Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Surface engineering of porous silicon to optimise therapeutic antibody loading and release†
Steven J. P.
McInnes
a,
Chris T.
Turner
b,
Sameer A.
Al-Bataineh
b,
Marta J. I.
Airaghi Leccardi
a,
Yazad
Irani
c,
Keryn A.
Williams
c,
Allison J.
Cowin
b and
Nicolas H.
Voelcker
*a
aARC Centre of Excellence in Convergent Bio-Nano Science and Technology, Mawson Institute, University of South Australia, Adelaide, South Australia 5001, Australia. E-mail: nico.voelcker@unisa.edu.au; Fax: +61 8 8302 5613; Tel: +61 8 8302 5508
bMawson Institute, University of South Australia, Adelaide, South Australia 5001, Australia
cDepartment of Ophthalmology, Flinders University, Bedford Park, South Australia, Australia
First published on 5th May 2015
Abstract
The proinflammatory cytokine, tumor necrosis factor-α (TNF-α), is elevated in several diseases such as uveitis, rheumatoid arthritis and non-healing chronic wounds. Adding Infliximab, a chimeric IgG1 monoclonal antibody raised against TNF-α, to chronic wound fluid can neutralise human TNF-α, thereby providing a potential therapeutic option for chronic wound healing. However, to avoid the need for repeated application in a clinical setting, and to protect the therapeutic antibody from the hostile environment of the wound, suitable delivery vehicles are required. Porous silicon (pSi) is a biodegradable high surface area material commonly employed for drug delivery applications. In this study, the use of pSi microparticles (pSi MPs) for the controlled release of Infliximab to disease environments, such as chronic wounds, is demonstrated. Surface chemistry and pore parameters for Infliximab loading are first optimised in pSi films and loading conditions are transferred to pSi MPs. Loading regimens exceeding 60 μg of Infliximab per mg of pSi are achieved. Infliximab is released with zero-order release kinetics over the course of 8 days. Critically, the released antibody remains functional and is able to sequester TNF-α over a weeklong timeframe; suitable for a clinical application in chronic wound therapy.
Introduction
Protein therapeutics are becoming more and more prevalent in the treatment of a variety of diseases and medical conditions.1,2 However, as proteins are relatively unstable and readily degraded, there is a need to develop alternative effective delivery systems.3 These delivery systems need to protect the protein from degradation by proteases and hydrolytic conditions whilst also being completely biodegradable naturally in the body after implantation or injection.3Porous silicon (pSi) is a biomaterial that is now under intense focus as it has several unique properties that make it very attractive for use in a wide variety of in vivo and ex vivo applications.4,5 Bulk crystalline silicon is converted into the high surface area and biocompatible pSi by means of anodisation in hydrofluoric acid (HF) solution. A broad range of porous structures can be generated by altering the wafer resistivity, HF concentrations and the applied current densities. The pore size can be tuned in diameter from a few nanometers to a few microns achieving surface areas of up to 800 m2 g−1.3,6 pSi degradation is tunable, from days to months,3,7,8 depending on the pore size and chemistry and produces non-toxic silicic acid.6,9–11 pSi has demonstrated in vivo biocompatibility in the subconjuctival space of rats12 and when injected into the vitreous of rabbit eyes.13 Bimbo et al. have also demonstrated the biodistribution of pSi nanoparticles (NPs) administered via oral, subcutaneous and intravenous administration and shown that the pSi NPs do not induce toxicity or inflammatory responses whilst displaying excellent in vivo stability in rats.14Ex vivo applications include optical biosensors that capitalise on the unique optical and photonic properties of the material and have been exploited in the fields of immunology,15 cancer diagnostics,16 chronic wound healing17,18 and infectious diseases.19 The ability of pSi to store and release various payloads of small molecular, oligonucleotide or even protein therapeutics has also been demonstrated.20–22
Immediately after fabrication, pSi is hydride-terminated and degrades within minutes when immersed in neutral aqueous medium. However, the surface chemistry of pSi can be easily modified by techniques such as oxidation, silanisation, hydrosilylation, hydrocarbonisation and electrografting in order to impart varying degrees of stability to the material in addition to introducing functional groups.23–26 Certain surface modifications including thermal hydrocarbonisation render pSi stable even in harsh conditions such as exposure to strong aqueous bases.27 The ability of pSi to degrade into non-toxic silicic acid upon exposure to physiological conditions is a key advantage over other biomaterials.28 The choice of surface chemistry also plays an important role in the ability to load different drugs and must therefore be chosen carefully.20,29 A range of in vivo and in vitro studies have demonstrated the biocompatibility of surface-modified pSi.11,28,30,31 We recently demonstrated composite pSi and poly(ε-caprolactone) (PCL) membranes can be implanted into the subconjunctival space of rats.32 These membranes did not erode or cause inflammatory responses in the tissue surrounding the implant and there was also no evidence of vascularisation.
