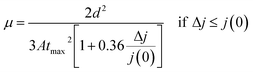Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ultra low band gap α,β-unsubstituted BODIPY-based copolymer synthesized by palladium catalyzed cross-coupling polymerization for near infrared organic photovoltaics†
Benedetta M.
Squeo
a,
Nicola
Gasparini
b,
Tayebeh
Ameri
b,
Alex
Palma-Cando
c,
Sybille
Allard
c,
Vasilis G.
Gregoriou
ad,
Christoph J.
Brabec
be,
Ullrich
Scherf
c and
Christos L.
Chochos
*a
aAdvent Technologies SA, Patras Science Park, Stadiou Street, Platani-Rio, 26504 Patra, Greece. E-mail: cchochos@advent-energy.com
bInstitute of Materials for Electronics and Energy Technology (I-MEET), Friedrich-Alexander-University Erlangen-Nuremberg, Martensstraße 7, 91058 Erlangen, Germany
cMacromolecular Chemistry Group (buwmakro) and Institute for Polymer Technology, Bergische Universität Wuppertal, Gaußstraße 20, D-42119 Wuppertal, Germany
dNational Hellenic Research Foundation (NHRF), 48 Vassileos Constantinou Avenue 11635, Athens, Greece
eBavarian Center for Applied Energy Research (ZAE Bayern), Haberstrasse 2a, 91058 Erlangen, Germany
First published on 13th July 2015
Abstract
A new ultra low band gap (LBG) α,β-unsubstituted BODIPY-based conjugated polymer has been synthesized by conventional cross coupling polymerization techniques (Stille cross coupling) for the first time. The polymer exhibits a panchromatic absorption spectrum ranging from 300 nm to 1100 nm and an optical band gap (Eoptg) of 1.15 eV, suitable for near infrared (NIR) organic photovoltaic applications as electron donor. Preliminary power conversion efficiency (PCE) of 1.1% in polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM) 1
[6,6]-phenyl-C71-butyric acid methyl ester (PC71BM) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 weight ratio bulk heterojunction (BHJ) solar cells has been achieved, demonstrating very interesting and promising photovoltaic characteristics, such as good fill factor (FF) and open circuit voltage (Voc). These results showing that by the proper chemical design, new α,β-unsubstituted BODIPY-based NIR copolymers can be developed in the future with suitable energy levels matching those of PC71BM towards more efficient NIR organic photovoltaics (OPVs).
3 weight ratio bulk heterojunction (BHJ) solar cells has been achieved, demonstrating very interesting and promising photovoltaic characteristics, such as good fill factor (FF) and open circuit voltage (Voc). These results showing that by the proper chemical design, new α,β-unsubstituted BODIPY-based NIR copolymers can be developed in the future with suitable energy levels matching those of PC71BM towards more efficient NIR organic photovoltaics (OPVs).
1. Introduction
Low band gap (LBG) organic materials that absorb into the near-infrared (NIR) are of great interest in the recent years for a number of potential applications.1 For example, the use of NIR-absorbing or NIR photovoltaic organic materials (small molecules or polymers) could extend the material's absorption into the NIR spectral region and even beyond 1000 nm wavelength, which in principle could enhance the current power conversion efficiency (PCE) of organic photovoltaics (OPVs).2Even though semiconducting polymers with ultra LBGs have been synthesized before,3–19 the challenge in designing and synthesizing materials that have a good photoresponse beyond 900 nm and an appreciable PCE in polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fullerene solar cells lies (among others) in the precise energy level control that is required. Two major methodologies are employed to fine tune the band gap and energy level alignment of conjugated polymers. One methodology relies on the donor–acceptor (D–A) approach and the other on the stabilization of the quinoid structure.20 D–A approach is the most common tool to synthesize NIR conjugated polymers due to the plethora of functional electron rich and electron deficient building blocks. In order to develop ultra LBG D–A polymers, usually strong electron rich units and strong electron deficient building blocks are used. Common monomer units function as strong electron donors include pyrrole, thiophene, ethylenedioxythiophene (EDOT), bridged bithiophenes derivatives etc.21 Moreover, the most well-known strong electron deficient units used to construct NIR D–A polymers are diketopyrrolopyrrole dyes,4c,13a,14c benzobisthiadiazole,3a,3g pyrazinoquinoxaline derivatives,9b,10a thiadiazoloquinoxaline derivatives,10a,11b,c annelated benzotriazole,10b thienoisoindigo,4b,13b emreladicene,3f cyclopentadithiophenone,14a tetraazabenzodifluoranthene diimides,8a–cetc.3g,22
fullerene solar cells lies (among others) in the precise energy level control that is required. Two major methodologies are employed to fine tune the band gap and energy level alignment of conjugated polymers. One methodology relies on the donor–acceptor (D–A) approach and the other on the stabilization of the quinoid structure.20 D–A approach is the most common tool to synthesize NIR conjugated polymers due to the plethora of functional electron rich and electron deficient building blocks. In order to develop ultra LBG D–A polymers, usually strong electron rich units and strong electron deficient building blocks are used. Common monomer units function as strong electron donors include pyrrole, thiophene, ethylenedioxythiophene (EDOT), bridged bithiophenes derivatives etc.21 Moreover, the most well-known strong electron deficient units used to construct NIR D–A polymers are diketopyrrolopyrrole dyes,4c,13a,14c benzobisthiadiazole,3a,3g pyrazinoquinoxaline derivatives,9b,10a thiadiazoloquinoxaline derivatives,10a,11b,c annelated benzotriazole,10b thienoisoindigo,4b,13b emreladicene,3f cyclopentadithiophenone,14a tetraazabenzodifluoranthene diimides,8a–cetc.3g,22
