Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electrical stimulation of human mesenchymal stem cells on biomineralized conducting polymers enhances their differentiation towards osteogenic outcomes†
John G.
Hardy
*abc,
Rushi C.
Sukhavasi
b,
David
Aguilar
Jr.
b,
Maria K.
Villancio-Wolter
a,
David J.
Mouser
b,
Sydney A.
Geissler
ab,
Lindsey
Nguy
b,
Jacqueline K.
Chow
b,
David L.
Kaplan
*c and
Christine E.
Schmidt
*ab
aJ. Crayton Pruitt Family Department of Biomedical Engineering, University of Florida, Gainesville, FL 32611, USA. E-mail: johnhardyuk@gmail.com; schmidt@bme.ufl.edu; Fax: +1-352-273-9221; Tel: +1-352-273-9222
bDepartment of Biomedical Engineering, The University of Texas at Austin, Austin, TX 78712, USA
cDepartment of Biomedical Engineering, Tufts University, Medford, MA 02155, USA. E-mail: david.kaplan@tufts.edu
First published on 23rd September 2015
Abstract
Tissue scaffolds allowing the behaviour of the cells that reside on them to be controlled are of particular interest for tissue engineering. Herein we describe biomineralized conducting polymer-based bone tissue scaffolds that facilitate the electrical stimulation of human mesenchymal stem cells, resulting in enhancement of their differentiation towards osteogenic outcomes.
Bone conditions requiring surgical intervention are of growing importance in societies with populations in which life expectancies are increasing, motivating the development of pro-regenerative biomaterials.1 Non-biodegradable materials (e.g. titanium), biodegradable materials (e.g. biopolymers, calcium phosphate cements) and multifunctional materials that combine habitats for the cells with the capability to deliver drugs, have been investigated as potential bone tissue scaffolds.1 Biomineralized materials are commonly investigated as bone tissue scaffolds, because the presence of the biomineral in the scaffold may promote osteogenesis.2
Conducting polymer (CP)-based biomaterials (such as derivatives of polyaniline, polypyrrole or polythiophene), have potential for both long term biomedical applications (e.g. electrodes) and short term biomedical applications (e.g. drug delivery or tissue engineering).3 CP-based scaffolds have been developed for the regeneration of bone, muscle and nerve tissue.3 Langer and coworkers first reported the use of CP-based materials for their application as bone tissue scaffolds.4 The application of a potential difference of 20 mV mm−1 over 2-dimensional polypyrrole films encouraged bone marrow-derived stromal cells to differentiate towards osteogenic outcomes (assayed as an increase in alkaline phosphatase (ALP) activity per cell relative to non-stimulated control substrates).4
A variety of research groups have reported further developments in conducting polymer-based materials for bone tissue engineering in the absence5 or presence6 of an electrical field, commonly finding improved osteogenesis for the electrically stimulated samples in vitro. Moreover, the success of inorganic bone substitutes in the clinic has led researchers to develop conducting polymer-based coatings for calcium phosphate-based,7 steel-based,8 and titanium-based9 biomaterials which offer a method of directly electrically stimulating cells residing on the materials, or delivering a drug from such a coating upon the application of an electrical stimulus.10
Here we describe the preparation of polycaprolactone (PCL) derivatives displaying pyrrole moieties from which conducting polymers (such as polypyrrole or polythiophene derivatives) can be grown (Fig. 1).