pSi is being exploited for applications in drug delivery including in several preclinical and clinical settings.12,33,34 A particularly suitable format of pSi for drug delivery are pSi microparticles (pSi MPs) that are fabricated to the desired size from “free-standing” membranes of pSi by sonication35–38 or other mechanical forces, such as ball milling.39 Given their fabrication from pSi films, those MPs have a characteristic plate shape. The Sailor laboratory has developed pSi MPs suitable for injection into the vitreous humor,13 has shown intravitreal drug release for up to 6 months33,40 and the ability to tune drug release by varying the pore size41 or polymer encapsulation of the pSi MPs.42 They have also extensively studied how the pSi dissolution by-products are cleared from the vitreous humor after injection of pSi MPs.43
Receptor proteins, including antibodies, can be both electrostatically bound or conjugated to pSi MPs or NPs for targeting44,45 or biosensing15,46 applications employing various chemistries such as thermal oxidation,46 amino-terminated silanes,47 or using semicarbazide,44 EDC/NHS48 or click49 chemistries. But very few examples exist in the literature describing the release of therapeutic proteins from pSi. One early example was performed by Foraker et al.50 who used micromachined pSi particles to deliver insulin across intestinal Caco-2 cell monolayers. They found that the flux of insulin from the pSi particles was nearly 10 times higher than from insulin in solution and 100 times higher when permeation-enhancing chemicals were omitted in that solution. Likewise, Wu and Sailor have used a polymeric chitosan-based hydrogel to cap the top of pSi films and demonstrate controlled release of insulin from the underlying porous structure in a switchable pH dependent fashion.51 In 2007, Prestidge et al.52 used pSi powders to load and release papain, a model hydrophilic protein. It was observed that the powders, anodised and stain-etched, used in this study varied in surface chemistry, which subsequently affected the loading and burst release of the enzyme from the material. More recently, Andrew et al.21 loaded a thermally oxidised pSi film with Avastin, a monoclonal antibody, and demonstrated a sustained release of 98% of active antibody after 1 month, but the authors did not translate this system to a fully degradable pSi MP format. The retention of the active form of the protein released from pSi MPs and NPs has also been demonstrated by other groups53 emphasising the careful choice of surface chemistry to minimise possible denaturation.54
Infliximab is a chimeric IgG1 monoclonal antibody raised against tumor necrosis factor-α (TNF-α). This antibody is clinically approved to treat Crohn's disease55 and rheumatoid arthritis.56 Infliximab functions by neutralising the function of TNF-α. Infliximab has also been studied for the treatment of psoriasis,57,58 ulcerative colitis,59,60 pyoderma gangrenosum,61 chronic venous ulcers62 and uveitis.63 Non-healing chronic wounds including venous leg ulcers affect 1–2% of the population in developed countries and place a significant burden on the community, estimated to cost $15 billion dollars per year worldwide.64 TNF-α has been demonstrated to be elevated in venous leg ulcers and Infliximab has proven successful in reducing TNF-α levels.62 Uveitis, also an inflammatory condition, occurs in the uveal tract and is responsible for 10% of visual loss in developing nations.65 Typical treatment is with topical and oral glucocorticosteroids and often patients receiving this treatment display significant side effects and insufficient therapeutic responses. Topically applied Infliximab has performed well in the reduction of scarring on the ocular surface in a mouse ocular surface scarring model.63
However, side effects of the systemic delivery of high-dose anti-TNF-α agents, including Infliximab and Etanercept, include drug-induced lupus66 and treatment must be discontinued in these cases. Hence, the development of better delivery systems for anti-TNF-α, particularly those that can sustain low-dose release over a prolonged timespan, could prove beneficial for patient well-being and continual treatment. Indeed, previous studies have shown that the topical application of 10 mg mL−1 Infliximab as a solution or gel and covered with an adhesive bandage could reduce ulcer healing time to 8 weeks in approximately 30% of cases, while the other 70% of ulcers treated had healed significantly, showing a 75% reduction in size.67 Similarly, the one off implantation or injection of a sustained drug delivery for Infliximab to the cornea and anterior segment may prove a safe and effective way to reduce the number of treatments required for anterior uveitis, hence improving patient care.
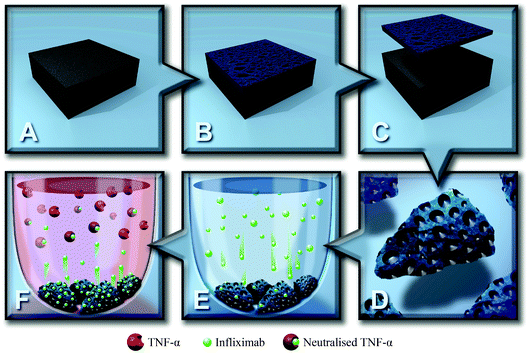
The current study aims to demonstrate the versatility of pSi as a reservoir for Infliximab delivery in both in vivo and ex vivo applications. pSi films with different pore size were prepared by electrochemical etching and thermally oxidised at a range of temperatures. The optimal etching conditions found for the films were then transferred to pSi MPs, which are fully degradable and, in contrast to pSi films, can be used for implantation or injection. pSi MPs were immersed in the antibody solution, allowing the positively charged protein to penetrate into the pores by electrostatic attraction with the negatively charged surface. Antibody loading was quantified using interferometric reflectance spectroscopy and UV-Vis spectroscopy. Infliximab released from pSi MPs remained active and was able to neutralise TNF-α (Scheme 1), potentially providing an improved therapeutic delivery system for the treatment of chronic wounds and ocular conditions such as uveitis.
Experimental details
Chemicals
Hydrofluoric acid (HF) 48% (Merck), dichloromethane (CH2Cl2, Labserv, analytical grade, 99.5%), methanol (Merck, analytical grade, 99.5%), acetone (Ajax, analytical grade, 99.5%), and ethanol (Ajax, absolute, 100%) were used without further purification. N,N-Dimethylformamide (DMF, EMD Chemicals, Belgium) was purified via standard laboratory protocols including drying over MgSO4 followed by distillation at reduced pressure.68 Milli-Q water was obtained from an Advantage A10 water purification system provided by Merck Millipore (water resistivity of 18.2 MΩ cm at 25 °C, TOC < 5 ppb). Phosphate buffered saline (PBS) solution was prepared by dissolving one PBS tablet (Sigma) in 200 mL of MilliQ water, giving a pH of 7.4.Infliximab (Remicade®) powder was purchased from Janssen, Australia. Each vial contains 100 mg of Infliximab, 6.1 mg of sodium phosphate dibasic dehydrate, 2.2 mg of sodium phosphate monobasic monohydrate, 500 mg of sucrose and 0.5 mg of polysorbate 80. The undiluted Infliximab powder was stored at 4 °C. Before use the Infliximab powder was diluted to 1 mg mL−1 with MilliQ water (10 mL).
pSi film preparation
Si wafers (p-type boron doped with a resistivity range of 0.00055–0.001 Ω cm and a 〈100〉 crystal orientation) were cut in 3–4 cm2 pieces, washed with high purity ethanol (Ajax, absolute, 100%) and placed into a Teflon cell, between two electrodes (a platinum mesh as cathode and an aluminum foil as anode for the back contact of Si). The exposed surface area was 1.767 cm2 and the distance between the silicon and the Pt cathode was approximately 1.5 cm. A Keithley 2425 100 W Source Meter was used for anodisation. Etching current and time were controlled by a custom written Labview 8.2 computer program. Polished silicon wafers (CZ process, diameter of 76.2 mm and thickness between 475–525 μm) were provided by Siltronix. The wafer pieces were etched in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 HF
1 HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (v/v) solution. One sacrificial etching step was carried out at 200 mA (113 mA cm−2) for 15 s and the freshly etched surface was washed with ethanol before treatment with 1 N sodium hydroxide for 1 min. The silicon surface was washed again with MilliQ water and ethanol and dried under nitrogen gas. The second etching process was performed with etching current densities ranging from 150 to 233 mA cm−2 and etching times of 15 to 291 s. After etching, washes were performed with ethanol and dichloromethane and dried with nitrogen gas.
ethanol (v/v) solution. One sacrificial etching step was carried out at 200 mA (113 mA cm−2) for 15 s and the freshly etched surface was washed with ethanol before treatment with 1 N sodium hydroxide for 1 min. The silicon surface was washed again with MilliQ water and ethanol and dried under nitrogen gas. The second etching process was performed with etching current densities ranging from 150 to 233 mA cm−2 and etching times of 15 to 291 s. After etching, washes were performed with ethanol and dichloromethane and dried with nitrogen gas.
pSi MP preparation
Microparticles were fabricated from p-type Si wafers (boron-doped, resistivity < 0.001 Ω cm, 〈100〉) supplied by Virginia Semiconductors (Fredericksburg, VA, USA). The wafer was anodised in an 18 cm2 etching cell in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 HF
1 HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (v/v) solution with a current density of 222 mA cm−2 for 4 min, and then electropolished for 30 s at 500 mA cm−2. Then, 20 min of sonication was performed (S30H Elmasonic, 280 W, Elma) to fracture the pSi membrane into MPs. The pSi MP suspension was filtered, washed with ethanol and dichloromethane before drying to completeness.
ethanol (v/v) solution with a current density of 222 mA cm−2 for 4 min, and then electropolished for 30 s at 500 mA cm−2. Then, 20 min of sonication was performed (S30H Elmasonic, 280 W, Elma) to fracture the pSi membrane into MPs. The pSi MP suspension was filtered, washed with ethanol and dichloromethane before drying to completeness.