One of the less explored electron deficient monomers for the synthesis of NIR conjugated polymers is the so-called, 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene, commonly known as BODIPY (Scheme 1).23 BODIPY dyes were first discovered in 1968 by Treibs and Kreuzer24 and exhibit unique properties such as large absorption coefficients, high fluorescence quantum yields, and remarkable photostability.23 In addition, straightforward chemical synthesis and structural robustness have enabled fine tuning of optical properties of BODIPY dyes via systematic structural variations.23 Owing to large extinction coefficients, intense absorption spectra that extend into the red region of the visible spectrum, and decent hole mobility, small-molecule BODIPY derivatives have been recently employed as p-type or donor materials in conjunction with PCBM in bulk heterojunction (BHJ) solar cells.25
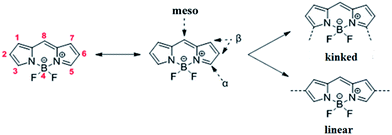
Despite the fact that BODIPY-based conjugated polymers have also been used in different applications,26–35 such as near-IR emitters, nonlinear optics, light harvesting, electrochromics and OPVs, limited examples have been presented up to now with ultra LBG conjugated polymers (Eg < 1.4–1.5 eV) consisting of the BODIPY core. As a matter of fact, Algi and Cihaner32 designed and synthesized a BODIPY-based polymer for electrochromic applications. In their study, a 1,3,5,7-tetramethyl substituted BODIPY derivative was used as an acceptor unit, featuring a non-planar repeating unit and distorted conjugation between EDOT (strong electron rich) and BODIPY (electron deficient) units due to the steric effect of the methyl groups. To avoid the steric effect of the methyl groups, Vobecka et al.33 and Samuel et al.34 have synthesized a BODIPY core with halogens at 3- and 5-positions (α-positions; Scheme 1) and effectively synthesized an EDOT-BODIPY-EDOT conjugated polymer both with electropolymerization and Stille cross coupling polymerization, respectively. However, the resulting copolymers are kinked because the linearity of the polymer was lost (Scheme 1). Last year, Stoddart et al.36 succeeded to synthesize the first ultra LBG DAD type α,β-unsubstituted BODIPY-based copolymer using EDOT as the electron rich (D) unit by electropolymerization which displays electrochromic behavior.
In this work, it is presented for the first time the successful synthesis and optoelectronic characterization of an ultra LBG (Eg = 1.15 eV) linear BODIPY-based copolymer by conventional cross coupling polymerization procedure (Stille cross coupling). The new synthesized NIR BODIPY copolymer exhibits a broad (panchromatic) absorption spectrum ranging from 300 nm to 1100 nm, while initial photovoltaic characterization as electron donor in BHJ OPVs reveals very interesting and promising photovoltaic characteristics such as good fill factor (FF) and open circuit voltage (Voc).
2. Results and discussion
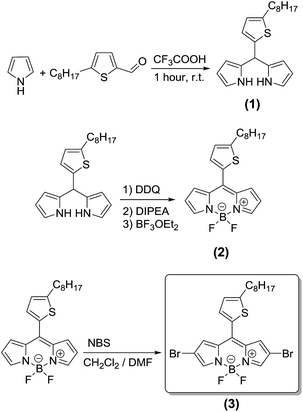
The preferred method of choice for the synthesis of α,β-unsubstituted BODIPY dyes is the combination of aldehydes and pyrrole under neat conditions.23,37 The aldehyde can be dissolved in excess pyrrole at room temperature, and the dipyrromethane intermediate (the reduced form of the dipyrromethene) is formed and isolated. The BODIPY core can then be obtained after oxidation and complexation with boron. In this work, 5-octylthiophene-2-carbaldehyde has been chosen as the aldehyde, where upon condensation with pyrrole, catalyzed by few drops of trifluoroacetic acid, the resulting 2,2′-((5-octylthiophen-2-yl)methylene)bis(1H-pyrrole) (1) is obtained (Scheme 2). The design concept of choosing an alkylthiophene-carbaldehyde is the formation of a functional BODIPY building block (3) with a 2D extension for the development of a series of BODIPY based copolymers soluble in common organic solvents. Treatment of monomer 1 with the strong oxidant 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), then diisopropylethylamine (Hünig's base) and finally with trifluoroborane dietherate [BF3O(Et)2] provided the corresponding borondipyrromethene monomer 2. Recently, new synthetic protocols to introduce bromine atoms at the 2- and 6-positions on the unsubstituted BODIPY core have been presented in the literature.38 In our case, the targeted dibromo borondipyrromethene monomer 3 is synthesized upon bromination with N-bromosuccinimide (NBS) using dimethylformamide (DMF)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methylene chloride (CH2Cl2) 1
methylene chloride (CH2Cl2) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as the solvent mixture similar to the conditions presented elsewhere.36,38c
1 as the solvent mixture similar to the conditions presented elsewhere.36,38c
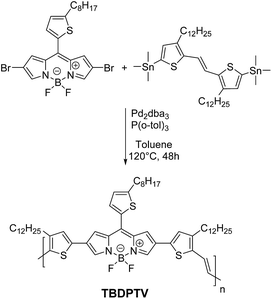
For the synthesis of TBDPTV, Stille cross-coupling polymerization using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 monomer feed ratios was used (Scheme 3). A solution of the commercially available (E)-1,2-bis(3-dodecyl-5-(trimethylstannyl)thiophen-2-yl)ethane and the dibromo borondipyrromethene monomer 3 were combined in dry deoxygenated toluene in the presence of tris(dibenzylideneacetone)dipalladium(0) [Pd2(dba)3] (5 mol%) and tri-o-tolylphosphine [P(o-Tol)3] (40 mol%) and the mixture was heated to ca. 120 °C for 48 h to provide the desired crude polymer. Purification was achieved by Soxhlet extraction with methanol (200 mL, 1 d), hexane (200 mL, 1 d) and chloroform (200 mL, 1 d). The chloroform fraction was then concentrated under reduced pressure, precipitated in methanol, filtered and dried in vacuum. The resulting TBDPTV is readily soluble in chloroform, chlorobenzene and o-dichlorobenzene (o-DCB). The polymer was characterized with 1H NMR spectroscopy and gel permeation chromatography (GPC) [see ESI (Fig. S1–S2†)]. The typical molecular weight characteristics of TBDPTV were estimated by GPC and presented in Table 1.
