Starburst 4,4′,4′′-tris(carbazol-9-yl)-triphenylamine-based deep-blue fluorescent emitters with tunable oligophenyl length for solution-processed undoped organic light-emitting diodes†
Abstract
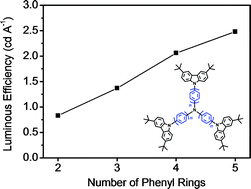
On the basis of a well-known hole transporting material, namely 4,4′,4′′-tris(carbazol-9-yl)-triphenylamine (TCTA), a series of star-shaped deep-blue fluorescent emitters (2P-TCTA, 3P-TCTA, 4P-TCTA and 5P-TCTA) have been successfully developed via a simple extension of the oligophenyl chain between two N atoms. When the number of phenyl rings increases, it is found that both the absorption and emission for these TCTA-based starbursts are red-shifted and finally become saturated for 5P-TCTA consisting of a pentaphenyl bridge. Interestingly, on going from 2P-TCTA to 5P-TCTA, the film photoluminescence quantum yield is gradually enhanced from 11.4% to 35.5%. The same trend is also observed for their corresponding solution-processed undoped OLEDs. As a consequence, 5P-TCTA shows the best device performance, revealing a maximum luminescence of 7300 cd m−2, and a peak luminous efficiency of 2.48 cd A−1 (2.15 lm W−1; 2.30%) together with CIE coordinates of (0.15, 0.09).
- This article is part of the themed collection: JMC C Top Picks collection: Recent progress in light emitting diodes

 Please wait while we load your content...
Please wait while we load your content...