Gravimetric analysis
The porosity of pSi was determined by weight measurements. To do this, the wafer is weighed before etching (m1), after etching (m2) and after the porous layer is dissolved from the bulk Si, with NaOH (m3). These three values can then be used to calculate the porosity using the following equation:5| Porosity (%) = (m1 − m2)/(m1 − m3) | (1) |
Zeta potential
The surface zeta (ζ)-potential of pSi MPs was determined by using a disposable zeta potential cell on a Zetasizer Nano ZS (Malvern Instruments). The analysis was carried out at a temperature of 25 °C using pSi MPs dispersed in PBS buffer at pHs ranging from 5.5 to 8.5. Zeta potential was acquired from 50 runs performed in triplicate for each sample.Thermal oxidation
A Labec horizontal tube furnace (heating rate of 20 °C min−1) was used to thermally oxidise the freshly etched pSi. Samples being oxidised were situated in the middle of the furnace and the ends of the tube were closed with ceramic caps. Various oxidation temperatures (300, 400 and 500 °C) were used. All thermal oxidations commenced at room temperature and the furnace was ramped to the desired temperature before remaining at that constant temperature for 1 h. The pSi samples were allowed to slowly cool to room temperature inside the furnace. The oxidised films were cut in two smaller pieces leaving out the rim of the etched area. The pieces were then washed in ethanol and dried with nitrogen gas before being loaded with Infliximab (see loading section below).Time-of-flight secondary ion mass spectrometry (TOF-SIMS)
ToF-SIMS measurements were performed using a Physical Electronics Inc. PHI TRIFT V nanoToF instrument (Chanhassen, MN, USA) equipped with a pulsed liquid metal Au+ primary ion gun (LMIG), operating at 30 kV. The extractor current of the ion source was maintained at 3 μA. Positive ion ToF-SIMS images (200 μm × 200 μm) were acquired on the unloaded and infliximab-loaded oxidised porous silicon macro-particles using ‘unbunched’ Au1 beam settings to deliver optimised spatial resolution. Positive ion mass spectra (200 μm × 200 μm) were acquired on the same surfaces using a ‘bunched’ Au1 beam setting for optimal mass resolution. The acquisition time for both images and spectra was 5 min each. Mass calibration of the spectra was done with CH3+, C2H5+, and C3H7+ ions. Experiments were performed a high vacuum (<10−8 Torr), in static mode (i.e. below 1012 ions per cm2) to minimise sample damage.Infrared spectroscopy
Attenuated total reflectance infrared (ATR-IR) spectra were obtained using a Bruker Hyperion 1000 IR microscope operating with a Bruker Vertex 80 IR spectrometer. The IR microscope was equipped with a liquid nitrogen cooled MCT detector. ATR spectra were collected over 64 scans, with a resolution of 4 cm−1, using a Ge ATR crystal. All spectra were background corrected with an unetched silicon wafer of the same type. Spectra of the pSi layers were recorded and analysed using OPUS version 7.0 software, in the range of 650–4000 cm−1. All IR spectra are presented with absorbance normalised to the Si–O peak at approximately 1100 cm−1.Scanning electron microscopy (SEM)
SEM was performed on a FEI Quanta 450 FEG environmental SEM fitted with an SSD detector, and operated at 30 keV with a spot size of 2 mm. To help facilitate the dissipation of charge build-up, samples were coated with 5 nm thick layer of Pt prior to analysis, according to our standard laboratory protocol.12 pSi MPs were dispersed directly onto conductive aluminium stubs for analysis, and were not coated for analysis.Loading and quantification of Infliximab
Infliximab powder was dissolved in 10 mL of sterile MilliQ water for injection, giving a drug concentration of 10 mg mL−1. The solution was subsequently diluted out in PBS to achieve a working concentration of 1 mg mL−1. The antibody solution was aliquoted and stored at −80 °C. The loading of Infliximab (1 mg mL−1, pH 7.4) into the oxidised pSi MPs was carried out using a sealed low protein binding Eppendorf tube. After loading the MPs were rinsed with PBS (pH 7.4, 15 min) to remove the weakly adsorbed antibody. The amount of protein loaded was determined from UV-Vis measurements of the supernatant before and after incubation with the pSi MPs.Interferometric reflectance spectroscopy (IRS) of pSi films
IRS was used to monitor the effective optical thickness (EOT) of the pSi layer in time-lapse mode. The experiments were performed using an interferometer with a bifurcated fiber on a motorised stage that allowed the same sample spots to be accurately analysed. The interferometer consisted of a tungsten light source and USB2000 CCD Detector (Ocean Optics, USA). For the EOT comparison, pSi substrates were placed directly on the motorised stage and monitored in air. For degradation studies, the pSi substrates were placed in a custom-built cell, described elsewhere,15 that allowed solutions to be flowed over the sample while monitoring the EOT in real time.Infliximab release (using ELISA and L929 assay)
Infliximab loaded pSi-MPs (15 mg) were incubated in 500 μL PBS, pH 7.2, for 2 or 4 weeks at 25 °C, to more closely mimic the skin surface temperature, which can vary significantly especially when wounded.69,70 At days 1, 2, 7, 14, 21 and 28 days, samples were spun briefly to pellet the pSi and then all of the supernatant was decanted. A 500 μL aliquot of fresh PBS, pH 7.4, was added to each sample, to continue the incubation. Each aliquot was tested for the amount of antibody release via ELISA (see Section, TNF-α ELISA, below) and TNF-α based bioassay (see Section, TNF-α cell-based bioassay, below).Infliximab release (using fluorimetry)
Infliximab (0.1 mg mL−1) was labeled with fluorescein isothiocyanate (FITC) for 4 h in a sodium carbonate buffer (100 mM, pH 9.5). After labeling the labeled protein was recovered using a Vivaspin 2 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MWCO spin tube (Sartorius Stedim) according to the manufacturer's instructions. This FITC labeled protein was then added to unlabeled Infliximab at a ratio of 1
000 MWCO spin tube (Sartorius Stedim) according to the manufacturer's instructions. This FITC labeled protein was then added to unlabeled Infliximab at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.85 and this stock was used to load pSi MPs as outlined above. Release was then monitored on an Agilent Technologies Cary Eclipse fluorimeter fitted with a Peltier temperature control system with a PMT of 650 V and excitation and emission slit widths of 5 nm. The emission was monitored at 525 nm and the excitation was performed at 490 nm. Data was recorded in an automated kinetic mode every 8 h for 7 d. The FITC signal was calibrated against a calibration curve constructed from dilutions of the FITC labeled Infliximab stock solution.
