Liposomal delivery of horseradish peroxidase for thermally triggered injectable hyaluronic acid–tyramine hydrogel scaffolds
Abstract
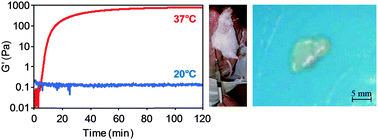
A thermoresponsive injectable hydrogel scaffold for tissue engineering has been developed, whereby the scaffold was injected as a liquid at room temperature, and gelled at the target site in response to the change in body temperature. Our approach involved suspending thermoresponsive liposomes, which encapsulated horseradish peroxidase (HRP), in a hyaluronic acid–tyramine (HA–Tyr) conjugate and hydrogen peroxide (H2O2) solution. At room temperature, HRP was separated from the HA–Tyr conjugate by the lipid membrane, and hence the precursor solution remained as a liquid and was injectable. Upon injection and exposure to body temperature, the lipids experienced a phase transition, which significantly increased the membrane permeability and led to the release of HRP and the oxidative coupling of Tyr moieties with H2O2, forming a crosslinked hydrogel scaffold. It was shown in this study that the precursor solution remained as a liquid on the order of hours at 20 °C, yet gelation could be induced within minutes by heating to 37 °C. Furthermore, it was shown that HRP release could be controlled by various material and processing parameters, and that the gelation rate could be adjusted to meet various clinical needs by adjusting HRP encapsulation, liposome concentration and HA–Tyr concentration.

 Please wait while we load your content...
Please wait while we load your content...