Covalently grafting nonmesogenic moieties onto polyoxometalate for fabrication of thermotropic liquid-crystalline nanomaterials†
Chang-Gen
Lin
,
Wei
Chen
,
Solomon
Omwoma
and
Yu-Fei
Song
*
State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: songyufei@hotmail.com; songyf@mail.buct.edu.cn; Fax: +86 10 64431832
First published on 27th October 2014
Abstract
We demonstrated herein the first class of polyoxometalate-containing thermotropic liquid-crystalline materials, in which the nonmesogenic organic moieties were covalently tethered onto the nanoscaled polyoxometalate. These materials showed the layered smectic A (SmA) phase when cooling from the isotropic state.
The liquid crystal (LC) state exists between the crystalline solid and the amorphous liquid.1 Combining both order and mobility, liquid crystals exhibit anisotropic and switching properties that are conducive to numerous applications.2 In the past decade, there has been increasing interest in the field of liquid crystal nanoscience, which primarily deals with the synergetic relationship between LCs and nanomaterials such as nanoparticles and nanotubes.3 Nanomaterials can introduce specific characteristics into LC systems and enhance the physical properties of LCs, while LCs provide a very good support for the self-assembly of nanomaterials into well-defined functional superstructures in multiple dimensions.4 Polyoxometalates (POMs) are a class of discrete anionic metal oxides with intriguing physical properties and unmatched ranges of structures that can range in size from the nano- to micrometer scale.5 Incorporation of the nanoscaled POMs into LCs generates novel functional liquid-crystalline nanomaterials and has received increasing attention over recent years.6–8 A widely used method to fabricate liquid-crystalline POM nanomaterials is the encapsulation of POM anions into mesogenic or nonmesogenic cationic surfactants.7 However, few examples based on the covalent modification have been reported,8 although the covalent approach indeed offers indisputable assets.9
In the search of POM-based advanced functional materials, herein we present the fabrication of liquid-crystalline nanomaterials based on [(C4H9)4N]4[(SiW11O39)O{Si(CH2)3–NH2·HCl}2] (SiW11–NH2) Keggin POM clusters (Scheme 1). The synthetic procedure is straightforward and consists of a one-step reaction of SiW11–NH2 with gallic acid derivatives that have been applied to obtain thermotropic mesophases with single-molecule magnets, octahedral metal atom clusters, etc.10–12
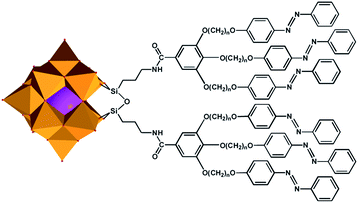 | ||
| Scheme 1 A schematic presentation of liquid-crystalline polyoxo-metalate hybrids 1a–c (a, n = 8; b, n = 10; c, n = 12) with WO6 indicated by gold octahedron and SiO4 indicated by pink sphere. | ||
The gallic acid derivatives were synthesized with long alkyl chains bearing azobenzene groups, which are widely used as rigid units in the fabrication of liquid-crystalline materials. In our case, differential scanning calorimetry (DSC) and optical polarized microscopy (OPM) experiments showed that the gallic acid derivatives did not show liquid-crystalline properties (see ESI†). Grafting these gallic acid derivatives onto POM clusters through amide bonds gave rise to compounds 1a–c.
