction. Following the general synthesis approach of MgF2-sols via the fluorolytic sol–gel route by reacting MgCl2 in EtOH with anhydrous HF,17 we started from CaCl2 in EtOH and reacted it with ethanolic HF solution to form nanoscopic calcium fluoride sols (eqn (2)).
| CaCl2 + 2HFEtOH → nano CaF2 + 2HCL | (2) |
The transparency of the CaF2-sol is opaque. By addition of 5 mol% tetramethyl orthosilicate (TMOS) or tetraethyl orthosilicate (TEOS) after fluorination the former opaque sol turned into a transparent CaF2-sol. The reaction of CaCl2 in ethanol with two equivalents of ethanolic HF solution leads to the formation of nanoscopic CaF2 particles. For good optical and mechanical properties of an AR-layer the sol should be transparent to achieve a homogenous layer on the substrate. Beside sol-transparency as an indication for homodispersed nano-particle distribution, volatility of the solvent is a further pre-condition to obtain homogeneous coatings. The higher the volatility of the solvent is, the faster a homogenous film is formed. Although methanol is more volatile than ethanol, it is toxic compared to ethanol. Hence, the latter was used in all experiments. The formation of calcium fluoride nanoparticles was investigated by 19F NMR spectroscopy. The amount of HF to calcium chloride was varied from 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
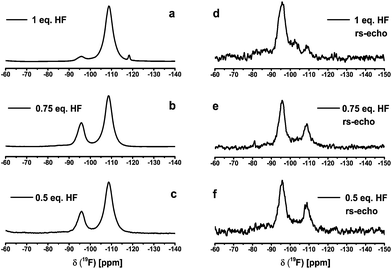
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in order to follow species that might be formed in the course of reaction. The 19F NMR spectra of the sols with different amount of HF are listed in Fig. 1. For a better illustration of the spectra without the known background signal in the 19F liquid NMR, the samples were measured statically by solid state NMR. The liquid 19F NMR spectra are included in the ESI.†Fig. 1 shows the typical signal for calcium fluoride at −108 ppm. Additionally to the CaF2 signal a second broad signal appears at about −95 ppm and −93 ppm in the 19F liquid NMR, respectively. We speculated, it may stand for another calcium fluoride species like calcium chloride fluoride (CaClF) or calcium alkoxide fluoride (CaF2−xORx), which could be a solvated species. For further information of this species 19F solid state NMR and XRD measurements of the xerogels were performed. Furthermore, the reaction of CaCl2 with two equivalents of HF exhibits a broad signal in the static solid state 19F NMR as well as in the liquid 19F NMR at about −180 ppm (line width ∼2000 Hz). Samples with under-stoichiometric amounts of fluorine also contain a signal between −174 ppm and −180 ppm in the liquid NMR. This signal corresponds to unreacted HF adsorbed to the particle surface.18,19 A similar observation was made in the synthesis of MgF2via the fluorolytic sol–gel route, with either Mg(OMe)2 or MgCl2 as reaction. Thus, we speculate that this corresponds to HF adsorbed at the precursor.17 During ageing of the MgF2-sol the signal vanishes.
1 in order to follow species that might be formed in the course of reaction. The 19F NMR spectra of the sols with different amount of HF are listed in Fig. 1. For a better illustration of the spectra without the known background signal in the 19F liquid NMR, the samples were measured statically by solid state NMR. The liquid 19F NMR spectra are included in the ESI.†Fig. 1 shows the typical signal for calcium fluoride at −108 ppm. Additionally to the CaF2 signal a second broad signal appears at about −95 ppm and −93 ppm in the 19F liquid NMR, respectively. We speculated, it may stand for another calcium fluoride species like calcium chloride fluoride (CaClF) or calcium alkoxide fluoride (CaF2−xORx), which could be a solvated species. For further information of this species 19F solid state NMR and XRD measurements of the xerogels were performed. Furthermore, the reaction of CaCl2 with two equivalents of HF exhibits a broad signal in the static solid state 19F NMR as well as in the liquid 19F NMR at about −180 ppm (line width ∼2000 Hz). Samples with under-stoichiometric amounts of fluorine also contain a signal between −174 ppm and −180 ppm in the liquid NMR. This signal corresponds to unreacted HF adsorbed to the particle surface.18,19 A similar observation was made in the synthesis of MgF2via the fluorolytic sol–gel route, with either Mg(OMe)2 or MgCl2 as reaction. Thus, we speculate that this corresponds to HF adsorbed at the precursor.17 During ageing of the MgF2-sol the signal vanishes.