5.85 and this stock was used to load pSi MPs as outlined above. Release was then monitored on an Agilent Technologies Cary Eclipse fluorimeter fitted with a Peltier temperature control system with a PMT of 650 V and excitation and emission slit widths of 5 nm. The emission was monitored at 525 nm and the excitation was performed at 490 nm. Data was recorded in an automated kinetic mode every 8 h for 7 d. The FITC signal was calibrated against a calibration curve constructed from dilutions of the FITC labeled Infliximab stock solution.
TNF-α ELISA
The Duo TNF-α ELISA kit (R&D Systems) was used to detect non-neutralised human TNF-α as per the manufacturer's instructions. TNF-α was evaluated in post-pSi supernatant and TNF-α spiked (1 μg mL−1) acute wound fluid (obtained with institutional ethics approvals Human Research Ethics Committee, The Queen Elizabeth Hospital, Lyell McEwin Hospital, Modbury Hospital (TQEH/LMH/MH) Ref#: HREC/12/TQEHLMH/107). The optical density of each well was determined immediately using a microplate reader set to 450 nm (Sunrise™, Tecan Group Ltd., Australia).TNF-α cell-based assay
A cell-based cytotoxic bioassay based on a subclone of the murine L929 fibroblast cell line (Sigma-Aldrich, Sydney, Australia) was used.71 Briefly, L929 cells were seeded at 2 × 104 cells per well in 96-well microtiter plates containing 50 μL of culture medium (Dulbecco's modified Eagle's medium containing 10% (v/v) fetal bovine serum) (Sigma-Aldrich, Sydney, Australia). The cells were incubated for 24 h before the addition of 50 μL test solution, containing from 1 mg mL−1 to 1 pg mL−1 recombinant human TNF-α (R&D Systems, Minneapolis, MN), and Infliximab released from pSi MPs to each well. Fresh Infliximab (1 to 1000 μg mL−1) was added to some wells as a positive control. After a further 24 h incubation, 20 μL of 2.5 mg mL−1 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma-Aldrich, Sydney, Australia) and 50 μL culture medium was added per well and incubated for 4 h before solubilisation in 100 μL per well 10% (w/v) sodium dodecyl sulfate-HCl. After a final overnight incubation, the blue formazan product was measured at 570 nm on a microplate reader (Sunrise™, Tecan Group Ltd., Australia).Statistical analysis
Statistical differences were determined using the Student's t-test or an ANOVA. For data not following a normal distribution, the Mann–Whitney U-test was performed. A P value of less than 0.05 was considered significant.Results
Infliximab (molecular weight of 149 kDa) has a hydrodynamic radius of 5–6 nm and an isoelectric point (pI) of approximately 8.3. The radius and the pI dictate that a >10 nm pore radius and a negative surface charge at neutral pH should be used to facilitate antibody loading and retention. When working with monoclonal antibodies, using a buffer with the correct pH and ionic strength is important.72 As the pI of Infliximab is 8.3 working at pH 7.4 which is below this pI should keep the Infliximab positively charged and subsequently less likely to aggregate, hence, helping the protein to remain in its fully active conformation.73 The original pSi etching conditions were adapted from previous work by Andrew et al.21 However, we observed that these conditions resulted in non-homogeneous pore sizes and a microporous layer formed when etching some wafers that possess a highly doped surface layer.74 In order to remove this microporous layer, a sacrificial etching step was applied (see Fig. S1, ESI†).74 After the sacrificial etching step, etching current densities from 233 to 150 mA cm−2 were used in combination with etching times from 15 to 240 s. Interferometric reflectance spectroscopy (IRS) analysis of the effective optical thickness (EOT) from 20 locations on each film showed that the 233 mA cm−2 etch for 20 s produced the most homogeneous surfaces with EOT variations of less than 0.20% across the scanned etched region (see Fig. S2, ESI†).Scanning electron microscopy (SEM) images of the pSi surfaces etched using the optimal conditions of 233 mA cm−2 for 20 s and oxidised at 400 °C (Fig. 1) revealed pore sizes are approximately 22.2 ± 4.4 nm. The thickness of the porous layer, measured via SEM, was 1.39 μm and the porosity, determined by means of IRS, was 76 ± 5%.
 | ||
| Fig. 1 Scanning electron microscopy (SEM) images of (A) cross-sectional view and (B) top down view of the oxidised (400 °C) pSi film. | ||
The temperature at which the pSi film surface is oxidised controls both the extent of antibody loading (assuming that a larger difference in net charge between the pSi surface and antibody encourages more protein binding), and the rate at which the pSi will degrade in aqueous buffers. It is known from previous work that pSi functionalised at 600 °C or above will not readily degrade in aqueous solutions either in vitro or in vivo.12 For this reason, we chose to investigate oxidation temperatures of 300, 400 and 500 °C with freshly etched pSi as a control. Fig. 2 shows the average degradation curves for each oxidation temperature over a 2 h period. Combining this analysis with the pre-determined film thickness, we can estimate the expected degradation time. As anticipated, the fastest surface to degrade was the freshly etched pSi surface at a rate of 18.13% EOT per h, resulting in complete degradation of the film in just 2.5 h (0.10 d). The time for degradation of the films oxidised at 300 °C extended to 73.8 h (3.1 d at a rate of 0.61% EOT per h). This degradation time increased further to 201.3 h (8.4 d at 0.22% EOT per h) for the film oxidised at 400 °C and to 790.0 h (32.91 d at 0.06% EOT per h) for the sample after 500 °C oxidation.
The optimal etching conditions were then used to produce pSi MPs that were thicker in nature than the pSi films (Fig. 3) and could be suitable for either implantation or injection. pSi MPs were etched using the conditions adapted from those optimised for the pSi films, with an etching time of 4 min and a subsequent 30 s electropolish at 500 mA cm−2 to lift off the film, which was then subsequently sonicated to generate particles. SEM revealed that the pSi MPs had a thickness of 23.4 ± 1.3 μm (Fig. 3A). Higher resolution SEM analysis showed an average pore diameter of 19.5 ± 8.2 nm (Fig. 3B), very similar to that of the pSi films (22.2 ± 4.4 nm, Fig. 1B). Typical particle sizes were in the range of 66.5 ± 20.9 μm. Gravimetric analysis determined the porosity of the pSi MPs to be 84.2 ± 2.0%, again very close to that of the pSi film preparations (76 ± 5%).