The molecular structures and conformations of 1a–c were investigated by different techniques. FT-IR spectra in 1a–c show typical amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations at ca. 1660 cm−1, and characteristic SiW11–NH2 stretching vibrations at ca. 1044 (Si–O–Si), 965 (W
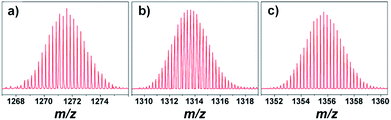
O stretching vibrations at ca. 1660 cm−1, and characteristic SiW11–NH2 stretching vibrations at ca. 1044 (Si–O–Si), 965 (W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 850 (W–O–W) cm−1, indicating that gallic acid derivatives have been grafted onto POMs. In the 1H NMR spectra of 1a–c, signals of the tetrabutylammonium cation and the gallic acid derivative are clearly observed and fit well with the molecular structures. The 29Si NMR spectra of 1a–c show two signals at ca. −52 ppm and −85 ppm, which can be assigned to the Si in the silane and the heteroatom of the mono-lacunary Keggin cluster of SiW11, respectively (Fig. S7–S9†). Although the above analytical methods gave a good indication for the formation of the desired compounds, electrospray ionization mass spectrometry (ESI-MS) provided further convincing structural information. ESI-MS spectra of 1a–c show several intense signals characterized by complex isotope patterns (Fig. S10–S12†). It is possible to assign all the signals (Tables S2–S4†). For example, ESI-MS peaks of 1a observed at m/z 1271.35 can be attributed to {[(SiW11O40){Si(CH2)3NHCOC6H2(OC8H16OC12H9N2)3}2] + HCOONa}4− (Fig. 1a), while peaks of 1b observed at m/z 1313.39 correspond to {[(SiW11O40){Si(CH2)3NHCOC6H2(OC10H20OC12–H9N2)3}2] + HCOONa}4− (Fig. 1b). In the case of 1c, ESI-MS peaks observed at m/z 1355.70 can be assigned to {[(SiW11O40)-{Si(CH2)3NHCOC6H2(OC12H24OC12H9N2)3}2] + HCOONa}4− (Fig. 1c).
O), and 850 (W–O–W) cm−1, indicating that gallic acid derivatives have been grafted onto POMs. In the 1H NMR spectra of 1a–c, signals of the tetrabutylammonium cation and the gallic acid derivative are clearly observed and fit well with the molecular structures. The 29Si NMR spectra of 1a–c show two signals at ca. −52 ppm and −85 ppm, which can be assigned to the Si in the silane and the heteroatom of the mono-lacunary Keggin cluster of SiW11, respectively (Fig. S7–S9†). Although the above analytical methods gave a good indication for the formation of the desired compounds, electrospray ionization mass spectrometry (ESI-MS) provided further convincing structural information. ESI-MS spectra of 1a–c show several intense signals characterized by complex isotope patterns (Fig. S10–S12†). It is possible to assign all the signals (Tables S2–S4†). For example, ESI-MS peaks of 1a observed at m/z 1271.35 can be attributed to {[(SiW11O40){Si(CH2)3NHCOC6H2(OC8H16OC12H9N2)3}2] + HCOONa}4− (Fig. 1a), while peaks of 1b observed at m/z 1313.39 correspond to {[(SiW11O40){Si(CH2)3NHCOC6H2(OC10H20OC12–H9N2)3}2] + HCOONa}4− (Fig. 1b). In the case of 1c, ESI-MS peaks observed at m/z 1355.70 can be assigned to {[(SiW11O40)-{Si(CH2)3NHCOC6H2(OC12H24OC12H9N2)3}2] + HCOONa}4− (Fig. 1c).
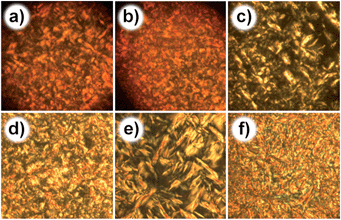
The thermal properties and LC behaviors of 1a–c were investigated by thermogravimetric analyses (TGA), DSC, OPM, and small-angle X-ray scattering (SAXS). TGA analyses reveal that all these hybrids are thermally stable up to 220 °C (Fig. S13†). DSC analyses of 1a–c show that a glass transition state exists in all these hybrids during the heating and cooling process (Fig. S14†). Specific exothermic and endothermic peaks, indicating the existence of liquid-crystalline phases, can be observed in the DSC curves of all the compounds except for 1a (Table S1†). However, all of them exhibit clear birefringence as observed by OPM measurements when directly cooling from the isotropic state (Fig. 2 and S15†). For 1a, a typical fan-shaped texture, which can be assigned to a layered smectic A (SmA) phase, was obtained when cooling from the isotropic state. A further decrease of temperature causes no significant change in texture until a frozen glass state appears. Similar situation can be observed for compounds 1b and 1c. However, some differences still exist. For example, during the cooling process of compound 1b or 1c, the fan-shaped texture gradually transfers to an irregular texture, indicating the thermal changes in enthalpy, which is in good accordance with the DSC measurements. These enthalpy changes, however, do not lead to phase transitions as revealed by the following SAXS studies.