 | ||
| Fig. 1 19F NMR spectra of the sols with different fluorine content (normalized to the CaF2 signal) carried out with ss-NMR spectrometer. | ||
The addition of TMOS or TEOS to the CaF2-sol vanishes this signal completely in a short time. The formation of SiOR4−xFx and SiF62− species (the second broad signal around −128 ppm could be CaSiF6 (Fig. 2)) after TMOS/TEOS addition are further evidences for unreacted HF in the sols at this stage of nanoparticles surface causes agglomeration, but consequently, removal of this leads to clear sols. Due to the line width of ∼4000 Hz the sol consists not only of calcium fluoride particles but also of calcium hexafluorosilicate or other fluorosilicate particles. The other small signals with line width of about 100 Hz are probably dissolved SiOR4−xFx species. Both species evidently are formed from unreacted HF and added Si(OMe)4. All as-prepared CaF2-sols with 5 mol% TMOS or TEOS are transparent and possess low viscosity. Without addition of TMOS or TEOS the sol remains turbid and tends to form a gel. After a few days the particles are aggregated, that a settle to the bottom of the flask is observed, which is referred to as sedimentation of the sol. According to Fig. 3 the mean particle diameter of a sol with 5 mol% TMOS was determined by dynamic light scattering. The intensity weighted particle distribution shows two size classes with mean particle diameters of 20 nm and 320 nm. Due to the Stokes–Einstein relation bigger particles scatter much more than smaller particles because the intensity of scattering is proportional to the sixth magnitude of its diameter (Rayleigh approximation). It follows that small particles are significantly under-estimated. Hence, calculating the volume weighted particle distribution from intensity weighted one shows that in fact just one class of particles with a mean particle diameter of approximately 10 nm is present. In addition, the particle size was also investigated by transmission electron microscopy and results are included in the ESI.† For the identification of species with the characteristic 19F NMR signal between −93 to −95 ppm 19F solid state NMR measurements were performed. For an exact identification of the unknown species, the CaF2-sol without the addition of TMOS was dried at room temperature in air and calcined at 600 °C in an electric furnace. The samples were compared with crystalline CaClF, which was synthesized by solid state reaction between CaCl2 and CaF2 in presence of NH4Cl as flux at 730 °C for 3 h in an electric furnace. Wenz et al. reported an eutectic point at 645 °C for a composition of 18.5 mol% CaCl2 and 81.5 mol% CaF2.20Fig. 4 shows the 19F MAS signal of the crystalline CaClF compound with a chemical shift of −83 ppm. The difference between the CaClF shift and the shift of the unknown species is about 12 ppm. Hence, the unknown species does not correspond to CaClF. The most plausible explanation is that this signal corresponds to an oxide fluoride species, which probably is caused by a preferential oxygen donation in the second coordination sphere as was also found for the MgF2 system.18 For comparison, the annealed CaF2 xerogel with one equivalent of HF shows a decrease of the signal at −95 ppm and an increase of the signal at −83 ppm in the spectrum unlike the un-annealed sample (Fig. 5). Thus, the formation of CaClF in the sol for the system CaCl2 in EtOH can be ruled out, it will only be formed at high temperature. Furthermore, the 19F signal at about −95 ppm also appears in the NMR spectra of CaF2-xerogels that have been prepared from other calcium precursors like CaBr2· H2O and CaO which do not contain any chloride. The NMR spectra are listed in the ESI (Fig. S1–S3†). In the 19F ss-NMR spectrum of the CaF2-xerogel obtained from CaO with two equivalents of HF appears the corresponding signal as shoulder of the main signal of CaF2. The difference between the signal shapes in the CaO–HF system and the CaCl2/CaBr2–HF system might be caused by dipole–dipole coupling. The shorter the dipole–dipole coupling is, the more is the signal separated from the main signal. Another interesting fact is also the different relaxation time T2 between the signal at −108 ppm and −95 ppm in the 19F NMR spectra. According to Fig. 6 