Supramolecular assemblies constructed from β-cyclodextrin-modified montmorillonite nanosheets as carriers for 5-fluorouracil
Abstract
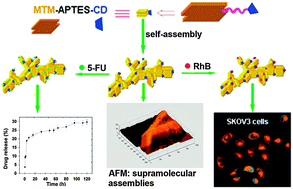
Supramolecular assemblies generated from self-assembling β-cyclodextrin-modified montmorillonite nanosheets were evaluated as anticancer drug carriers in vitro. The results showed that the assemblies had a high loading capacity of 5-fluorouracil (5-FU) under the optimized conditions that included a temperature of 80 °C and a pH level of 11. Scanning electron microscopy (SEM) images showed no morphological changes in the assemblies even after 20 days of storage at room temperature. Moreover, SEM and atomic force microscopy (AFM) observations revealed that the incorporation of 5-FU hardly affected the morphology of the assemblies. Furthermore, the assemblies showed sustained release behavior in vitro, and SEM and AFM analyses indicated that the kinetics of 5-FU release were closely associated with morphological changes in the surface of the assemblies during drug release. Cell viability assays showed that the blank assemblies had low cytotoxicity against A549 cells, while the inhibitory effects of 5-FU-loaded assemblies against A549 cells increased significantly with an increased concentration. More importantly, fluorescence microscopy imaging and transmission electron microscopy (TEM) demonstrated that both blank assemblies and 5-FU-loaded assemblies can easily penetrate cultured human ovarian cancer SKOV3 cells. These results suggest that the supramolecular assemblies may potentially be used as building blocks for the development of new anti-cancer drug delivery systems.

 Please wait while we load your content...
Please wait while we load your content...