1 monomer feed ratios was used (Scheme 3). A solution of the commercially available (E)-1,2-bis(3-dodecyl-5-(trimethylstannyl)thiophen-2-yl)ethane and the dibromo borondipyrromethene monomer 3 were combined in dry deoxygenated toluene in the presence of tris(dibenzylideneacetone)dipalladium(0) [Pd2(dba)3] (5 mol%) and tri-o-tolylphosphine [P(o-Tol)3] (40 mol%) and the mixture was heated to ca. 120 °C for 48 h to provide the desired crude polymer. Purification was achieved by Soxhlet extraction with methanol (200 mL, 1 d), hexane (200 mL, 1 d) and chloroform (200 mL, 1 d). The chloroform fraction was then concentrated under reduced pressure, precipitated in methanol, filtered and dried in vacuum. The resulting TBDPTV is readily soluble in chloroform, chlorobenzene and o-dichlorobenzene (o-DCB). The polymer was characterized with 1H NMR spectroscopy and gel permeation chromatography (GPC) [see ESI (Fig. S1–S2†)]. The typical molecular weight characteristics of TBDPTV were estimated by GPC and presented in Table 1.
| Polymer | M n (g mol−1) | M w (g mol−1) | λ solmax (nm) | λ filmmax (nm) | E optg (eV) | E AAPPSHOMO (eV) | E CVHOMO (eV) | E CVLUMO (ev) |
|---|---|---|---|---|---|---|---|---|
| TBDPTV | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 (UV) 700 (UV) |
70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 (UV) 300 (UV) |
444![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 817 817 |
451![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 848 848 |
1.15 | −5.29 | −5.16 | −4.02 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (RI) 000 (RI) |
71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (RI) 100 (RI) |
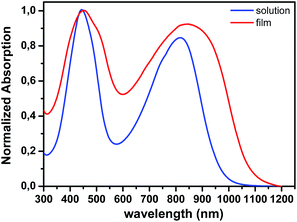
The normalized UV-vis absorption spectra of TBDPTV in chloroform solution and in the solid state are presented in Fig. 1, and the corresponding optical properties are summarized in Table 1.
Two major absorption bands can be observed in solution, a feature, which is commonly observed for alternating D–A copolymers. The low-wavelength peak observed at 444 nm for can be attributed to a π–π* transition, while the high-wavelength transition with maximum absorption at 817 nm is believed to be related to an intramolecular charge transfer (ICT) between the electron donor and the electron deficient of the repeating unit.
Passing from solution to the solid state, the absorption spectrum of TBDPTV becomes broader covering all the range from 300 nm to 1100 nm. All the absorption bands are red-shifted when passing from solution to the solid state, the peak at 444 nm in solution is situated at 451 nm in the solid state whereas the peak at 817 nm in solution has been shifted to 848 nm in the solid state, indicating the presence of strong intermolecular π–π interactions. The optical band gap (Eoptg) deduced from the absorption onset in the solid state is estimated at 1.15 eV.
The energy levels of TBDPTV have been determined utilizing both cyclic voltammetry (CV) and atmospheric pressure photoelectron spectroscopy (AAPPS, AC-2). TBDPTV exhibits reduction and oxidation peak potentials versus saturated calomel electrode (SCE) at −0.68 V and 0.46 V, respectively as obtained by cyclic voltammetry (Fig. 2). The resulting HOMO (EHOMO) and LUMO (ELUMO) energy levels as derived from the equations EHOMO = −(4.7 + Eoxonset) eV and ELUMO = −(4.7 + Eredonset) eV are −5.16 eV and −4.02 eV vs. vacuum, respectively. The electrochemical bandgap (ECVg) of 1.14 eV is in excellent agreement with the Eoptg. The HOMO level as calculated by AAPPS (Fig. S3 in ESI†) is situated at −5.29 eV, slightly different from the one obtained by cyclic voltammetry.
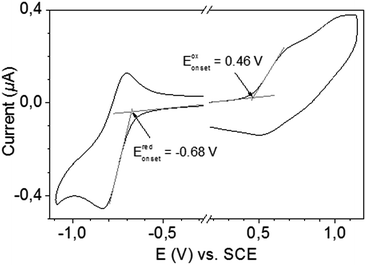 | ||
| Fig. 2 Cyclic voltammogram of TBDPTV in chloroform (containing 0.1 M tetrabutylammonium perchlorate). | ||
An attempt to probe the photovoltaic properties of the synthesized polymer was carried out. Organic BHJ solar cells were fabricated in inverted device structure consisting of ITO/ZnO/TBDPTV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71B/MoOx/Ag, using the three different D
PC71B/MoOx/Ag, using the three different D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A composition ratio (1
A composition ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). The active layer solution was spin coated under inert atmosphere condition using 97 to 3 vol% of chlorobenzene (CB) and 1,8 diiodooctane (DIO). Fig. 3a and b show the J–V curves of the optimized BHJ solar cells under simulated AM 1.5G solar irradiance (100 mW cm−2) and in the dark, respectively. A PCE of 1.1% was obtained for the 1
4). The active layer solution was spin coated under inert atmosphere condition using 97 to 3 vol% of chlorobenzene (CB) and 1,8 diiodooctane (DIO). Fig. 3a and b show the J–V curves of the optimized BHJ solar cells under simulated AM 1.5G solar irradiance (100 mW cm−2) and in the dark, respectively. A PCE of 1.1% was obtained for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio, with a short circuit current density (Jsc) of 3.39 mA cm−2, Voc of 0.59 V and FF of 0.56. Using the same fabrication conditions, solar cells containing 1
3 ratio, with a short circuit current density (Jsc) of 3.39 mA cm−2, Voc of 0.59 V and FF of 0.56. Using the same fabrication conditions, solar cells containing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 D
4 D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratio achieved a maximum efficiency of 1.03 and 1.01, respectively. A strong correlation between the charge carrier mobility and FFs is also obtained for these devices. In fact, as shown in Table 2, solar cells based on 1
A ratio achieved a maximum efficiency of 1.03 and 1.01, respectively. A strong correlation between the charge carrier mobility and FFs is also obtained for these devices. In fact, as shown in Table 2, solar cells based on 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio, display the highest FF of 0.56 and 0.57, respectively. On the other hand, the 1
4 ratio, display the highest FF of 0.56 and 0.57, respectively. On the other hand, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 based devices depicted a lower FF of 0.52, costing with the lower charge carrier mobility obtained. It is worth to mention that for all the devices, Jsc values are similar and this can be possible attributed to the not perfect energy alignment with PC71BM for achieving efficient exciton splitting rather than to morphological issues as can be seen on Fig. 5. Furthermore, external quantum efficiency (EQE) measurements have been carried out (Fig. S4†). An EQE signal around 950–1000 nm, even though very weak, is observed, suggesting an existing path towards charge generation from the polymer. As reported by Janssen et al.,39 a fast recombination path becomes essential when the LUMO of the acceptor become too shallow, leading to low Jsc values. Noticeably, the rectification behavior of the device slightly improved with increasing the D
2 based devices depicted a lower FF of 0.52, costing with the lower charge carrier mobility obtained. It is worth to mention that for all the devices, Jsc values are similar and this can be possible attributed to the not perfect energy alignment with PC71BM for achieving efficient exciton splitting rather than to morphological issues as can be seen on Fig. 5. Furthermore, external quantum efficiency (EQE) measurements have been carried out (Fig. S4†). An EQE signal around 950–1000 nm, even though very weak, is observed, suggesting an existing path towards charge generation from the polymer. As reported by Janssen et al.,39 a fast recombination path becomes essential when the LUMO of the acceptor become too shallow, leading to low Jsc values. Noticeably, the rectification behavior of the device slightly improved with increasing the D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratio (Fig. 3b). The Jsc calculated from the EQE is 3.08 mA cm−3, which is less than 10% smaller compared to the Jsc obtained from the solar simulator.