the influence of the relaxation time T2 of under-stoichiometric xerogels reveals that the intensity of the signal at −108 ppm decreases and the intensity of the signal at −95 ppm increases. Another time-dependent NMR measurement was also performed with a CaF2-xerogel derived from CaCl2 with H2O as solvent (Fig. 7). In the case of H2O as solvent the 19F NMR spectra exhibit another signal at −83 ppm beside the main signal and the signal at −95 ppm. This signal at −83 ppm corresponds to CaClF, which has the same NMR shift like the reference sample of CaClF. It is obvious that the 19F signal at about −95 ppm does not correspond to the CaClF species but rather to an oxide fluoride species like CaF2−xORx (R = H, Et). An explanation of the formation of an oxide fluoride species from CaCl2 in EtOH or in H2O with ethanolic/aqueous HF-solution as fluorine agent could be either the preferential oxygen donation in the second coordination sphere like mentioned before or the use of only 96% CaCl2 as precursor, which includes impurities like Ca(OH)2 or CaClOH. These impurities are caused by the manufacturing process and subsequent dehydration of CaCl2· xH2O.21 Thus, it could be possible that after dehydration of CaCl2· xH2O such oxygenated species are still present. If a certain amount of oxygen is incorporated in the CaF2-film, the refractive index of the film will increase, and thus, the optical properties of such films decline. However, the percentage of oxygen is not only unwanted in CaF2-films but also in ceramics due to the loss of laser oscillation as a result of the scattering at the grain boundaries, which occurs in the ceramics.4 It is known that CaF2 nanocrystals prepared by CaCl2 in EtOH with NH4F as fluorine agent can be also obtained via co-precipitation and hydrothermal synthesis.22
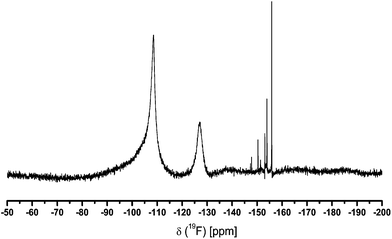 | ||
| Fig. 2 19F NMR spectrum of a CaF2-sol with 5 mol% of TMOS. Two broad signals at −108 ppm (CaF2) and −128 ppm (SiF62−, SiFx) are observed. No evidence for adsorbed HF (liquid NMR spectrometer). | ||
 | ||
| Fig. 3 Hydrodynamic diameter of particles of CaF2-sol with 5 mol% TMOS measured by dynamic light scattering (intensity and volume weighted particle distribution). | ||
 | ||
| Fig. 4 19F MAS NMR spectrum (νrot = 20 kHz) of crystalline CaClF (rorisite) prepared from 40 mol% CaCl2 and 60 mol% CaF2 with NH4Cl as flux at 730 °C for 3 h. | ||
 | ||
| Fig. 5 19F MAS NMR spectra of CaF2-xerogel prepared from CaCl2 with one equivalent of HF(EtOH) (νrot = 20 kHz) dried at 40 °C (a) and (νrot = 19 kHz) 600 °C for 2 h (b). | ||
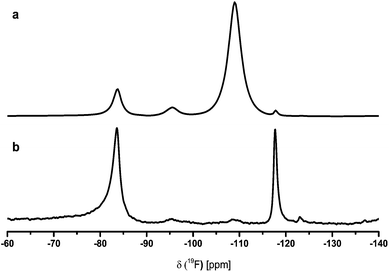 | ||
| Fig. 7 19F MAS NMR spectra of CaF2-xerogel derived from CaCl2 in H2O with two equivalents of HF(EtOH) (a) νrot = 25 kHz, D1 = 5 s, NS = 64 and (b) rs-echo, νrot = 20 kHz, L0 = 20, D1 = 5 s, NS = 256. | ||
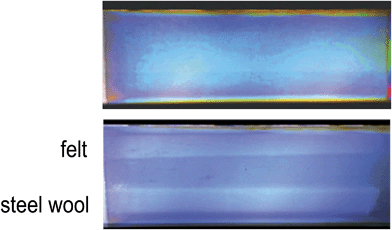
However, a certain amount of oxygen is present in the CaF2 powder, which occurs from the evaporation of the precipitate. Apparently, the percentage of oxygen in the sample is induced by the drying process. Unfortunately, no 19F MAS NMR spectra are listed in this publication, so that a comparison with the signal at −95 ppm of our shown spectra is not possible. The reflections in the X-ray pattern of the xerogels in Fig. 8 reveal CaF2 and CaClF. In the diffractograms as well as in the 19F NMR spectra, it is notable that the CaClF species is formed at 600 °C. Xerogels prepared from CaCl2 as precursor with just one equivalent of HF show after annealing reflections of CaClF (rorisite, PDF 24-0815) and CaF2 (fluorite, PDF 35-0816). In contrast, un-annealed as well as annealed xerogels obtained from stoichiometric Ca2+ to HF ratio show reflections of CaF2 only. Obviously, the nominal fluorine stoichiometric content in the reaction of CaCl2 with HF is crucial whether CaClF or CaF2 is formed. The powder diffractograms show that CaF2 is more crystalline in comparison to MgF2. The crystallite sizes of the un-annealed CaF2-xerogel derived from the Scherrer equation is about 17 nm. In contrast, the crystallite sizes of a MgF2-xerogel annealed at 300 °C is only about 11 nm.17 Moreover, the chloride concentration of the xerogels, which is given in Table 1, reveals that chloride is still present. It is