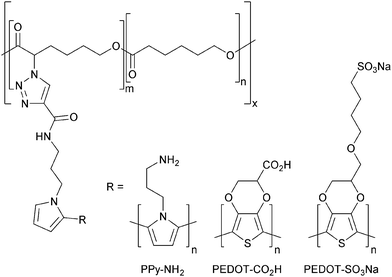 | ||
| Fig. 1 Conducting polymers enabling biomineralization with silica (R–NH2), or calcium carbonate/phosphate (R–CO2H or R–SO3Na). | ||
PCL derivatives displaying amines (e.g. PPy–NH2, Fig. 1) facilitate the mineralization of silica through interactions between the amine which is positively charged under physiological conditions and negatively charged silicic acid precursor to silica; whereas those PCL derivatives displaying carboxylates or sulfonates (e.g. PEDOT–CO2H or PEDOT–SO3Na, Fig. 1) facilitate the mineralization of calcium carbonates or phosphates through interactions of the negatively charged carboxylate/sulfonate moieties with Ca2+ (which subsequently encourages the deposition of carbonates or phosphates). Biomineralized biomaterials have been shown to promote osteogenic differentiation of stem cells,2 as indeed have conducting biomaterials,6 yet this is to the best of our knowledge the first report of biomineralization of conducting polymer-based bone tissue scaffolds which yield biomaterials that combine these attractive features. We demonstrate that such composite biomaterials can be used as bone tissue scaffolds that facilitate electrical stimulation of human mesenchymal stem cells which promotes their differentiation towards osteogenic outcomes in vitro.
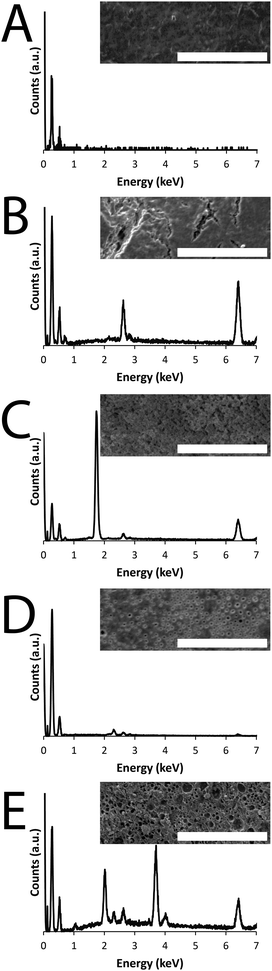
In this study we report a variety of new conducting PCL derivatives. We began by synthesizing PCL derivatives displaying azides to facilitate the coupling of an alkyne-displaying pyrrole derivative which may act as a site from which to grow conducting polymers. Propiolic acid was coupled to aminopropylpyrrole11,12 by carbodiimide-mediated peptide coupling (Scheme S1, ESI†), and these were coupled to PCL derivatives displaying azide moieties13 by Cu(I)-mediated triazole formation13 (Scheme S2, ESI†), after which the copper was removed by incubation in a solution of ethylenediaminetetraacetic acid (EDTA).14 The material was extensively washed to remove traces of EDTA and vacuum dried yielding pyrrole-displaying PCL derivative (depicted in Fig. 1) with Mn = 5.0 kDa and Mw/Mn of 1.95 (Fig. S1, ESI†) in the form of a light brown powder. Films of the resulting polymer were solution cast on either commercially available tissue-culture treated Corning® Costar® tissue culture plates (TCP) or glass. An interpenetrating network of either amine displaying polypyrrole derivative (PPy–NH2, Fig. 1) or carboxylate displaying poly(3,4-ethylenedioxythiophene) derivative (PEDOT–CO2H, Fig. 1) were generated by incubation of the pyrrole-functionalized PCL films in aqueous solutions of the appropriate pyrrole and EDOT derivatives in the presence of the initiators ammonium persulfate and ferric chloride (Scheme S3 and S4, ESI,† respectively).15 Films of the amine or carboxylate derivative displaying films were washed thoroughly with water to remove the by-products (e.g. initiators, monomers, oligomers and polymers) and vacuum dried. The brown-black PPy–NH2 films were biomineralized with silica and those of the blue-grey PEDOT–CO2H were biomineralized with calcium phosphate. Energy dispersive X-ray (EDX) analysis of the films confirms that their surface chemistry is different. Peaks in the EDX spectra of the PCL derivatives displaying pyrrole moieties have lines at 0.277 and 0.525 keV that are the characteristic Kα emissions of carbon and oxygen, respectively, and the very weak emission at 0.392 keV is the Kα emission of nitrogen (Fig. 2A–E). The peaks in the spectra of the films after the polymerization reactions at 2.621 and 6.398 keV are characteristic Kα emission lines of chlorine and iron, the peak at 0.705 keV is the Lα emission line of iron (Fig. 2B–E), and the peak at 2.307 keV is the Kα emission line of sulphur present in the backbone of the PEDOT–CO2H (Fig. 2D and E). The successful biomineralization of the PPy–NH2 films (Fig. 2B) with silica is clear from the appearance of the Kα emission peak of silicon at 1.739 keV (Fig. 2C). Likewise, the successful biomineralization of the PEDOT–CO2H films (Fig. 2D) with calcium phosphate is clear from the appearance of the peaks at 2.013 and 3.690 keV, that are characteristic of the Kα emissions of phosphorous and calcium, respectively (Fig. 2E). The inset SEM images show the surface morphologies of the films (Fig. 2A–E), with nanometer to micrometer scale pores present on the surface of the biomineralized films (Fig. 2B–E).