To gain more insights into the arrangement of the molecules in the mesophase, variable-temperature SAXS measurements were carried out (Fig. 3). The SAXS patterns of 1a shown in Fig. 3a are nearly independent of temperature (Table S5†), and can be characterized by five reflexes at q = 1.12, 2.24, 3.41, 4.62, and 5.68 nm−1 with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, indicating a highly ordered lamellar structure. The interlayer distance d001 (d001 = 2π/q1; 56.0 Å) is comparable with the molecular length in the extended molecular conformation (56.2 Å, calculated by the MM2 force field method). This fact strongly suggests the existence of a lamellar SmA phase on the cooling process. The SAXS patterns of 1b and 1c are almost the same with those of 1a. Five equidistant reflexes are found in all temperature ranges (Fig. 3b and c). The interlayer distances (d001) of 1b and 1c are calculated to be 59.1 Å and 62.2 Å, respectively, which are very close to the extended molecular lengths (59.0 Å for 1b and 61.6 Å for 1c). The interlayer distances of 1b and 1c determined by SAXS are also found to be nearly independent of temperature, which is in agreement with the nature of the SmA phase.13 The reflex (q = 5.06 nm−1) shown in all SAXS patterns corresponds to a distance of d′ = 12.4 Å, presumably caused by neighboring POM clusters.14,15
5, indicating a highly ordered lamellar structure. The interlayer distance d001 (d001 = 2π/q1; 56.0 Å) is comparable with the molecular length in the extended molecular conformation (56.2 Å, calculated by the MM2 force field method). This fact strongly suggests the existence of a lamellar SmA phase on the cooling process. The SAXS patterns of 1b and 1c are almost the same with those of 1a. Five equidistant reflexes are found in all temperature ranges (Fig. 3b and c). The interlayer distances (d001) of 1b and 1c are calculated to be 59.1 Å and 62.2 Å, respectively, which are very close to the extended molecular lengths (59.0 Å for 1b and 61.6 Å for 1c). The interlayer distances of 1b and 1c determined by SAXS are also found to be nearly independent of temperature, which is in agreement with the nature of the SmA phase.13 The reflex (q = 5.06 nm−1) shown in all SAXS patterns corresponds to a distance of d′ = 12.4 Å, presumably caused by neighboring POM clusters.14,15
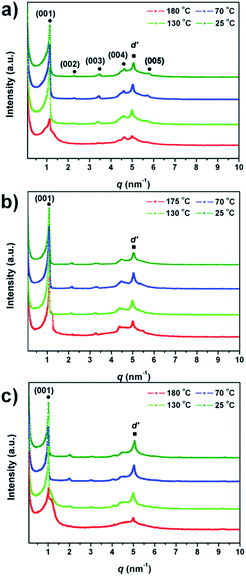 | ||
| Fig. 3 Variable-temperature small-angle X-ray scattering patterns of (a) 1a, (b) 1b, and (c) 1c on the cooling process. | ||
We also employed TEM measurements to confirm the lamellar structures of 1a–c. It can be seen clearly that the alternating patterns of the bright and dark streaks correspond to the gallic acid derivatives and POM clusters, respectively (Fig. S18†). The lamellar distances are estimated to be 5.7, 6.0, and 6.2 nm for 1a–c, respectively, which are consistent with the interlayer distances obtained in the SAXS characterization. As the interlayer distances measured by SAXS and TEM are nearly the same with the fully extended molecular lengths, the molecules are assumed to be oriented in a head-to-tail fashion13,14 within the smectic layers and the alkyl chains are deeply interdigitated for efficient space filling. This proposed model is further confirmed by HR-TEM. As shown in Fig. 4b, well-ordered POM clusters within the POM-containing layer can be observed. On the basis of the above results, we propose a model for the packing structures of the SmA phase (Fig. 4c), in which the organic moieties are located in between the POM layers.