A ratio (Fig. 3b). The Jsc calculated from the EQE is 3.08 mA cm−3, which is less than 10% smaller compared to the Jsc obtained from the solar simulator.
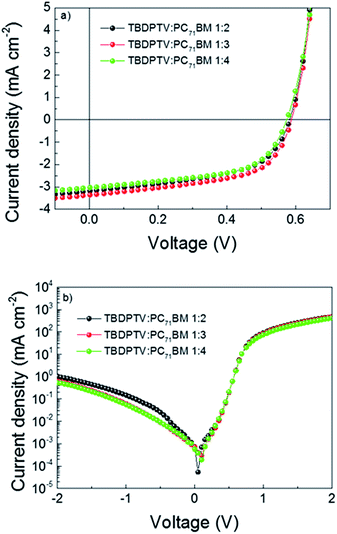 | ||
| Fig. 3 Current density–voltage characteristics under 100 mW cm−2 illumination (AM 1.5G) (a) in linear graph and (b) in semilogarithmic graph. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM system in different composition ratio
PC71BM system in different composition ratio
TBDPTV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM PC71BM |
V oc [V] | J sc [mA cm−2] | FF [%] | η [%] |
|---|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.58 (0.58 ± 0.00) | 3.32 (3.27 ± 0.32) | 53.45 (51.54 ± 1.82) | 1.03 (0.97 ± 0.04) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.59 (0.59 ± 0.00) | 3.39 (3.16 ± 0.17) | 56.18 (55.52 ± 0.58) | 1.10 (1.03 ± 0.06) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
0.59 (0.59 ± 0.00) | 3.12 (2.86 ± 0.24) | 56.51 (56.14 ± 0.37) | 1.01 (0.93 ± 0.07) |
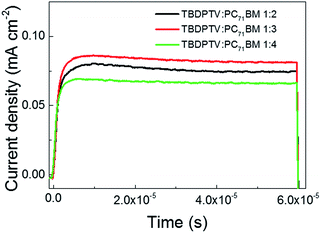
In order to understand the transport properties of TBDPTV-based solar cells, the charge carrier mobility μ of the aforementioned devices were determined by employing the technique of photoinduced charge carrier extraction by linearly increasing voltage (photo-CELIV).40 Charges are photogenerated by a strongly absorbed laser pulse and extracted after an adjustable delay time. Fig. 4 shows the photo-CELIV transients of the TBDPTV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM system in the three different composition ratio (1
PC71BM system in the three different composition ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), which were recorded by applying a 2 V/20 μs linearly increasing reverse bias pulse and a delay time (td) of 1 μs. From the measured photocurrent transients, the charge carrier mobility (μ) is calculated using the following equation:
4), which were recorded by applying a 2 V/20 μs linearly increasing reverse bias pulse and a delay time (td) of 1 μs. From the measured photocurrent transients, the charge carrier mobility (μ) is calculated using the following equation:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 weight ratio. As a result, the calculated charge carrier mobility (μ) is higher for the 1
4 weight ratio. As a result, the calculated charge carrier mobility (μ) is higher for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio (1.05 ± 0.2) × 10−5 cm2 V−1 s−1, then for the 1
4 ratio (1.05 ± 0.2) × 10−5 cm2 V−1 s−1, then for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio (9.18 ± 0.2) × 10−6 cm2 V−1 s−1, and finally for the 1
3 ratio (9.18 ± 0.2) × 10−6 cm2 V−1 s−1, and finally for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio (7.50 ± 0.1) × 10−6 cm2 V−1 s−1, respectively.
2 ratio (7.50 ± 0.1) × 10−6 cm2 V−1 s−1, respectively.