The electrical sheet resistance of the biomineralized samples was measured in accordance with the method described by Schmidt11,16 and Zhang.17 The PPy–NH2 films had sheet resistances of 8.8 ± 3.6 kΩ which increase to 31.6 ± 9.1 kΩ after biomineralization with silica; whereas the sheet resistances of PEDOT–CO2H films was 2.7 ± 0.4 MΩ which decreased to 248.6 ± 71.8 kΩ after biomineralizion with calcium phosphate; and these values are of a similar order of magnitude to interpenetrating networks of polypyrrole and polystyrenesulfonate in PCL (68.0 ± 18.1 kΩ) that we have previously used to electrically simulate cells.16 While the electrochemical stability of the polypyrrole and PEDOT derivatives are known to decrease over long periods of time which may be problematic for biointerfaces intended for long term use,18 we and others have found them to be acceptable for the short term stimulation of cells residing in tissue scaffolds such as those reported here.3,4,6,11,16,17
To investigate the potential of the biomineralized CPs to act as bone tissue scaffolds, we seeded human mesenchymal stem cells (HMSCs) on their surfaces and cultured them in osteogenic medium for 3 weeks in vitro (testing necessary prior to in vivo testing). We seeded six different systems: (1) cells seeded on TCP controls; (2) cells seeded on PCL (80 kDa); (3) cells seeded on silica-biomineralized PPy–NH2 films without electrical stimulation; (4) cells seeded on silica-biomineralized PPy–NH2 films with electrical stimulation; (5) cells seeded on silica-biomineralized PEDOT–CO2H films without electrical stimulation; (6) cells seeded on silica-biomineralized PEDOT–CO2H films with electrical stimulation. Those samples that were electrically stimulated were cultured for 2 days without stimulation, followed by four periods of stimulation at 10 mV mm−1 for 8 hours then 40 hours without stimulation, and no stimulation thereafter; a stimulation paradigm analogous to that of Langer and coworkers4 and indeed ourselves16).