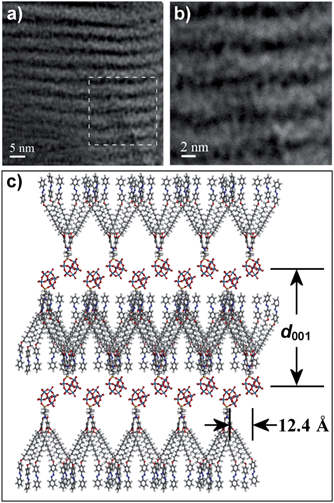 | ||
| Fig. 4 (a) HR-TEM image of 1c; (b) magnified HR-TEM image of 1c; and (c) the suggested model describing the lamellar SmA structure. | ||
Conclusions
In conclusion, we reported for the first time the synthesis and characterization of covalently modified POM-containing thermotropic liquid-crystalline nanomaterials, in which the nonmesogenic organic moieties were tethered onto the nanoscaled POM cluster. These hybrids were found to be able to self-organize into a well-defined smectic lamellar structure, as confirmed by SAXS and HR-TEM measurements. Combining the advantages of organic moieties and tremendous properties of POMs, these hybrids allow the development of promising LCs in the field of smart multi-responsive materials and catalysts. As such, this work provides fascinating perspectives in the design and elaboration of novel POMs-containing liquid-crystalline nanomaterials and may open a new pathway in the development of POM-containing multifunctional nanosystems.Acknowledgements
This research was supported by the National Basic Research Program of China (973 program, 2014CB932104), National Science Foundation of China (21222104), the Fundamental Research Funds for the Central Universities (RC1302, YS1406), Changjiang Scholars and Innovative Research Team in University. The authors appreciate the financial support from Beijing Engineering Center for Hierarchical Catalysts.Notes and references
- D. Demus, J. W. Goodby, G. W. Gray, H.-W. Spiess, and V. Vill, Handbook of Liquid Crystals, Wiley-VCH, Weinheim, Germany, 1998 Search PubMed.
- T. Kato, N. Mizoshita and K. Kishimoto, Angew. Chem., Int. Ed., 2006, 45, 38 CrossRef CAS PubMed; J. W. Goodby, I. M. Saez, S. J. Cowling, V. Gortz, M. Draper, A. W. Hall, S. Sia, G. Cosquer, S.-E. Lee and E. P. Raynes, Angew. Chem., Int. Ed., 2008, 47, 2754 CrossRef PubMed; C. Tshierske, Chem. Soc. Rev., 2007, 36, 193 Search PubMed; B. Donnio, S. Buathong, I. Bury and D. Guillon, Chem. Soc. Rev., 2007, 36, 1495 RSC.
- H. Krishna and S. Kumar, Chem. Soc. Rev., 2011, 40, 306 RSC.
- T. Hegmann and H. Qi, J. Mater. Chem., 2006, 16, 4197 RSC; T. Hegmann, H. Qi and V. M. Marx, J. Inorg. Organomet. Polym. Mater., 2007, 17, 483 CrossRef CAS PubMed.