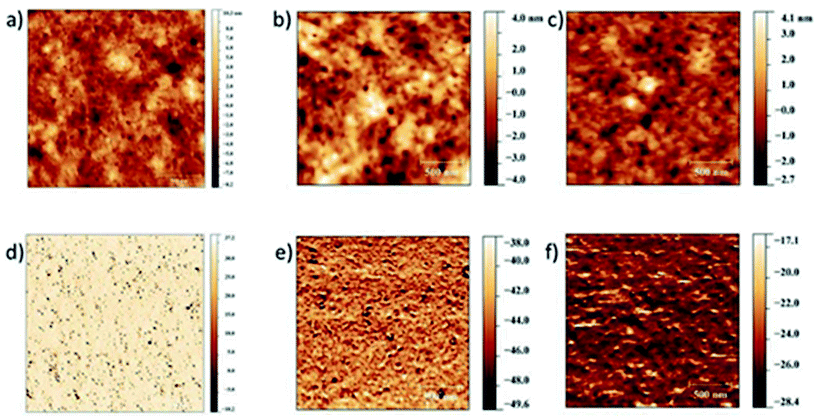
Investigations on the surface microstructure were also carried out through intermitted contact mode atomic force microscopy (AFM, Fig. 5). A good intermixing between the donor and the acceptor materials is obtained, consequentially the topography and the phase images do not reveal obvious differences between the three systems studied, supporting the hypothesis that the main limitations on TBDPTV-based solar cells are attributed to the low LUMO offset rather than to morphological issues.
3. Conclusions
A dibromo α,β-unsubstituted BODIPY building block suitable for conventional catalyzed cross coupling polymerizations has been effectively synthesized. In order to confirm the proof of concept, the dibromo BODIPY monomer 3 has been successfully polymerized through Stille cross coupling with (E)-1,2-bis(3-dodecyl-5-(trimethylstannyl)thiophen-2-yl)ethane as the comonomer providing the new ultra LBG (Eoptg = 1.15 eV) TBDPTV copolymer. To the best of our knowledge TBDPTV is the first α,β-unsubstituted BODIPY-based NIR copolymer synthesized by conventional catalyzed cross coupling polymerization methods. TBDPTV exhibits a panchromatic absorption spectrum ranging from 300 nm to 1100 nm, and a promising PCE of 1.1% in NIR BHJ OPVs using PC71BM as the electron acceptor with very interesting photovoltaic characteristics, such as good fill factor (FF) and open circuit voltage (Voc).4. Experimental
All reactions were treated as air and light sensitive and performed under argon and in the dark. All glassware used were washed using teepol surfactant, rinsing with excess water, acetone and methylene dichloride and dried in an oven at 120 °C. All solvents and reagents were sourced commercially from Aldrich, except (E)-1,2-bis(3-dodecyl-5-(trimethylstannyl)thiophen-2-yl)ethane which was obtained from Solarmer Materials Inc.Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as eluent. The yellow-green oil product obtained with a yield of 71% (2.40 g).
1 as eluent. The yellow-green oil product obtained with a yield of 71% (2.40 g).
1H NMR (CDCl3, 400 MHz): δ 8.0 (s, 2H), 6.70 (dd, 3J = 6.70 Hz, 2H), 6.60 (d, 1H), 6.17 (dd, 3J = 6.17 Hz, 2H), 6.04 (s, 2H), 5.66 (s, 1H), 2.73 (t, 2H), 1.63 (m, 2H), 1.29 (m, 10H), 0.89 (t, 3H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane 2
hexane 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as eluent. The product obtained as a red-orange oil with a yield of 40% (1.43 g).
1 as eluent. The product obtained as a red-orange oil with a yield of 40% (1.43 g).
1H NMR (CDCl3, 400 MHz): δ 7.9 (s, 2H), 7.44 (d, J = 3.6 Hz, 2H), 7.32 (d, J = 3.6 Hz, 2H), 6.97 (d, J = 3.2 Hz, 1H), 6.57 (m, 2H), 2.92 (t, J = 7.6 Hz, 2H), 1.76 (m, 2H), 1.29 (m, 10H), 0.89 (t, J = 6.8 Hz, 3H).
13C NMR (CDCl3, 100 MHz): δ 154.21, 143.11, 133.79, 132.13, 131.30, 125.88, 118.19, 31.83, 31.49, 29.26, 28.18, 22.85, 14.19, 0.99.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 hexane/methylene chloride. The product obtained as a pink solid with yield of 50% (0.120 g).
3 hexane/methylene chloride. The product obtained as a pink solid with yield of 50% (0.120 g).
1H NMR (CDCl3, 400 MHz): δ 7.8 (s, 2H), 7.48 (d, J = 3.7 Hz, 1H), 7.32 (s, 2H), 7.0 (d, J = 3.6 Hz, 1H), 2.93 (t, J = 7.7 Hz, 2H), 1.77 (m, 2H), 1.38 (m, 10H), 0.89 (t, J = 6.4 Hz, 3H).
13C NMR (CDCl3, 100 MHz): δ 156.36, 142.84, 135.02, 131.40, 126.61, 31.83, 31.46, 30.55, 29.24, 29.17, 22.65, 14.11.
MALDI m/z, calcd for C21H23BBr2F2N2S (M+): 543.10; found: 543.14.
Synthesis of TBDPTV
Dibromo-BODIPY monomer 3 (0.2022 mmol, 110 mg) and the distannyl-derivative (0.2022 mmol, 172.8 mg) were dissolved in dry toluene (8.1 mL). Then, Pd2dba3 (0.0101 mmol, 9.3 mg) and P(o-tol)3 (0.0808 mmol, 24.6 mg) were added and the reaction mixture was stirred at 120 °C under argon atmosphere for 48 h. Then, the toluene solution was evaporated, the mixture was solubilized in CHCl3. The polymer was purified by precipitation in methanol, filtered and washed on Soxhlet apparatus with methanol, hexane and chloroform. The chloroform fraction was evaporated under reduced pressure and the polymer was precipitated in acetone, filtered and finally dried under high vacuum, providing a greenish solid (52 mg).Instrumentation and materials characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC70BM (20 g L−1). To complete the fabrication of the devices 10 nm of MoOx and 100 nm of Ag were thermally evaporated through a mask (with a 10.4 mm2 active area opening) under a vacuum of ∼5 × 10−6 mbar.
PC70BM (20 g L−1). To complete the fabrication of the devices 10 nm of MoOx and 100 nm of Ag were thermally evaporated through a mask (with a 10.4 mm2 active area opening) under a vacuum of ∼5 × 10−6 mbar.