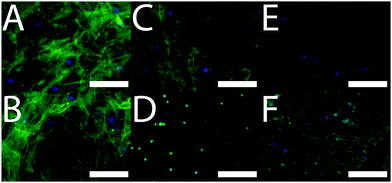
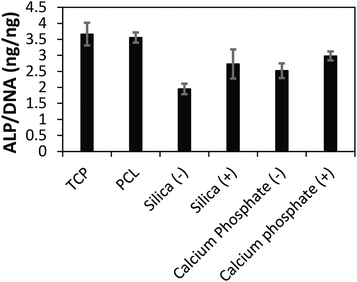
After 3 weeks of in vitro culture, cells were fixed with paraformaldehyde and cell nuclei and actin filaments within cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) and Alexa Fluor® 488 Phalloidin, respectively. We observed that cells were homogeneously distributed on the TCP and PCL controls, and that cells had infiltrated the biomineralized CP films (Fig. 3) which is promising for their integration in the body where infiltration of cells such as macrophages and osteoclasts facilitates remodelling of implanted biomaterials.19 The differentiation of the cell population towards osteogenic fates in vitro was shown using a biochemical assay for alkaline phosphatase (ALP) activity which is a characteristic marker of bone formation. To within experimental error, ALP activity of cells cultured on the TCP and PCL control substrates was the same (Fig. 4). ALP activity for cells cultured on the conductive biomineralized scaffolds in vitro was reduced relative to the TCP and PCL control substrates, which is likely to be because of subtle differences in cell–matrix interactions as observed for analogous systems.20 Interestingly, ALP activity of cells cultured on the scaffolds mineralized with calcium phosphate was slightly higher than for cells cultured on the scaffolds mineralized with silica, which is likely to be because the calcium phosphate acts as a source of calcium and phosphate ions enabling the production of calcified extracellular matrix.21 Furthermore, the ALP activity of cells cultured on the conductive biomineralized scaffolds was increased after electrical stimulation (four periods during which a potential step of 10 mV mm−1 was applied across the conductive substrates for 8 hours), which is in line with reports by Langer4 and others.6 Therefore, our biochemical analysis reveals that while the non-conductive scaffolds support differentiation of HMSCs towards osteogenic outcomes, the application of an electrical stimulus to HMSCs residing in a conductive scaffold in vitro enhances levels of ALP activity which is a hallmark of bone tissue formation.
In the long term we believe it should be possible to prepare a variety of conductive biomineralized tissue scaffolds by chemical modification of the scaffolds with peptides directing the mineralization (e.g. FHRRIKA),22 and potentially also peptides that control other aspects of cell behaviour (e.g. RGD, YIGSR or KRSR for cell adhesion, and NSPVNSKIPKACCVPTELSAI for osteoinduction),22 thereby allowing us to tailor the properties of the scaffold to specific niche applications (and potentially specific patients).
Conclusions
Pro-regenerative biomaterials for the treatment of bone conditions and disorders that require surgical intervention are of growing importance in modern societies in which life expectancies are increasing. Bone tissue scaffolds that control the behaviour of cells residing on them are particularly interesting for such applications.Calcium carbonate is increasingly interesting in biomedicine as novel materials for bone tissue engineering,23 and it is possible to biomineralize PEDOT–CO2H films with calcium carbonate (Fig. S2, ESI†). While it is possible to biomineralize analogous materials incorporating interpenetrating networks of sulfonate displaying PEDOT–SO3Na (Fig. 1 and Scheme S5, ESI†)24 with calcium-based biominerals we found them to be mechanically unstable during long term in vitro cell culture experiments. PEDOT–SO3Na is the most hydrophilic/water soluble of the conducting polymers tested, which is likely to increase rates of enzymatic degradation of the PCL matrix as we have observed for interpenetrating networks of PCL with water insoluble polyplexes of polypyrrole/polystyrenesulfonate.16 Moreover, we know that such PCL/polypyrrole/polystyrenesulfonate-based materials are stable to long term cell culture in vitro,16 and allow the growth of calcium-based biominerals such as calcium carbonate (Fig. S3, ESI†).
Herein we report the first examples of bone tissue scaffolds that combine the attractive properties of biomineralized substrates and electrical conductivity. Controlling the surface chemistry of the substrate enables us to impart electrical conductivity to a biodegradable polymer-based substrate (i.e. PCL) by functionalizing it with conducting polymers (e.g. polypyrrole and poly(3,4-ethylenedioxythiophene) derivatives. Appropriate choice of monomers constituting the conducting polymers enables us to display moieties that facilitate the deposition of specific biominerals; conducting polymers displaying amines that are positively charged under physiological conditions interact with silicic acid which is a precursor to silica formation, whereas those displaying carboxylates or sulfonates which are negatively charged under physiological conditions interact with Ca2+ encouraging their mineralization with calcium phosphate or indeed calcium carbonate, all of which have been shown to be beneficial for bone tissue engineering. Finally, we show that the electrical stimulation of HMSCs residing on our biomineralized conducting polymer-based substrates in vitro enhances levels of ALP activity, which represents an important step towards the formation of bone tissue. We believe that such materials represent useful prototypes for conducting bone tissue scaffolds and warrant further development.