- D.-L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736 CrossRef CAS PubMed; Y.-F. Song and R. Tsunashima, Chem. Soc. Rev., 2012, 41, 7384 RSC; H. N. Miras, J. Yan, D.-L. Long and L. Cronin, Chem. Soc. Rev., 2012, 41, 7403 RSC; S.-T. Zheng and G.-Y. Yang, Chem. Soc. Rev., 2012, 41, 7623 RSC; B. Hasenknopf, Front. Biosci., 2005, 10, 275 CrossRef PubMed; C. L. Hill, Chem. Rev., 1998, 98, 1 CrossRef PubMed; S. Omwoma, W. Chen, R. Tsunashima and Y.-F. Song, Coord. Chem. Rev., 2014, 258–259, 58 CrossRef PubMed.
- S. Polarz, B. Smarsly and M. Antonietti, ChemPhysChem, 2001, 7, 457 CrossRef CAS; T. Zhang, C. Spitz, M. Antonietti and C. F. J. Faul, Chem.–Eur. J., 2005, 11, 1001 CrossRef PubMed; T. Zhang, S. Liu, D. G. Kurth and C. F. J. Faul, Adv. Funct. Mater., 2009, 19, 642 CrossRef.
- W. Li, S. Yin, J. Wang and L. Wu, Chem. Mater., 2008, 20, 514 CrossRef CAS; Y. Jiang, S. Liu, S. Li, J. Miao, J. Zhang and L. Wu, Chem. Commun., 2011, 47, 10287 RSC; Y. Jia, H.-Q. Tan, Z.-M. Zhang and E.-B. Wang, J. Mater. Chem. C, 2013, 1, 3681 RSC.
- S. Landsmann, C. Lizandara-Pueyo and S. Polarz, J. Am. Chem. Soc., 2010, 132, 5315 CrossRef CAS PubMed; S. Landsmann, M. Wessig, M. Schmid, H. Cölfen and S. Polarz, Angew. Chem., Int. Ed., 2012, 51, 5995 CrossRef PubMed.
- A. Proust, B. Matt, R. Villanneau, G. Guillemot, P. Gouzerh and G. Izzet, Chem. Soc. Rev., 2012, 41, 7605 RSC; A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009 CrossRef CAS PubMed.
- E. Terazzi, C. Bourgogne, R. Welter, J.-L. Gallani, D. Guillon, G. Rogez and B. Donnio, Angew. Chem., Int. Ed., 2008, 47, 490 CrossRef CAS PubMed; E. Terazzi, G. Rogez, J.-L. Gallani and B. Donnio, J. Am. Chem. Soc., 2013, 135, 2708 CrossRef PubMed.
- Y. Molard, F. Dorson, V. Cîrcu, T. Roisnel, F. Artzner and S. Cordier, Angew. Chem., Int. Ed., 2010, 49, 3351 CrossRef CAS PubMed; A. S. Mocanu, M. Amela-Cortes, Y. Molard, V. Cîrcu and S. Cordier, Chem. Commun., 2011, 47, 2056 RSC.
- R. Deschenaux, B. Donnio and D. Guillon, New J. Chem., 2007, 31, 1064 RSC.
- S. Campidelli, J. Lenoble, J. Barberá, F. Paolucci, M. Marcaccio, D. Paolucci and R. Deschenaux, Macromolecules, 2005, 38, 7915 CrossRef CAS.
- M.-B. Hu, Z.-Y. Hou, W.-Q. Hao, Y. Xiao, W. Yu, C. Ma, L.-J. Ren, P. Zheng and W. Wang, Langmuir, 2013, 29, 5714 CrossRef CAS PubMed.
- T. Nakanishi, T. Michinobu, K. Yoshida, N. Shirahata, K. Ariga, H. Möhwald and D. G. Kurth, Adv. Mater., 2008, 20, 443 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures; NMR, FT-IR, and ESI-MS spectra; TGA, DSC, OPM, and TEM. See DOI: 10.1039/c4tc02142h |
| This journal is © The Royal Society of Chemistry 2015 |