Acknowledgements
This project has received funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under the Grant Agreement no. 607585. The authors acknowledges the financial support of a Marie Curie Initial Training Network (FP7-PEOPLE-2013-ITN) project OSNIRO. The authors thank Anke Helfer and Sylwia Adamczyk for performing the GPC- and PESA measurements.Notes and references
- G. Qian and Z. Y. Wang, Chem.–Asian J., 2010, 5, 1006 CrossRef CAS PubMed.
- A. J. Heeger, Adv. Mater., 2014, 26, 10 CrossRef CAS PubMed.
- (a) J. D. Yuen, R. Kumar, D. Zakhidov, J. Seifter, B. Lim, A. J. Heeger and F. Wudl, Adv. Mater., 2011, 23, 3780 CAS; (b) J. Fan, J. D. Yuen, M. Wang, J. Seifter, J.-H. Seo, A. R. Mohebbi, D. Zakhidov, A. Heeger and F. Wudl, Adv. Mater., 2012, 24, 2186 CrossRef CAS PubMed; (c) J. D. Yuen, J. Fan, J. Seifter, B. Lim, R. Hufschmid, A. J. Heeger and F. Wudl, J. Am. Chem. Soc., 2011, 133, 20799 CrossRef CAS PubMed; (d) C. Yang, S. Cho, R. C. Chiechi, W. Walker, N. E. Coates, D. Moses, A. J. Heeger and F. Wudl, J. Am. Chem. Soc., 2008, 130, 16524 CrossRef CAS PubMed; (e) W. Cui and F. Wudl, Macromolecules, 2013, 46, 7232 CrossRef CAS; (f) A. R. Mohebbi, J. Yuen, J. Fan, C. Munoz, M. F. Wang, R. S. Shirazi, J. Seifter and F. Wudl, Adv. Mater., 2011, 23, 4644 CrossRef CAS PubMed; (g) J. D. Yuen and F. Wudl, Energy Environ. Sci., 2013, 6, 392 RSC.
- (a) M. M. Wienk, M. G. R. Turbiez, M. P. Struijk, M. Fonrodona and R. A. J. Janssen, Appl. Phys. Lett., 2006, 88, 153511 CrossRef PubMed; (b) G. W. P. van Pruissen, F. Gholamrezaie, M. M. Wienk and R. A. J. Janssen, J. Mater. Chem., 2012, 22, 20387 RSC; (c) K. H. Hendriks, W. Li, M. M. Wienk and R. A. J. Janssen, J. Am. Chem. Soc., 2014, 136, 12130 CrossRef CAS PubMed; (d) A. P. Zoombelt, M. Fonrodona, M. M. Wienk, A. B. Sieval, J. C. Hummelen and R. A. J. Janssen, Org. Lett., 2009, 11, 903 CrossRef CAS PubMed.
- (a) Y. Yao, Y. Liang, V. Shrotriya, S. Xiao, L. Yu and Y. Yang, Adv. Mater., 2007, 19, 3979 CrossRef CAS PubMed; (b) L. Dou, C.-C. Chen, K. Yoshimura, K. Ohya, W.-H. Chang, J. Gao, Y. Liu, E. Richard and Y. Yang, Macromolecules, 2013, 46, 3384 CrossRef CAS; (c) L. Dou, W.-H. Chang, J. Gao, C.-C. Chen, J. You and Y. Yang, Adv. Mater., 2013, 25, 825 CrossRef CAS PubMed.
- Y. Xia, L. Wang, X. Deng, D. Li, X. Zhu and Y. Cao, Appl. Phys. Lett., 2006, 89, 081106 CrossRef PubMed.
- (a) P. M. Beaujuge, S. Ellinger and J. R. Reynolds, Nat. Mater., 2008, 7, 795 CrossRef CAS PubMed; (b) T. T. Steckler, X. Zhang, J. Hwang, R. Honeyager, S. Ohira, X.-H. Zhang, A. Grant, S. Ellinger, S. A. Odom, D. Sweat, D. B. Tanner, A. G. Rinzler, S. Barlow, J.-L. Brédas, B. Kippelen, S. R. Marder and J. R. Reynolds, J. Am. Chem. Soc., 2009, 131, 2824 CrossRef CAS PubMed.
- (a) P.-T. Wu, F. S. Kim and S. A. Jenekhe, Chem. Mater., 2011, 23, 4618 CrossRef CAS; (b) H. Li, F. S. Kim, G. Ren and S. A. Jenekhe, J. Am. Chem. Soc., 2013, 135, 14920 CrossRef CAS PubMed; (c) H. Li, Y.-J. Hwang, T. Earmme, R. C. Huber, B. A. E. Courtright, C. O'Brien, S. H. Tolbert and S. A. Jenekhe, Macromolecules, 2015, 48, 1759 CrossRef CAS; (d) Y.-J. Hwang, F. S. Kim, H. Xin and S. A. Jenekhe, Macromolecules, 2012, 45, 3732 CrossRef CAS.
- (a) T. T. Steckler, P. Henriksson, S. Mollinger, A. Lundin, A. Salleo and M. R. Andersson, J. Am. Chem. Soc., 2014, 136, 1190 CrossRef CAS PubMed; (b) E. Wang, L. Hou, Z. Wang, S. Hellström, W. Mammo, F. Zhang, O. Inganäs and M. R. Andersson, Org. Lett., 2010, 12, 4470 CrossRef CAS PubMed.
- (a) T. L. D. Tam, T. Salim, H. Li, F. Zhou, S. G. Mhaisalkar, H. Su, Y. M. Lam and A. C. Grimsdale, J. Mater. Chem., 2012, 22, 18528 RSC; (b) T. L. D. Tam, W. Ye, H. H. R. Tan, F. Zhou, H. Su, S. G. Mhaisalkar and A. C. Grimsdale, J. Org. Chem., 2012, 77, 10035 CrossRef CAS PubMed.
- (a) M. Li, C. An, T. Marszalek, X. Guo, Y.-Z. Long, H. Yin, C. Gu, M. Baumgarten, W. Pisula and K. Müllen, Chem. Mater., 2015, 27, 2218 CrossRef CAS; (b) T. Dallos, D. Beckmann, G. Brunklaus and M. Baumgarten, J. Am. Chem. Soc., 2011, 133, 13898 CrossRef CAS PubMed; (c) C. An, S. R. Puniredd, X. Guo, T. Stelzig, Y. Zhao, W. Pisula and M. Baumgarten, Macromolecules, 2014, 47, 979 CrossRef CAS.