apparent that un-annealed as well as annealed xerogels with a stoichiometric Ca2+ to HF ratio still have a small chloride percentage. It shows that the fluorination of CaCl2 is not complete. In case of one equivalent of HF the discrepancy of the experimentally found percentage of chloride in un-annealed and annealed xerogels is more pronounced. A plausible explanation for this effect is that the un-annealed samples contain a certain amount of water, which is caused by the hygroscopic nature of CaClF.23 It is evident that the deviation from the experimental to the theoretical chloride percentage results from the moisture content of the environment. CaF2-sols were used for the deposition of CaF2-films on borosilicate glass with subsequent thermal treatment at 500 °C for 15 min. Films prepared from sols with stoichiometric fluorine content (F−/Ca2+ = 2) were characterised regarding their optical and mechanical properties. The optical data varied depending on the CaF2-sol synthesis and post-treatment of the coated layer. E.g., the refractive index, determined by spectroscopic ellipsometry, of a 165.50 nm thick CaF2-film on a silicon-wafer and a post-calcination at 500 °C for 15 minutes was 1.37 at 500 nm. For CaF2-coatings on glass the reflection and absorption of the deposited CaF2-film were determined by UV-Vis-spectroscopy. As can be seen in Fig. 9, the reflectance at 600 nm reached almost nearly 0% and the absorbance of the layer between 400 and 900 nm is about 0% as well. Transmittance measurements of the CaF2 film and the glass substrate were not performed for this glass sample. However, AR-layers on float glass samples from different batches showed transmission values at 600 nm repeatedly between 98.4 to 98.7%. Hence, we assume similar transmissions for this sample. Hence, the layer absorbance of CaF2 which is given in Fig. 9 holds for the transmittance of the CaF2 film on glass. It is the difference between the absorbance of the glass substrate and the absorbance of the glass substrate with CaF2 film. To illustrate the morphology of the film, the cross-section image of the CaF2-film derived from high resolution scanning electron microscopy (HR-SEM) is shown in Fig. 10. The measured thickness of the film is approximately 170 nm, and thus, corresponds very well to that determined by ellipsometry. This is in good agreement with the conditions of antireflective coatings. The diameter of the particles is between 10 and 30 nm. This corresponds well with the crystallite sizes of the xerogel determined by XRD. Furthermore, the film is very porous due to the pores, which completely proceed through the whole film. Apparently, if chloride is still present in the film, a certain amount of CaClF might be formed. Hence, the sintering process of a CaClF-containing CaF2-film might be rendered, which is reflected by the high porosity as well mechanical abrasion (Fig. 11). Another fact causing creation of porosity could be also the release of the organic solvent together with HCl formed by the reaction of HF with CaCl2. Thus, these pores filled with air (n0 ≈ 1.0) are the reason for the low refractive index of the CaF2-film. Similar porous coating films are well known for SiO2-films on glass.25,26 Since CaF2 exhibits a lower refractive index than SiO2, it was expected that CaF2-AR-layers with lower porosity but equally good refractive indices possess higher mechanical stability. Therefore, mechanical abrasion of the films was tested too. For that purpose, the CaF2-films annealed at 500 °C were subjected to the Crockmeter test. Fig. 11 is showing the CaF2-film before and after abrasion with felt and steel wool. After 100 cycles with felt and 25 cycles with steel wool the film has only a slight scratch track. Under these conditions, the films are almost robust against abrasion. Just the very surface near region seems to show some little damages without losing the optical performance. There is no visual difference in the abrasion with felt or steel wool. In conclusion, the mechanical stability of CaF2-films is better than that of other CaF2-films, which were derived from calcium acetate as mentioned above. All in all, the CaF2-films show not only excellent optical performance, but show at the same time also good mechanical stability.