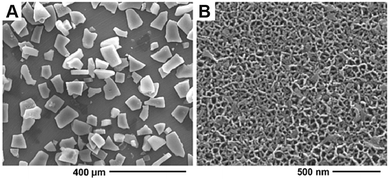 | ||
| Fig. 3 (A) SEM micrograph showing the size distribution of the pSi MPs and (B) higher resolution SEM micrograph showing mesopores of the pSi MPs. | ||
Zeta potential investigations into the pSi MPs prepared with various oxidation conditions at pH 7.4 revealed that the surface was negatively charged at about −20 mV for all oxidation temperatures (Table 1).
| pSi oxidation temperature (°C) | Zeta potential (mV) |
|---|---|
| 300 | −20.3 ± 0.9 |
| 400 | −19.4 ± 1.5 |
| 500 | −20.3 ± 1.7 |
This is in line with the literature and is attributed to the presence of Si–OH on the surface.53 Next, Infliximab binding was analysed overnight at room temperature by observing the change in zeta potential of the pSi MPs before and after the injection of Infliximab at 266 μg mL−1 (Fig. 4A).
It was observed that the zeta potential of the MPs after overnight incubation decreased significantly for all oxidation conditions, due to the adsorption of protein. Loading of the Infliximab into the pSi MPs during the zeta measurements was also confirmed by the UV-Vis spectroscopy of the supernatant before and after the loading experiment. The supernatant after loading showed complete removal of the protein peak at 280 nm (Fig. 4B), suggesting that the protein was completely sequestered by the pSi MPs.
Considering the degradation and zeta potential data, we chose to use 400 °C oxidised pSi MPs to perform binding Infliximab experiments since this sample was stable over several days (the desired timeframe of drug release) and was negatively charged where Infliximab at pH 7.4 is positively charged.73
Antibody loading experiments performed at pH 6.5 and 5.5 showed a similar trend in both zeta potential measurements and UV-Vis analysis (see Fig. S3, ESI†), suggesting no advantage of loading at more acidic pH values. Subsequently, our typical loadings of the pSi MPs were performed with approximately 15 mg of pSi and 1 mg (at 1 mg mL−1) Infliximab in PBS at 7.4. Loading values were individually assessed by UV-Vis spectrophotometry of the supernatant before and after for each particle preparation used, and were typically in the range of 0.063 ± 0.010 mg mg−1 similar to literature values.52 Loadings could be further improved by the use of higher concentrations of Infliximab (see Table S1, ESI†).
Successful loading of Infliximab was also confirmed by infrared (IR) spectroscopy. The IR in attenuated total reflection (ATR) spectra of oxidised pSi (Fig. 5, 400 °C oxidised pSi) showed a broad, intense peak centered at 1047 cm−1 attributed to the asymmetric stretching of Si–O–Si groups75 and at 887 cm−1 due to Si–O bending in O–Si–O.76 The shoulder located at approximately 1182 cm−1 was attributed to the stretching of surface oxide species including O–Si–O.77–79 After loading of Infliximab, into the oxidised pSi (Fig. 5, 400 °C oxidised pSi with Infliximab), the spectra still showed surface peaks characteristic of oxidised pSi in addition to new peaks at 1465 cm−1 from the asymmetric CH3 deformation and dual peaks at 2850–3000 cm−1 for the C–H stretching vibrations of the protein. Two prominent peaks at 1542 cm−1 and 1639 cm−1 were attributed to C–N–H bending vibrations (amide II) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations (amide I) of the peptide bonds, respectively.80 The 1247 cm−1 peak could also be ascribed to amide III of the protein,81 while the secondary amine (N–H) stretching appeared at 3290 cm−1.80 The IR results therefore confirm the presence of Infliximab on the pSi surface. X-ray photoelectron spectroscopy (XPS) further corroborated those results (Table S2, ESI†).
O stretching vibrations (amide I) of the peptide bonds, respectively.80 The 1247 cm−1 peak could also be ascribed to amide III of the protein,81 while the secondary amine (N–H) stretching appeared at 3290 cm−1.80 The IR results therefore confirm the presence of Infliximab on the pSi surface. X-ray photoelectron spectroscopy (XPS) further corroborated those results (Table S2, ESI†).
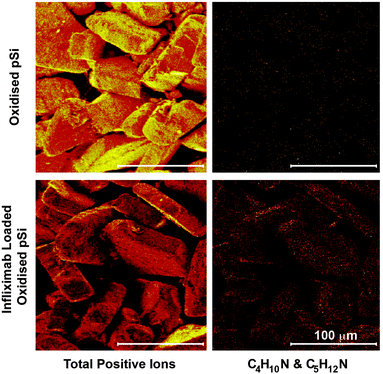
To verify that Infliximab diffused completely through the oxidised pSi layer, the cross-sections of pSi films before and after Infliximab loading were imaged by time-of-flight secondary ion mass spectrometry (ToF-SIMS) to detect characteristic positive ion fragments, appearing in the mass spectra after loading Infliximab into pSi. Fig. 6 shows ToF-SIMS images of the total positive ions and the total intensity of the selected positive ion fragments C4H10N+ (m/z 72.081) and C5H12N+ (m/z 86.096) characteristic of the amino acids valine and leucine/isoleucine, respectively82 (see Fig. S4, ESI† for ToF-SIMS mass spectra and Table S3, ESI† for mass peak assignments). As expected, no signal was detected for the C4H10N+ & C5H12N+ positive fragments within the oxidised porous layer of the unloaded sample In contrast, both positive ion fragments were observed after Infliximab loading. The ToF-SIMS imaging also showed that the protein was present throughout the porous layer although signal intensity decreased with increasing depth. It should be noted that in order to facilitate the ToF-SIMS imaging, a very thick pSi film (80 μm) was used, much thicker than what was used for pSi MPs (23.4 μm). Indeed, ToF-SIMS imaging of oxidised pSi MPs after loading with Infliximab showed representative positive ion fragments, C4H10N+ (m/z 72.081) and C5H12N+ (m/z 86.096) over across the MPs (Fig. 7). As expected, however, mapping these fragments on the unloaded oxidised pSi MPs showed a very weak intensity. While it was impractical to cross-section the oxidised pSi MPs, we expected MPs with open pores on both sides would allow antibody loading easier than pSi films.
To obtain the antibody release kinetics from the 400 °C pSi MPs, we followed the emission of FITC-labeled Infliximab releasing into solution via fluorimetry (Fig. 8A). The release kinetics appeared initially to show a small burst release (approx. 5.3% at 8 h) followed by a near linear release profile (R2 = 0.976) release profile. This is desirable to maximise the therapeutic benefits of a localised drug delivery platform.83 It was observed that the optimally loaded pSi MPs released the Infliximab at a rate of 22.56 μg of Infliximab per day. These results show that the release of Infliximab should continue for approximately 14 d (47.34% release observed at 7 d).
