The position and frequency of fluorine atoms changes the electron donor/acceptor properties of fluorophenoxy silicon phthalocyanines within organic photovoltaic devices†
Abstract
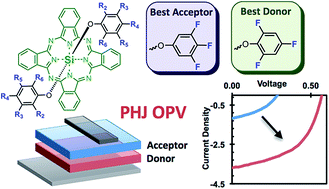
In a previous study we have shown the first example of silicon phthalocyanines (SiPcs) applied within organic photovoltaic (OPV) devices. In that study we showed the electronic performance of a SiPc is significantly increased by replacing the axial chloride groups with pentafluoro phenoxy moieties. It was further demonstrated that bis(pentafluoro phenoxy) SiPc (F10-SiPc) is best applied as an electron accepting material within a fullerene-free planar heterojunction (PHJ) OPV. Within this study we have synthesized a new series of fluorophenoxy silicon phthalocyanines ((XF)2-SiPc) whereby the frequency of the fluorine atoms on the fluorophenoxy groups was systematically reduced from 5. These relatively small changes resulted in small changes in UV-Vis absorption properties, thermal stability, electrochemical behavior, ultraviolet photoelectron spectroscopy characteristics and solid state arrangement in both single crystals and in thin films obtained by thermal evaporation. Single crystal X-ray diffraction determined that all (XF)2-SiPcs have significantly enhanced π–π interactions compared with dichlorosilicon phthalocyanine (Cl2-SiPc) and depending on the position and frequency of the fluorine atoms, the solid state arrangement varied significantly. The complete series of (XF)2-SiPcs were then introduced into PHJ OPVs as both electron accepting and electron donating materials when paired with pentacene, α-sexithiophene or C60. It was found that depending on the structure of the (XF)2-SiPcs the most favourable role for the material would either be as an electron donating or electron accepting material and in most cases the (XF)2-SiPcs outperformed Cl2-SiPc and F10-SiPc. One material, (246F)2-SiPc, did however emerge as one material that has dual functionality. Unoptimized PHJ OPV devices were generally characterized with open circuit voltages (VOC) as high as 0.94 V and power conversion efficiencies >2.0%. As only the second example of SiPcs being integrated into PHJ OPV devices, these results show that versatile phenoxylation chemistry can impact the application and performance and therefore also demonstrates great promise for this class of significantly understudied organic electronic materials. Finally these results give indications of the direction phenoxy-SiPc molecular design should take in order to potentially further improve their overall performance in PHJ OPV devices.

 Please wait while we load your content...
Please wait while we load your content...