Acknowledgements
We thank the University of Texas at Austin for financial support of David J. Mouser and Rushi C. Sukhavasi in the form of Undergraduate Research Fellowships. We thank Professor Christopher W. Bielawski for access to the GPC and Dr Robert J. Ono and Dr C. Daniel Varnado for training. SEM and EDX were carried out at the Texas Materials Institute and we thank Dr Shouliang Zhang for training. We thank the University of Florida for financial support in the form of startup resources.References
- A. Atala, J. Tissue Eng. Regener. Med., 2007, 1, 83 CrossRef CAS PubMed; J. O. Hollinger, S. Winn and J. Bonadio, Tissue Eng., 2000, 6, 341 CrossRef PubMed; C. T. Laurencin, A. M. A. Ambrosio, M. D. Borden and J. A. Cooper, Jr., Annu. Rev. Biomed. Eng., 1999, 1, 19 CrossRef PubMed; J. R. Porter, T. T. Ruckh and K. C. Popat, Biotechnol. Prog., 2009, 25, 1539 Search PubMed; M. A. Fernandez-Yague, S. A. Abbah, L. McNamar, D. I. Zeugolis, A. Pandit and M. J. Biggs, Adv. Drug Delivery Rev., 2015, 84, 1 CrossRef PubMed; D. Marolt, M. Knezevic and G. Vunjak-Novakovic, Stem Cell Res. Ther., 2010, 1, 10 CrossRef PubMed; W. L. Grayson, T. P. Martens, G. M. Eng, M. Radisic and G. Vunjak-Novakovic, Semin. Cell Dev. Biol., 2009, 20, 665 CrossRef PubMed; M. Fröhlich, W. L. Grayson, L. Q. Wan, D. Marolt, M. Drobnic and G. Vunjak-Novakovic, Curr. Stem Cell Res. Ther., 2008, 3, 254 CrossRef.
- N. M. Alves, I. B. Leonor, H. S. Azevedo, R. L. Reis and J. F. Mano, J. Mater. Chem., 2010, 20, 2911 RSC; S. V. Dorozhkin and M. Epple, Angew. Chem., Int. Ed., 2002, 41, 3130 CrossRef CAS; A. R. Boccaccini, M. Erol, W. J. Stark, D. Mohn, Z. Hong and J. F. Mano, Compos. Sci. Technol., 2010, 70, 1764 CrossRef PubMed; F. C. Meldrum and H. Cölfen, Chem. Rev., 2008, 108, 4332 CrossRef PubMed; S. Heinemann, T. Coradin and M. F. Desimone, Biomater. Sci., 2013, 1, 688 RSC; C. Li and D. L. Kaplan, Curr. Opin. Solid State Mater. Sci., 2003, 7, 265 CrossRef PubMed; N. M. Alves, I. B. Leonor, H. S. Azevedo, R. L. Reis and J. F. Mano, J. Mater. Chem., 2010, 20, 2911 RSC; A. Dey, G. de With and N. A. J. M. Sommerdijk, Chem. Soc. Rev., 2010, 39, 397 RSC; A. J. Salinas, P. Esbrit and M. Vallet-Regí, Biomater. Sci., 2013, 1, 40 RSC; E. Ko and S.-W. Cho, Int. J. Stem Cells, 2013, 6, 87 CrossRef.