To test the functionality of the Infliximab released from the pSi MPs oxidised at 400 °C, we carried out a TNF-α neutralisation ELISA periodically over 14 d. Infliximab released from pSi was functional with >90% TNF-α neutralisation observed for the day 1 and 2 samples (Fig. 8B, black bars). However, the activity of the released Infliximab diminished over time, with no effect observed past the day 7 time-point. Infliximab functionality when incubated in the absence of pSi (Fig. 8B, grey bars) (at an equivalent concentration to the amount released from pSi) was less than observed for antibody released from pSi, with no activity detected after the 2 day time-point. This indicated that the incubation of low concentrations of Infliximab in PBS at 25 °C leads to degradation, providing a limitation for the assay, but importantly provided evidence that pSi protected Infliximab from degradation prior to release.
To confirm that Infliximab released from pSi MPs neutralises TNF-α, whilst showing efficacy in a cellular environment, a L929 cell bioassay was used.71,84 Recovery of TNF-α treated L929 cells was assessed after 7, 14, 21 and 28 d of release. Recombinant TNF-α was cytotoxic to L929 cells in a dose dependent manner (p < 0.005) (Fig. S5, ESI†) and this cytotoxicity could subsequently be inhibited by Infliximab at ≥5 μg mL−1 (Fig. S6, ESI†). The addition of supernatant from Infliximab-loaded pSi MPs was demonstrated to increase L929 cell viability for up to 21 d (Fig. 9A). It should be noted that the MTT assay (Fig. 9A) showed the presence of functional Infliximab in samples up to day 28, longer than could be detected by ELISA (Fig. 8B). The discrepancy may be caused by differences in the sensitivity of these two assays and variations in the strength of TNF-α–Infliximab binding due to the different buffers, pH values and incubation times required. Together, the ELISA and L929 cell bioassay was able to confirm that Infliximab released from pSi MPs remained both stable and active.
The applicability of this system with conditions closer to a wound environment was demonstrated via the neutralisation of TNF-α spiked into acute wound fluid (AWF) from 3 different patients (Fig. 9B). Time points of 1, 2, 7 and 14 d were analysed with the L929 cell bioassay. L929 cells could recover >25% when exposed to supernatant incubated for 1 and 2 d. This result suggests that the pSi MPs loaded with Infliximab were able to neutralise the TNF-α in actual wound fluid samples. Additionally, the range of conditions in which Infliximab remained active was tested via the L929 assay. We observed that the L929 cells were able to respond to Infliximab treatment when high concentrations (1 mg mL−1) of Infliximab were held at pH values ranging from 4.5–8.5 and temperatures ranging from 4 °C to 37 °C for up to 7 d (see Fig. S7, ESI†). It was observed that even at the extreme pH values and temperatures approximately 70% cell recovery was observed.
The data presented here demonstrates that Infliximab is able to bind to TNF-α in a wound environment and subsequently reduces its activity. These results suggest the wide range and applicability of the delivery of Infliximab for applications ranging from wound healing to uveitis.
Conclusions
We demonstrate that oxidised pSi films and MPs have a high loading capacity for Infliximab and extend antibody release in vitro. In particular, we show near linear release kinetics of Infliximab from oxidized pSi MPs over 8 days. The released Infliximab was able to prevent apoptosis of L929 cells in acute wound fluid by sequestering TNF-α over 7 days, a period suitable for a clinical application. Hence, with optimised tuning of the porous structure and its surface, the pSi films and MPs described here may represent a form of resorbable and biocompatible therapeutic carrier for extended drug delivery requiring no surgical removal. It is envisaged that pSi MPs can be incorporated into wound dressings materials and deliver Infliximab to wound fluid in order to improve chronic wound healing. We also believe that the same Infliximab-releasing pSi MP format may be fit for the purpose of treating uveitis.Acknowledgements
Funding from the NHMRC Project Grant 595901 is acknowledged. We thank intern students Hiten Lad and Laura Rollinger for assistance with experiments as well as Roshan Vasani for XPS analysis, Stephanie Pace for assistance in interpreting the IRS results, John Denman for assistance with acquiring ToF-SIMS spectra and Stuart McClure for performing the SEM imaging. This research was in part conducted and funded by the Australian Research Council Centre of Excellence in Convergent Bio-Nano Science and Technology (project number CE140100036). The authors also wish to thank Marc Cirera for his design of the TOC graphic and Scheme 1.Notes and references
- S. Farajnia, V. Ahmadzadeh, A. Tanomand, K. Veisi, S. A. Khosroshahi and L. Rahbarnia, Immunopharmacol. Immunotoxicol., 2014, 36, 297–308 CrossRef CAS PubMed.
- P. Chames, M. Van Regenmortel, E. Weiss and D. Baty, Br. J. Pharmacol., 2009, 157, 220–233 CrossRef CAS PubMed.
- J. K. Tessmar and A. M. Göpferich, Adv. Drug Delivery Rev., 2007, 59, 274–291 CrossRef CAS PubMed.
- H. A. Santos, Porous silicon for biomedical applications, Woodhead Publishing Limited, 2014 Search PubMed.
- L. T. Canham, Properties of Porous Silicon, Short Run Press, London, 2006 Search PubMed.
- A. Loni, in Properties of Porous Silicon, ed. L. Canham, Short Run Press, London, 2006 Search PubMed.
- S. J. P. McInnes, E. J. Szili, S. A. Al-Bataineh, J. Xu, M. E. Alf, K. K. Gleason, R. D. Short and N. H. Voelcker, ACS Appl. Mater. Interfaces, 2012, 4, 3566–3574 CAS.
- S. J. P. McInnes, H. Thissen, N. R. Choudhury and N. H. Voelcker, J. Colloid Interface Sci., 2009, 332, 336–344 CrossRef CAS PubMed.
- L. T. Canham, Adv. Mater., 1995, 7, 1033–1037 CrossRef CAS.
- L. A. R. Canham, Phys. World, 2001, 27–31 CrossRef CAS.
- S. D. Alvarez, A. M. Derfus, M. P. Schwartz, S. N. Bhatia and M. J. Sailor, Biomaterials, 2009, 30, 26–34 CrossRef CAS PubMed.
- S. P. Low, N. H. Voelcker, L. T. Canham and K. A. Williams, Biomaterials, 2009, 30, 2873–2880 CrossRef CAS PubMed.
- L. Cheng, E. J. Anglin, F. Cunin, D. Kim, M. J. Sailor, I. Falkenstein, A. Tammewar and W. R. Freeman, Br. J. Ophthalmol., 2008, 92, 705–711 CrossRef CAS PubMed.
- L. M. Bimbo, M. Sarparanta, H. A. Santos, A. J. Airaksinen, E. Mäkilä, T. Laaksonen, L. Peltonen, V.-P. Lehto, J. Hirvonen and J. Salonen, ACS Nano, 2010, 4, 3023–3032 CrossRef CAS PubMed.