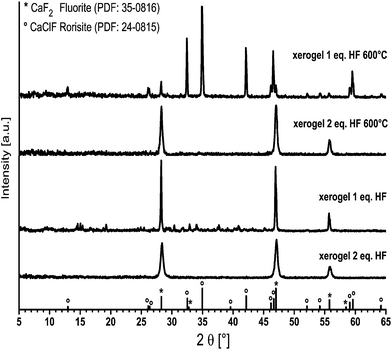 | ||
| Fig. 8 X-ray diffraction patterns of un-annealed and annealed CaF2-xerogels with one and two equivalents of HF(EtOH). | ||
| Xerogel | Theoretical Cl [%] | Experimental Cl [%] |
|---|---|---|
| 1 eq. HF after 40 °C | 37.50 | 23.42 |
| 1 eq. HF after 600 °C | 37.50 | 34.43 |
| 2 eq. HF after 40 °C | 0.0 | 4.16 |
| 2 eq. HF after 600 °C | 0.0 | 2.21 |
 | ||
| Fig. 9 Reflectance and absorption spectra of the CaF2-film annealed at 500 °C by UV-Vis-spectroscopy and refractive index of CaF2 film and CaF2 bulk24 according to the wavelength. | ||
Conclusions
The synthesis of CaF2-sols containing nanoscopic particles was successfully performed by reaction of CaCl2 as inexpensive precursor with anhydrous HF in ethanol. Addition of up to 5 mol% TMOS or TEOS after fluorination always causes a rapid formation of a transparent sol with small particle diameter in the range of 10 to 20 nm. This coating solution is stable for several weeks. No sedimentation was observed. TMOS not only causes a fast clearing up of the sol but also stabilizes the CaF2-particles in the sol. Apparently, addition of TMOS leads to a de-agglomeration of the particles probably due to the reaction with the traces of un-reacted HF in the reaction system. We speculate that the electrostatic repulsion of the particles is changed due to the formation of Si(OR)4−xFx-species – even the formation of SiF4 – as intermediate product cannot be ruled out. Since these are strong Lewis acids, the formation of a CaSiF6 species might be favoured. Further investigations are necessary to study the real rule of TMOS in the CaF2 system. We also evidenced the formation of CaClF at 600 °C beside the formation of an oxide fluoride species during the fluorination. Interestingly, the formation of CaClF is also favoured by using H2O as solvent with two equivalents of aqueous HF-solution and subsequent drying at room temperature. The observation for the oxide fluoride species was not only recorded for CaCl2 as precursor but also for other calcium precursors like calcium bromide or calcium oxide. The percentage of oxygen in the samples could be explained by dehydration of CaCl2 · xH2O. It is also known that CaF2 nanocrystals derived from CaCl2 in EtOH with NH4F, which are prepared by co-precipitation and solvothermal technique, possess impurities of oxygen too. That could be a problem for laser applications because it leads to optical scattering losses, and hence, prohibit laser oscillation. Hence, it is difficult to completely exclude oxygen in CaF2 nanocrystals by sol–gel as well as other synthesis techniques. The CaF2-films exhibit excellent antireflective properties. The mechanical resistance of such films compared to CaF2-films, which were derived from calcium acetate, is significantly better. Only corresponding MgF2-films show marginal better mechanical resistance. It is worth noting that a further improvement of the mechanical properties of these antireflective layers is essential for applications, where a mechanical resistance is indispensable. However, due to the lower solubility as compared to MgF2, CaF2 is an interesting alternative candidate for the manufacture of antireflective coatings.Acknowledgements
This work was financed by the graduate school GRK 1582 “Fluorine as a Key Element” by the Deutsche Forschungsgemeinschaft (DFG). Furthermore, we thank Sigrid Benemann (Federal Institute for Materials Research and Testing, Berlin) for HR-SEM measurements, Dr Gianvito Caputo (HU-Berlin) for TEM measurements, Dr Andrea Zehl (HU-Berlin) for elemental analysis, PD Dr Gudrun Scholz for the discussion of MAS NMR spectra and Dr Birgit Lintner (Prinz Optics GmbH) for UV-Vis-spectroscopy measurements.Notes and references
- I. H. Malitson, Appl. Opt., 1963, 2, 1103–1107 CrossRef CAS.