- B. Guo, L. Glavas and A. C. Albertsson, Prog. Polym. Sci., 2013, 38, 1263 CrossRef CAS PubMed; M. Muskovich and C. J. Bettinger, Adv. Healthcare Mater., 2012, 1, 248 CrossRef PubMed; M. Berggren and A. Richter-Dahlfors, Adv. Mater., 2007, 19, 3201 CrossRef PubMed; R. Balint, N. J. Cassidy and S. H. Cartmell, Acta Biomater., 2014, 10, 2341 CrossRef PubMed; R. A. Green, N. H. Lovell, G. G. Wallace and L. A. Poole-Warren, Biomaterials, 2008, 29, 3393 CrossRef PubMed; M. Irimia-Vladu, Chem. Soc. Rev., 2014, 43, 588 RSC; N. K. Guimard, N. Gomez and C. E. Schmidt, Prog. Polym. Sci., 2007, 32, 876 CrossRef PubMed; J. G. Hardy, J. Y. Lee and C. E. Schmidt, Curr. Opin. Biotechnol., 2013, 24, 847 CrossRef PubMed; D. Svirskis, J. Travas-Sejdic, A. Rodgers and S. Garg, J. Controlled Release, 2010, 146, 6 CrossRef PubMed; J. G. Hardy, D. J. Mouser, N. Arroyo-Currás, S. Geissler, J. K. Chow, L. Nguy, J. M. Kim and C. E. Schmidt, J. Mater. Chem. B, 2014, 2, 6809 RSC; T. F. Otero and J. G. Martinez, J. Mater. Chem. B, 2013, 1, 26 RSC; R. Gracia and D. Mecerreyes, Polym. Chem., 2013, 4, 2206 RSC; T. H. Qazi, R. Rai and A. R. Boccaccini, Biomaterials, 2014, 35, 9068 CrossRef PubMed; J. Zimmerman, R. Parameswaran and B. Tian, Biomater. Sci., 2014, 2, 619 RSC.
- V. P. Shastri, N. Rahman, I. Martin and R. Langer, Mater. Res. Soc. Symp. Proc., 1999, 550, 215 CrossRef CAS.
- C. Rincón and J. C. Meredith, Macromol. Biosci., 2010, 10, 258 CrossRef CAS PubMed; C. Rincón, C.-C. Chen and J. C. Meredith, Macromol. Biosci., 2010, 10, 1536 CrossRef PubMed; E. De Giglio, S. Cometa, C.-D. Calvano, L. Sabbatini, P. G. Zambonin, S. Colucci, A. Di Benedetto and G. Colaianni, J. Mater. Sci.: Mater. Med., 2007, 18, 1781 CrossRef PubMed; B. Lakard, L. Ploux, K. Anselme, F. Lallemand, S. Lakard, M. Nardin and J. Y. Hihn, Bioelectrochemistry, 2009, 75, 148 CrossRef PubMed; J. S. Moreno, S. Panero, S. Materazzi, A. Martinelli, M. G. Sabbieti, D. Agas and G. Materazzi, J. Biomed. Mater. Res., 2009, 88A, 832 CrossRef PubMed; H. Castano, E. A. O'Rear, P. S. McFetridge and V. I. Sikavitsas, Macromol. Biosci., 2004, 4, 785 CrossRef PubMed.
- J. Cao, Y. Man and L. Li, Biomed. Rep., 2013, 1, 428 Search PubMed; L. Liu, P. Li, G. Zhou, M. Wang, X. Jia, M. Liu, X. Niu, W. Song, H. Liu and Y. Fan, J. Biomed. Nanotechnol., 2013, 9, 1532 CrossRef CAS PubMed; W.-W. Hu, Y.-T. Hsu, Y.-C. Cheng, C. Li, R.-C. Ruaan, C.-C. Chien, C.-A. Chung and C.-W. Tsao, Mater. Sci. Eng., C, 2014, 37, 28 CrossRef PubMed; G. Jin and G. Kim, J. Mater. Chem. B, 2013, 1, 1439 RSC; J. Zhang, K. G. Neoh, X. Hu, E.-T. Kang and W. Wang, Biotechnol. Bioeng., 2013, 110, 1466 CrossRef PubMed; Y. Liu, H. Cui, X. Zhuang, Y. Wei and X. Chen, Acta Biomater., 2014, 10, 5074 CrossRef PubMed; H. Cui, Y. Liu, M. Deng, X. Pang, P. Zhang, X. Wang, X. Chen and Y. Wei, Biomacromolecules, 2012, 13, 2881 CrossRef PubMed; H. Cui, Y. Wang, L. Cui, P. Zhang, X. Wang, Y. Wei and X. Chen, Biomacromolecules, 2014, 15, 3146 CrossRef PubMed; S. Meng, M. Rouabhia and Z. Zhang, Bioelectromagnetics, 2013, 34, 189 CrossRef PubMed; S. Meng, Z. Zhang and M. Rouabhia, J. Bone Miner. Metab., 2011, 29, 535 CrossRef PubMed.