- E. J. Szili, A. Jane, S. P. Low, M. Sweetman, P. Macardle, S. Kumar, R. S. C. Smart and N. H. Voelcker, Sens. Actuators, B, 2011, 160, 341–348 CrossRef CAS.
- M. J. Sailor and J.-H. Park, Adv. Mater., 2012, 24, 3779–3802 CrossRef CAS PubMed.
- F. S. H. Krismastuti, S. Pace, E. Melville, A. J. Cowin, T. R. Dargaville and N. H. Voelcker, Aust. J. Chem., 2013, 66, 1428–1434 CrossRef CAS.
- S. Pace, R. B. Vasani, F. Cunin and N. H. Voelcker, New J. Chem., 2012, 37, 228–235 RSC.
- A. M. Rossi, L. Wang, V. Reipa and T. E. Murphy, Biosens. Bioelectron., 2007, 23, 741–745 CrossRef CAS PubMed.
- J. Salonen, L. Laitinen, A. Kaukonen, J. Tuura, M. Bjorkqvist, T. Heikkila, K. Vaha-Heikkila, J. Hirvonen and V.-P. Lehto, J. Controlled Release, 2005, 108, 362–374 CrossRef CAS PubMed.
- J. S. Andrew, E. J. Anglin, E. C. Wu, M. Y. Chen, L. Cheng, W. R. Freeman and M. J. Sailor, Adv. Funct. Mater., 2010, 20, 4168–4174 CrossRef CAS PubMed.
- J. Rytkönen, P. Arukuusk, W. Xu, K. Kurrikoff, Ü. Langel, V.-P. Lehto and A. Närvänen, Mol. Pharmaceutics, 2014, 11, 382–390 CrossRef PubMed.
- Z. P. Xu, Q. H. Zeng, G. Q. Lu and A. B. Yu, New J. Chem., 2003, 27, 1027–1040 Search PubMed.
- Y.-L. Khung, S. D. Graney and N. H. Voelcker, Biotechnol. Prog., 2008, 22, 1388–1393 CrossRef PubMed.
- A. Janshoff, K.-P. S. Dancil, C. Steinem, D. P. Greiner, V. S. Y. Lin, C. Gurtner, K. Motesharei, M. J. Sailor and M. R. Ghadiri, J. Am. Chem. Soc., 1998, 120, 12108–12116 CrossRef CAS.
- M. P. Stewart and J. M. Buriak, Adv. Mater., 2000, 12, 859–869 CrossRef CAS.
- J. Salonen and V.-P. Lehto, Chem. Eng. J., 2008, 137, 162–172 CrossRef CAS.
- S. Low, K. Williams, L. Canham and N. H. Voelcker, Biomaterials, 2006, 27, 4538–4546 CrossRef CAS PubMed.
- L. A. Perelman, C. Pacholski, Y. Y. Li, M. S. VanNieuwenhze and M. J. Sailor, Nanomedicine, 2008, 3, 31–43 CrossRef CAS PubMed.
- S. Bayliss, L. Buckberry, P. Harris and M. Tobin, J. Porous Mater., 1999, 7, 191–195 CrossRef.
- Y. L. Khung, G. Barritt and N. H. Voelcker, Exp. Cell Res., 2008, 314, 789–800 CrossRef CAS PubMed.
- S. Kashanian, F. Harding, Y. Irani, S. Klebe, K. Marshall, A. Loni, L. Canham, D. Fan, K. A. Williams, N. H. Voelcker and J. L. Coffer, Acta Biomater., 2010, 6, 3566–3572 CrossRef CAS PubMed.
- J. Chhablani, A. Nieto, H. Hou, E. C. Wu, W. R. Freeman, M. J. Sailor and L. Cheng, Invest. Ophthalmol. Visual Sci., 2013, 54, 1268–1279 CAS.
- K. Zhang, S. L. Loong, C. Connor, S. W. Yu, T. SY, R. T. Ng, K. M. Lee, L. T. Canham and P. K. Chow, Clin. Cancer Res., 2005, 11, 7532–7537 CrossRef CAS PubMed.
- J. R. Dorvee, A. M. Derfus, S. N. Bhatia and M. J. Sailor, Nat. Mater., 2004, 3, 896–899 CrossRef CAS PubMed.
- J. Link and M. Sailor, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10607–10610 CrossRef CAS PubMed.
- J. C. Thomas, C. Pacholski and M. J. Sailor, Lab Chip, 2006, 6, 782–787 RSC.
- E. C. Wu, J.-H. Park, J. Park, E. Segal, F. Cunin and M. J. Sailor, ACS Nano, 2008, 2, 2401–2409 CrossRef CAS PubMed.
- M. G. Donato, M. A. Monaca, G. Faggio, L. De Stefano, P. H. Jones, P. G. Gucciardi and O. M. Marago, Nanotechnology, 2011, 22, 505704 CrossRef PubMed.
- K. I. Hartmann, A. Nieto, E. C. Wu, W. R. Freeman, J. S. Kim, J. Chhablani, M. J. Sailor and L. Cheng, J. Ocul. Pharmacol. Ther., 2013, 29, 493–500 CrossRef CAS PubMed.
- H. Hou, A. Nieto, F. Ma, W. R. Freeman, M. J. Sailor and L. Cheng, J. Controlled Release, 2014, 178, 46–54 CrossRef CAS PubMed.
- K. Nan, F. Ma, H. Hou, W. R. Freeman, M. J. Sailor and L. Cheng, Acta Biomater., 2014, 10, 3505–3512 CrossRef CAS PubMed.
- A. Nieto, H. Hou, M. J. Sailor, W. R. Freeman and L. Cheng, Exp. Eye Res., 2013, 116, 161–168 CrossRef CAS PubMed.
- E. Secret, K. Smith, V. Dubljevic, E. Moore, P. Macardle, B. Delalat, M.-L. Rogers, T. G. Johns, J.-O. Durand, F. Cunin and N. H. Voelcker, Adv. Healthcare Mater., 2013, 2, 718–727 CrossRef CAS PubMed.
- B. Guan, A. Magenau, S. Ciampi, K. Gaus, P. J. Reece and J. J. Gooding, Bioconjugate Chem., 2014, 25, 1282–1289 CrossRef CAS PubMed.
- C. Pacholski, M. Sartor, M. J. Sailor, F. Cunin and G. M. Miskelly, J. Am. Chem. Soc., 2005, 127, 11636–11645 CrossRef CAS PubMed.
- N. Naveas, V. T. Costa, D. Gallach, J. Hernandez-Montelongo, R. J. M. Palma, J. P. Garcia-Ruiz and M. Manso-Silván, Sci. Technol. Adv. Mater., 2012, 13, 045009 CrossRef.
- L. R. Clements, P.-Y. Wang, F. Harding, W.-B. Tsai, H. Thissen and N. H. Voelcker, Phys. Status Solidi A, 2010, 208, 1440–1445 CrossRef.