- (a) H. Usta, C. Newman, Z. Chen and A. Facchetti, Adv. Mater., 2012, 24, 3678 CrossRef CAS PubMed; (b) H. Huang, Z. Chen, R. P. Ortiz, C. Newman, H. Usta, S. Lou, J. Youn, Y.-Y. Noh, K.-J. Baeg, L. X. Chen, A. Facchetti and T. Marks, J. Am. Chem. Soc., 2012, 134, 10966 CrossRef CAS PubMed.
- (a) R. S. Ashraf, I. Meager, M. Nikolka, M. Kirkus, M. Planells, B. C. Schroeder, S. Holliday, M. Hurhangee, C. B. Nielsen, H. Sirringhaus and I. McCulloch, J. Am. Chem. Soc., 2015, 137, 1314 CrossRef CAS PubMed; (b) R. S. Ashraf, A. J. Kronemeijer, D. I. James, H. Sirringhaus and I. McCulloch, Chem. Commun., 2012, 48, 3939 RSC; (c) H. Bronstein, E. Collado-Fregoso, A. Hadipour, Y. W. Soon, Z. Huang, S. D. Dimitrov, R. S. Ashraf, B. P. Rand, S. E. Watkins, P. S. Tuladhar, I. Meager, J. R. Durrant and I. McCulloch, Adv. Funct. Mater., 2013, 23, 5647 CrossRef CAS PubMed; (d) I. Meager, M. Nikolka, B. C. Schroeder, C. B. Nielsen, M. Planells, H. Bronstein, J. W. Rumer, D. I. James, R. S. Ashraf, A. Sadhanala, P. Hayoz, J.-C. Flores, H. Sirringhaus and I. McCulloch, Adv. Funct. Mater., 2014, 24, 7109 CAS; (e) J. W. Rumer, M. Levick, S.-Y. Dai, S. Rossbauer, Z. Huang, L. Biniek, T. D. Anthopoulos, J. R. Durrant, D. J. Procter and I. McCulloch, Chem. Commun., 2013, 49, 4465 RSC; (f) H. Bronstein, Z. Chen, R. S. Ashraf, W. Zhang, J. Du, J. R. Durrant, P. S. Tuladhar, K. Song, S. E. Watkins, Y. Geerts, M. M. Wienk, R. A. J. Janssen, T. Anthopoulos, H. Sirringhaus, M. Heeney and I. McCulloch, J. Am. Chem. Soc., 2011, 133, 3272 CrossRef CAS PubMed.
- (a) Z. Fei, X. Gao, J. Smith, P. Pattanasattayavong, E. B. Domingo, N. Stingelin, S. E Watkins, T. D. Anthopoulos, R. J. Kline and M. Heeney, Chem. Mater., 2013, 25, 59 CrossRef CAS; (b) M. Shahid, T. McCarthy-Ward, J. Labram, S. Rossbauer, E. B. Domingo, S. E. Watkins, N. Stingelin, T. D. Anthopoulos and M. Heeney, Chem. Sci., 2012, 3, 181 RSC; (c) A. J. Kronemeijer, E. Gili, M. Shahid, J. Rivnay, A. Salleo, M. Heeney and H. Sirringhaus, Adv. Mater., 2012, 24, 1558 CrossRef CAS PubMed.
- (a) C. P. Yau, Z. Fei, R. S. Ashraf, M. Shahid, S. E. Watkins, P. Pattanasattayavong, T. D. Anthopoulos, V. G. Gregoriou, C. L. Chochos and M. Heeney, Adv. Funct. Mater., 2014, 24, 678 CrossRef CAS PubMed; (b) C. L. Chochos, J. K. Kallitsis, P. E. Keivanidis, S. Baluschev and V. G. Gregoriou, J. Phys. Chem. B, 2006, 110, 4657 CrossRef CAS PubMed.
- (a) S. Cho, J. Lee, M. Tong, J. H. Seo and C. Yang, Adv. Funct. Mater., 2011, 21, 1910 CrossRef CAS PubMed; (b) P. Sonar, S. P. Singh, Y. Li, M. S. Soh and A. Dodabalapur, Adv. Mater., 2010, 22, 5409 CrossRef CAS PubMed.
- (a) C. V. Hoven, X.-D. Dang, R. C. Coffin, J. Peet, T.-Q. Nguyen and G. C. Bazan, Adv. Mater., 2010, 22, E63 CrossRef CAS PubMed; (b) G. C. Welch and G. C. Bazan, J. Am. Chem. Soc., 2011, 133, 4632 CrossRef CAS PubMed; (c) L. Ying, B. B. Y. Hsu, H. Zhan, G. C. Welch, P. Zalar, L. A. Perez, E. J. Kramer, T.-Q. Nguyen, A. J. Heeger, W.-Y. Wong and G. C. Bazan, J. Am. Chem. Soc., 2011, 133, 18538 CrossRef CAS PubMed.
- (a) Z. Cai, H. Luo, P. Qi, J. Wang, G. Zhang, Z. Liu and D. Zhang, Macromolecules, 2014, 47, 2899 CrossRef CAS; (b) M. Karakawa and Y. Aso, Macromol. Chem. Phys., 2013, 214, 2388 CrossRef CAS PubMed.
- (a) R. Tautz, E. Da Como, T. Limmer, J. Feldmann, H.-J. Egelhaaf, E. von Hauff, V. Lemaur, D. Beljonne, S. Yilmaz, I. Dumsch, S. Allard and U. Scherf, Nat. Commun., 2012, 3, 970 CrossRef PubMed; (b) H. Reisch, U. Wiesler, U. Scherf and N. Tuytuylkov, Macromolecules, 1996, 29, 8204 CrossRef CAS; (c) C. J. Kudla, D. Dolfen, K. J. Schottler, J.-M. Koenen, D. Breusov, S. Allard and U. Scherf, Macromolecules, 2010, 43, 7864 CrossRef CAS.