- S. Yala, H. Khireddine, D. Sidane, S. Ziani and F. Bir, J. Mater. Sci., 2013, 48, 7215 CrossRef CAS; Y. Liu, H. Cui, X. Zhuang, P. Zhang, Y. Cui, X. Wang, Y. Wei and X. Chen, Macromol. Biosci., 2013, 13, 356 CrossRef PubMed.
- D. Gopi, S. Ramya, D. Rajeswari, M. Surendiran and L. Kavitha, Colloids Surf., B, 2014, 114, 234 CrossRef CAS PubMed.
- J. Liao, H. Pan, C. Ning, G. Tan, Z. Zhou, J. Chen and S. Huang, Macromol. Rapid Commun., 2014, 35, 574 CrossRef CAS PubMed; J. Liao, Y. Zhu, Z. Yin, G. Tan, C. Ning and C. Mao, J. Mater. Chem. B, 2014, 2, 7872 RSC; E. De Giglio, M. R. Guascito, L. Sabbatini and G. Zambonin, Biomaterials, 2001, 22, 2609 CrossRef; E. De Giglio, L. Sabbatini and P. G. Zambonin, J. Biomater. Sci., Polym. Ed., 1999, 10, 845 CrossRef PubMed; E. De Giglio, L. Sabbatini, S. Colucci and G. Zambonin, J. Biomater. Sci., Polym. Ed., 1999, 10, 1073 CrossRef PubMed; E. De Giglio, L. De Genarro, L. Sabbatini and G. Zambonin, J. Biomater. Sci., Polym. Ed., 2001, 12, 63 CrossRef PubMed; E. De Giglio, S. Cometa, C.-D. Calvano, L. Sabbatini, P. G. Zambonin, S. Colucci, A. Benedetto and G. Colaianni, J. Mater. Sci.: Mater. Med., 2007, 18, 1781 CrossRef PubMed.
- S. Sirivisoot, R. A. Pareta and T. J. Webster, Solid State Phenom., 2009, 151, 197 CrossRef CAS; S. Sirivisoot, R. A. Pareta and T. J. Webster, Nanotechnology, 2011, 22, 085101 CrossRef PubMed.
- J. Y. Lee, C. A. Bashur, A. S. Goldstein and C. E. Schmidt, Biomaterials, 2009, 30, 4325 CrossRef CAS PubMed.
- J. Y. Lee and C. E. Schmidt, J. Biomed. Mater. Res., Part A, 2015, 103, 2126 CrossRef CAS PubMed.
- S. Lenoir, R. Riva, X. Lou, C. Detrembleur, R. Jérôme and P. Lecomte, Macromolecules, 2004, 37, 4055 CrossRef CAS; R. Riva, P. Lussis, S. Lenoir, C. Jérôme, R. Jérôme and P. Lecomte, Polymer, 2008, 49, 2023 CrossRef PubMed; R. Riva, S. Schmeits, F. Stoffelbach, C. Jérôme, R. Jérôme and P. Lecomte, Chem. Commun., 2005, 5334 RSC.
- M. Malkoch, R. Vestberg, N. Gupta, L. Mespouille, P. Dubois, A. F. Mason, J. L. Hedrick, Q. Liao, C. W. Frank, K. Kingsbury and C. J. Hawker, Chem. Commun., 2006, 2774 RSC.
- R. H. Karlsson, A. Herland, M. Hamedi, J. A. Wigenius, A. Åslund, X. Liu, M. Fahlman, O. Inganas and P. Konradsson, Chem. Mater., 2009, 21, 1815 CrossRef CAS.
- J. G. Hardy, R. C. Cornelison, R. C. Sukhavasi, R. J. Saballos, P. Vu, D. L. Kaplan and C. E. Schmidt, Bioengineering, 2015, 2, 15 CrossRef PubMed.