- P. A. Popov, K. V. Dukel'skii, I. A. Mironov, A. N. Smirnov, P. L. Smolyanskii, P. P. Fedorov, V. V. Osiko and T. T. Basiev, Dokl. Phys., 2007, 52, 7–9 CrossRef CAS.
- I. Nicoara, M. Stef and A. Pruna, J. Cryst. Growth, 2008, 310, 1470–1475 CrossRef CAS PubMed.
- A. Ikesue and Y. L. Aung, Nat. Photonics, 2008, 2, 721–727 CrossRef CAS.
- M. J. Dodge, Appl. Opt., 1984, 23, 1980–1985 CrossRef CAS.
- H. K. Pulker, Coating on glass, Elsevier Amsterdam, 1999 Search PubMed.
- C. Barta, F. Fendrych, K. Recker, A. Triska and F. Wallrafen, Cryst. Res. Technol., 1990, 25, 1287–1298 CrossRef CAS.
- M. Tada, S. Fujihara and T. Kimura, J. Mater. Res., 1999, 14, 1610–1616 CrossRef CAS.
- S. Fujihara, Y. Kadota and T. Kimura, J. Sol-Gel Sci. Technol., 2002, 24, 147–154 CrossRef CAS.
- E. Kemnitz, U. Gross, S. Rudiger and C. S. Shekar, Angew. Chem., Int. Ed., 2003, 42, 4251–4254 CrossRef CAS PubMed.
- R. Sokoll, H. J. Tiller and T. Hoyer, J. Electrochem. Soc., 1991, 138, 2150–2153 CrossRef CAS PubMed.
- F. Sayilkan, M. Asilturk, H. Sayilkan, Y. Onal, M. Akarsu and E. Arpac, Turk. J. Chem., 2005, 29, 697–706 CAS.
- B. E. Yoldas, Am. Ceram. Soc. Bull., 1975, 54, 289–290 CAS.
- Y. L. Diao, W. P. Walawender, C. M. Sorensen, K. J. Klabunde and T. Ricke, Chem. Mater., 2002, 14, 362–368 CrossRef CAS.
- N. Y. Turova, E. P. Turevskaya, V. G. Kessler, A. I. Yanovsky and Y. T. Struchkov, J. Chem. Soc., Chem. Commun., 1993, 21–23 RSC.
- A. Rehmer, in Department of chemistry, Humboldt-Universität zu Berlin, 2012, p. 74 Search PubMed.
- J. Noack, K. Scheurell, E. Kemnitz, P. Garcia-Juan, H. Rau, M. Lacroix, J. Eicher, B. Lintner, T. Sontheimer, T. Hofmann, J. Hegmann, R. Jahn and P. Lobmann, J. Mater. Chem., 2012, 22, 18535–18541 RSC.
- G. Scholz, C. Stosiek, J. Noack and E. Kemnitz, J. Fluorine Chem., 2011, 132, 1079–1085 CrossRef CAS PubMed.
- M. Karg, G. Scholz, R. Konig and E. Kemnitz, Dalton Trans., 2012, 41, 2360–2366 RSC.
- D. A. Wenz, I. Johnson and R. D. Wolson, J. Chem. Eng. Data, 1969, 14, 250–252 CrossRef CAS.
- K. M. Allal, J. C. Doliginier and G. Martin, Oil Gas Sci. Technol., 1997, 52, 361–368 CAS.
- C. Pandurangappa, B. N. Lakshminarasappa and B. M. Nagabhushana, J. Alloys Compd., 2010, 489, 592–595 CrossRef CAS PubMed.
- H. P. Beck, J. Solid State Chem., 1976, 17, 275–282 CrossRef CAS.
- Schott Optical Glass Catalogue on August 2010.
- Y. Liu, W. Ren, L. Y. Zhang and X. Yao, Thin Solid Films, 1999, 353, 124–128 CrossRef CAS.
- N. K. Park, Y. S. Kim, M. J. Kim, T. J. Lee, S. H. Lee and S. H. Lee, J. Nanosci. Nanotechnol., 2013, 13, 7493–7497 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4tc02510e |
| This journal is © The Royal Society of Chemistry 2015 |