- (a) C. L. Chochos and S. A. Choulis, Prog. Polym. Sci., 2011, 36, 1326 CrossRef CAS PubMed; (b) J. Roncali, Macromol. Rapid Commun., 2007, 28, 1761 CrossRef CAS PubMed.
- (a) P.-L. T. Boudreault, A. Najari and M. Leclerc, Chem. Mater., 2011, 23, 456 CrossRef CAS; (b) X. Guo, M. Baumgarten and K. Müllen, Prog. Polym. Sci., 2013, 38, 1832 CrossRef CAS PubMed; (c) A. Facchetti, Chem. Mater., 2011, 23, 733 CrossRef CAS.
- C. L. Chochos, N. Tagmatarchis and V. G. Gregoriou, RSC Adv., 2013, 3, 7160 RSC.
- (a) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184 CrossRef CAS PubMed; (b) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS PubMed; (c) M. Benstead, G. H. Mehl and R. W. Boyle, Tetrahedron, 2011, 67, 3573 CrossRef CAS PubMed.
- A. Treibs and F. H. Kreuzer, Justus Liebigs Ann. Chem., 1968, 718, 208 CrossRef CAS PubMed.
- (a) C. Qin, A. Mirloup, N. Leclerc, A. Islam, A. El-Shafei, L. Han and R. Ziessel, Adv. Energy Mater., 2014, 4, 1400085 Search PubMed; (b) T. Rousseau, A. Cravino, T. Bura, G. Ulrich, R. Ziessel and J. Roncali, Chem. Commun., 2009, 1673 RSC.
- S. P. Economopoulos, C. L. Chochos, H. A. Ioannidou, M. Neophytou, C. Charilaou, G. A. Zissimou, J. M. Frost, T. Sachetan, M. Shahid, J. Nelson, M. Heeney, D. D. C. Bradley, G. Itskos, P. A. Koutentis and S. A. Choulis, RSC Adv., 2013, 3, 10221 RSC.
- (a) B. Kim, B. W. Ma, V. R. Donuru, H. Y. Liu and J. M. J. Fréchet, Chem. Commun., 2010, 46, 4148 RSC; (b) R. Yoshii, H. Yamane, K. Tanaka and Y. Chujo, Macromolecules, 2014, 47, 3755 CrossRef CAS.
- B. C. Popere, A. M. Della Pelle and S. Thayumanavan, Macromolecules, 2011, 44, 4767 CrossRef CAS.
- (a) C. Thivierge, A. Loudet and K. Burgess, Macromolecules, 2011, 44, 4012 CrossRef CAS PubMed; (b) A. B. Nepomnyashchii, M. Bröring, J. Ahrens and A. J. Bard, J. Am. Chem. Soc., 2011, 133, 8633 CrossRef CAS PubMed.
- M. Zhu, L. Jiang, M. J. Yuan, X. F. Liu, C. B. Ouyang, H. Y. Zheng, X. D. Yin, Z. C. Zuo, H. B. Liu and Y. L. Li, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7401 CrossRef CAS PubMed.
- S. L. Zhu, N. Dorh, J. T. Zhang, G. Vegesna, H. H. Li, F. T. Luo, A. Tiwari and H. Y. Liu, J. Mater. Chem., 2012, 22, 2781 RSC.
- F. Algi and A. Cihaner, Org. Electron., 2009, 10, 453 CrossRef CAS PubMed.
- J. C. Forgie, P. J. Skabara, I. Stibor, F. Vilela and Z. Vobecka, Chem. Mater., 2009, 21, 1784 CrossRef CAS.
- D. Cortizo-Lacalle, C. T. Howells, S. Gambino, F. Vilela, Z. Vobecka, N. J. Findlay, A. R. Inigo, S. A. J. Thomson, P. J. Skabara and I. D. W. Samuel, J. Mater. Chem., 2012, 22, 14119 RSC.
- H. Usta, M. D. Yilmaz, A.-J. Avestro, D. Boudinet, M. Denti, W. Zhao, J. F. Stoddart and A. Facchetti, Adv. Mater., 2013, 25, 4327 CrossRef CAS PubMed.
- M. D. Yilmaz, T. Aytun, M. Frasconi, S. I. Stupp and F. Stoddart, Synth. Met., 2014, 197, 52 CrossRef CAS PubMed.
- R. W. Wagner and J. S. Lindsey, Pure Appl. Chem., 1996, 68, 1373 CrossRef CAS.
- (a) L. Wang, J.-W. Wang, A.-J. Cui, X.-X. Cai, Y. Wan, Q. Chen, M.-Y. He and W. Zhang, RSC Adv., 2013, 3, 9219 RSC; (b) L. Jiao, W. Pang, J. Zhou, Y. Wei, X. Mu, G. Bai and E. Hao, J. Org. Chem., 2011, 76, 9988 CrossRef CAS PubMed; (c) Y. Hayashi, S. Yamaguchi, W. Y. Cha, D. Kim and H. Shinokubo, Org. Lett., 2011, 13, 2992 CrossRef CAS PubMed.
- D. D. Nuzzo, G.-J. A. H. Wetzelaer, R. K. M. Bouwer, V. S. Gevaerts, S. C. J. Meskers, J. C. Hummelen, P. W. M. Blom and R. A. J. Janssen, Adv. Energy Mater., 2013, 3, 85 CrossRef PubMed.
- G. J. R. O. A. Pivrikas and N. S. Sariciftci, Progress in Photovoltaics: Research and Applications, 2007, 15, 677 CrossRef PubMed.
- A. Mozer, G. Dennler, N. Sariciftci, M. Westerling, A. Pivrikas, R. Österbacka and G. Juška, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 035217 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Gel Permeation Chromatography (GPC), 1H-NMR spectra, atmospheric pressure photoelectron spectroscopy graph of the studied material and external quantum efficiency graph. See DOI: 10.1039/c5ta04229a |
| This journal is © The Royal Society of Chemistry 2015 |