- X. P. Jiang, D. Tessier, L. H. Dao and Z. Zhang, J. Biomed. Mater. Res., 2002, 62, 507 CrossRef CAS PubMed.
- A. Kros, N. A. J. M. Sommerdijk and R. J. M. Nolte, Sens. Actuators, B, 2005, 106, 289 CrossRef CAS PubMed.
- P. M. Mountziaris and A. G. Mikos, Tissue Eng., Part B, 2008, 14, 179 CrossRef CAS PubMed; L. J. Raggatt and N. C. Partridge, J. Biol. Chem., 2010, 285, 25103 CrossRef PubMed; S. Hofmann, M. Hilbe, R. J. Fajardo, H. Hagenmuller, K. Nuss, M. Arras, R. Muller, B. von Rechenberg, D. L. Kaplan, M. P. Merkle and L. Meinel, Eur. J. Pharm. Biopharm., 2013, 85, 119 CrossRef PubMed; D. J. Hadjidakis and I. I. Androulakis, Ann. N. Y. Acad. Sci., 2006, 1092, 385 CrossRef PubMed; J. P. Santerre, R. S. Labow and E. L. Boynton, Can. J. Surg., 2000, 43, 173 Search PubMed; N. A. Sims and T. J. Martin, BoneKEy Rep., 2014, 3, 481 Search PubMed; J. C. Crockett, M. J. Rogers, F. P. Coxon, L. J. Hocking and M. H. Helfrich, J. Cell Sci., 2011, 124, 991 CrossRef PubMed.
- G. G. Wallace, M. J. Higgins, S. E. Moulton and C. Wang, Nanoscale, 2012, 4, 4327 RSC; M. J. Higgins, P. J. Molino, Z. Yue and G. G. Wallace, Chem. Mater., 2012, 24, 828 CrossRef CAS; A. Gelmi, M. K. Ljunggren, M. Rafat and E. W. H. Jager, J. Mater. Chem. B, 2014, 2, 3860 RSC.
- Y.-R. V. Shih, Y. Hwang, A. Phadke, H. Kang, N. S. Hwang, E. J. Caro, S. Nguyen, M. Siu, E. A. Theodorakis, N. C. Gianneschi, K. S. Vecchio, S. Chien, O. K. Lee and S. Varghese, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 990 CrossRef CAS PubMed.
- K. G. Sreejalekshmi and P. D. Nair, J. Biomed. Mater. Res., Part A, 2011, 96A, 477 CrossRef CAS PubMed; H. Shin, S. Jo and A. G. Mikos, Biomaterials, 2003, 24, 4353 CrossRef.
- C. Combes, B. Miaoa, R. Bareilleb and C. Rey, Biomaterials, 2006, 27, 1945 CrossRef CAS PubMed; W. Schneiders, A. Reinstorf, W. Pompe, R. Grass, A. Biewener, M. Holch, H. Zwipp and S. Rammelt, Bone, 2007, 40, 1048 CrossRef PubMed; K. Sariibrahimoglu, S. C. G. Leeuwenburgh, J. G. C. Wolke, L. Yubao and J. A. Jansen, J. Biomed. Mater. Res., Part A, 2012, 100A, 712 CrossRef PubMed; A. H. Dewi, I. D. Ana, J. Wolke and J. Jansen, J. Biomed. Mater. Res., Part A, 2013, 101A, 2143 CrossRef PubMed; H. Zhu, J. Schulz and H. Schliephake, Clin. Oral Implants Res., 2010, 21, 182 CrossRef PubMed.
- O. Stéphan, P. Schottland, P.-Y. Le Gall, C. Chevrot, C. Mariet and M. Carrier, Electroanal. Chem., 1998, 443, 217 CrossRef CAS; M. Yamada, N. Ohnishi, M. Watanabe and Y. Hino, Chem. Commun., 2009, 7203 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, supplementary schemes and figures. See DOI: 10.1039/c5tb00714c |
| This journal is © The Royal Society of Chemistry 2015 |