- A. Schlossbauer, D. Schaffert, J. Kecht, E. Wagner and T. Bein, J. Am. Chem. Soc., 2008, 130, 12558–12559 CrossRef CAS PubMed.
- A. Foraker, R. Walcazak, M. Cohen, T. Boiarski, C. Grove and P. Swaan, Pharm. Res., 2003, 20, 110–116 CrossRef CAS.
- J. Wu and M. J. Sailor, Adv. Funct. Mater., 2009, 19, 733–741 CrossRef CAS.
- C. A. Prestidge, T. J. Barnes, A. Mierczynska-Vasilev, W. Skinner, F. Peddie and C. Barnett, Phys. Status Solidi A, 2007, 204, 3361–3366 CrossRef CAS.
- K. L. Jarvis, T. J. Barnes and C. A. Prestidge, Langmuir, 2010, 26, 14316–14322 CrossRef CAS PubMed.
- K. L. Jarvis, T. J. Barnes and C. A. Prestidge, Langmuir, 2008, 24, 14222–14226 CrossRef CAS PubMed.
- F. I. Scott and M. T. Osterman, Clin. Colon Rectal Surg., 2013, 26, 67–74 CrossRef PubMed.
- X. Ma and S. Xu, Biomed. Rep., 2012, 1, 177–184 Search PubMed.
- L. H. Kircik and J. Q. Del Rosso, J. Drugs Dermatol., 2009, 8, 546–559 Search PubMed.
- E. O. Demirsoy, R. Kiran, S. Salman, C. Caglayan, A. S. Akturk, D. Bayramgurler and N. Bilen, J. Drugs Dermatol., 2013, 12, 1039–1043 Search PubMed.
- D. J. Gracie and A. C. Ford, Minerva Gastroenterol. Dietol., 2012, 58, 87–99 CAS.
- S. J. Mehta, A. R. Silver and J. O. Lindsay, Aliment. Pharmacol. Ther., 2013, 38, 77–97 CrossRef CAS PubMed.
- Y. J. Tai and R. Kelly, Australas. J. Dermatol., 2005, 46, 161–164 CrossRef PubMed.
- M. A. Murphy, W. P. Joyce, C. Condron and D. Bouchier-Hayes, Eur. J. Vasc. Endovasc., 2002, 23, 349–352 CrossRef CAS PubMed.
- G. Ferrari, F. Bignami, C. Giacomini, S. Franchini and P. Rama, Invest. Ophthalmol. Visual Sci., 2013, 54, 1680–1688 CAS.
- J. Said, C. C. Dodoo, M. Walker, D. Parsons, P. Stapleton, A. E. Beezer and S. Gaisford, Int. J. Pharm., 2014, 462, 123–128 CrossRef CAS PubMed.
- J. R. Smith, R. D. Smith, G. N. Holland, D. A. Jabs, M. R. Robinson, S. M. Whitcup and J. T. Rosebaum, Arthritis Care Res., 2001, 45, 252–257 CrossRef CAS.
- M. De Bandt, J. Sibilia, X. Le Loët, S. Prouzeau, B. Fautrel, C. Marcelli, E. Boucquillard, J. Siame and X. Mariette, Arthritis Res. Ther., 2005, 7, R545–R551 CAS.
- M. Streit, Z. Beleznay and L. R. Braathen, Int. Wound J., 2006, 3, 171–179 CrossRef PubMed.
- W. Armarego and D. Perrin, Purification of laboratory chemicals, Butterworth-Heinemann, 4th edn, 1996 Search PubMed.
- W. McGuiness, E. Vella and D. Harrison, J. Wound Care, 2004, 13, 383–385 CrossRef CAS PubMed.
- P. Abraham, M. Bourgeau, M. Camo, A. Humeau-Heurtier, S. Durand, P. Rousseau and G. Mahe, Microvasc. Res., 2013, 88, 56–60 CrossRef CAS PubMed.
- B. J. Sugarman, B. B. Aggarwal, P. E. Hass, I. S. Figari, M. A. Palladino Jr and H. M. Shepard, Science, 1985, 230, 943–945 CAS.
- P. Kheddo, M. Tracka, J. Armer, R. J. Dearman, S. Uddin, C. F. van der Walle and A. P. Golovanov, Int. J. Pharm., 2014, 473, 126–133 CrossRef CAS PubMed.
- R. Chaudhuri, Y. Cheng, C. R. Middaugh and D. B. Volkin, AAPS J., 2013, 16, 48–64 CrossRef PubMed.
- B. Sciacca, E. Secret, S. Pace, P. Gonzalez, F. Geobaldo, F. Quignard and F. Cunin, J. Mater. Chem., 2011, 21, 2294–2302 RSC.
- S. A. Mirji, S. B. Halligudi, N. Mathew, V. Ravia, N. E. Jacob and K. R. Patil, Colloids Surf., A, 2006, 287, 51–58 CrossRef CAS.
- O. Bisi, S. Ossicini and L. Pavesi, Surf. Sci. Rep., 2000, 38, 1–126 CrossRef CAS.
- K. L. Pong, S.-C. Chen and K. W. Cheah, Solid State Commun., 1996, 99, 887–890 CrossRef CAS.
- F. X. Qiu, P. P. Li and D. Y. Yang, eXPRESS Polym. Lett., 2007, 1, 150–156 CrossRef CAS.
- E. Kondoha, T. Asano, A. Nakashima and M. Komatu, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.–Process., Meas., Phenom., 2000, 18, 1276–1280 CrossRef.
- M. E. Alf, T. A. Hatton and K. K. Gleason, Thin Solid Films, 2011, 1–3 Search PubMed.
- D. Zhao, G. Liu, D. Song, J.-H. Liu, Y. Zhou, J. Ou and S. Sun, SPIE, ed. G. von Bally and Q. Luo, 2006, vol. 6026, p. 60260H Search PubMed.
- H. E. Canavan, D. J. Graham, X. Cheng, B. D. Ratner and D. G. Castner, Langmuir, 2007, 23, 50–56 CrossRef CAS PubMed.
- S. J. P. McInnes and N. H. Voelcker, Future Med. Chem., 2009, 1, 1051–1074 CrossRef CAS PubMed.
- A. J. Cowin, N. Hatzirodos, J. Rigden, R. Fitridge and D. A. Belford, Wound Repair Regen., 2006, 14, 421–426 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Infliximab loading optimisation results, XPS analysis of Infliximab loaded pSi, L929 bioassay experimental details, ToF-SIMS data, microporous pSi layer characterisation, EOT optimisation for pSi films, zeta potential measurements and UV-Vis loadings at pH 6.5 and 5.5, ToF-SIMS mass spectra, L929 cell assay optimisation and L929 assay results for incubations of Infliximab at different pH and temperature. See DOI: 10.1039/c5tb00397k |
| This journal is © The Royal Society of Chemistry 2015 |