Open Access Article
Open Access ArticleA global view of the phase transitions of SnO2 in rechargeable batteries based on results of high throughput calculations
Yingchun
Cheng
a,
Anmin
Nie
b,
Li-Yong
Gan
a,
Qingyun
Zhang
a and
Udo
Schwingenschlögl
*a
aDepartment of Materials Science and Engineering, King Abdullah University of Science & Technology, Thuwal, 23955-6900, Kingdom of Saudi Arabia. E-mail: udo.schwingenschlogl@kaust.edu.sa
bDepartment of Mechanical Engineering-Engineering Mechanics, Michigan Technological University, 1400 Townsend Dive, Houghton, Michigan 49931, USA
First published on 28th August 2015
Abstract
Lithium, sodium and magnesium have attracted wide attention as potential ions for rechargeable batteries. The Materials Project database of high throughput first principles calculations is used to investigate the phase transitions of SnO2 during ion intercalation and extraction. Various intermediate phases are predicted to be formed during the first intercalation, whereas in later cycles other intermediate phases are encountered. The volume expansions after intercalation and extraction are analyzed. We show that different lithium and sodium oxide products found in recent experiments are due to different oxygen chemical potentials.
Introduction
Lithium ion batteries are key components in current energy conversion and storage technologies because of their high energy density and long cycle life. However, the ever-growing need for high-capacity storage in applications ranging from portable electronic devices and electric vehicles to green energy requires new battery concepts. Recently, sodium1–3 and magnesium4 ion batteries have attracted a lot of interest because of limited lithium reserves and the associated high costs. Alternative active anodes, such as SnO2, are extensively investigated both experimentally and theoretically with respect to the intercalation, conversion, and alloying mechanisms.5–13 In this context, SnO2 possesses a low reaction potential and high theoretical capacity, being cheap and environmentally friendly.11–16 Due to the relatively short history of SnO2 based batteries, however, fundamental understanding of the reaction mechanism is missing. Experimentally, the reaction products can be investigated with respect to their short- and long-range atomic structures by X-ray, neutron, and electron diffraction, as well as by nuclear magnetic resonance and other methods.9–13In situ observation of ion intercalation and extraction by transmission electron microscopy (TEM) has provided atomic scale insight into the reaction dynamics,9,15–25 whereas theoretical transition barriers and the mechanical failure mechanism have been investigated by first principles calculations.15–18 Yet, the understanding of the phase transitions in batteries based on SnO2 is far from complete.Using the Inorganic Crystal Structure Database,26 the total energies and electronic structures of a large number of compounds have been determined by the Materials Project27 and AFLOWLIB consortium28 by high throughput schemes, making it possible to screen for specific properties29 and new materials.30,31 In this work, we demonstrate that the results of high throughput calculations can be used to construct phase diagrams by analyzing the relative thermodynamic stability of different phases using a free energy model.27,32,33 This allows us to clarify the phase transitions of SnO2 in Li/Na/Mg ion batteries and particularly to identify intermediate phases, which is key for understanding the battery processes.
Results and discussion
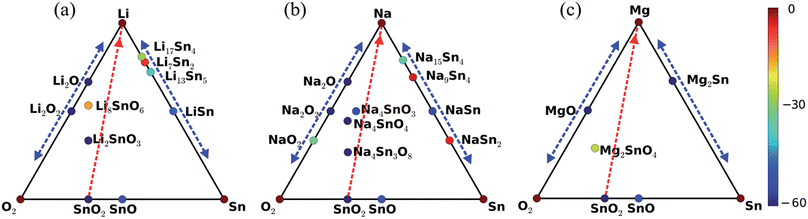
The high throughput data forming the basis of our investigation are taken from the Materials Project database and were obtained by density functional theory as implemented in the Vienna Ab initio Simulation Package,34 using the generalized gradient approximation without and with the onsite Coulomb interaction.35 The calculations took into account spin-polarization and employed an energy cutoff of 520 eV. The number of points in the k-mesh was set to 1000 divided by the number of atoms in the unit cell. The Gibbs free energy is defined as G(P, T) = U + PV − TS, where U, P, V, T, and S are the internal energy, pressure, volume, temperature, and entropy, respectively. In the alkali battery reactions, no gas phase reactants or products are involved and surfaces effects of the electrodes are not considered, so that P = 0 GPa and T = 0 K are valid approximations.32,36–42 Therefore, G is given by the internal energy, which corresponds to the total energy of density functional theory.We used the total energies Ei (per atom) of the involved elements as the reference to define the formation energy E = (Etot − ∑NiEi)/∑Ni of a compound, where Etot is the total energy of the compound and Ni is the amount of atoms in the compound. The structures of bulk lithium, sodium, and magnesium are shown in Fig. 1(a)–(c), respectively. It has been recently found that the reaction product after several cycles of lithiation and delithiation of β-Sn, SnS, and SnO2 contains a mixture of β-Sn and α-Sn,34 which are the two Sn polymorphs at ambient pressure. α-Sn (Fig. 1(d)) is stable at temperatures below 13.2 °C and β-Sn (Fig. 1(e)) at temperatures up to 232 °C. We note that cubic α-Sn is favored at small sizes.9 Sn nanostructures as a function of temperature and size have been investigated recently in ref. 38. The phase diagram of α-Sn, O, and Li/Na/Mg is shown in Fig. 2 including all stable compounds found in high throughput calculations.27,28 The color indicates the formation energy per atom (E).
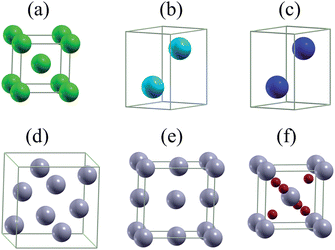 | ||
| Fig. 1 Unit cells of (a) Li, (b) Na, (c) Mg, (d) α-Sn, (e) β-Sn, and (f) SnO2. Green, cyan, blue, gray, and red balls represent Li, Na, Mg, Sn, and O atoms. | ||
It has been conjectured15 that the first step of the lithiation process is the insertion of Li+ into the SnO2 lattice: SnO2 + xLi+ + xe− ↔ LixSnO2. However, what is the detailed phase evolution? The first lithiation line in Fig. 2(a) shows that there exist two lithium tin oxides, Li2SnO3 and Li8SnO6, see Fig. 3(c) and (d), which have not been characterized experimentally so far. Li2SnO3 is obtained before Li8SnO6. Meanwhile, Sn and LixSn are formed by means of irreversible conversion of SnO2 into Sn and lithium oxide: SnO2 + 4Li+ + 4e− → 2Li2O + Sn. Finally, there is a reversible alloying reaction: Sn + xLi+ + xe− ↔ LixSn. All encountered structures are shown in Fig. 3. For sodiation there exist three intermediate phases (Na4Sn3O8, Na4SnO4, and Na4SnO3), see Fig. 4. Theoretically, all mentioned phases can form in Li/Na ion batteries, whereas experimentally it is challenging to identify them by conventional techniques, such as Raman spectroscopy and X-ray diffraction. However, identification can be achieved by in situ TEM, though the lithiation/sodiation front is tiny and the process is fast.15–18
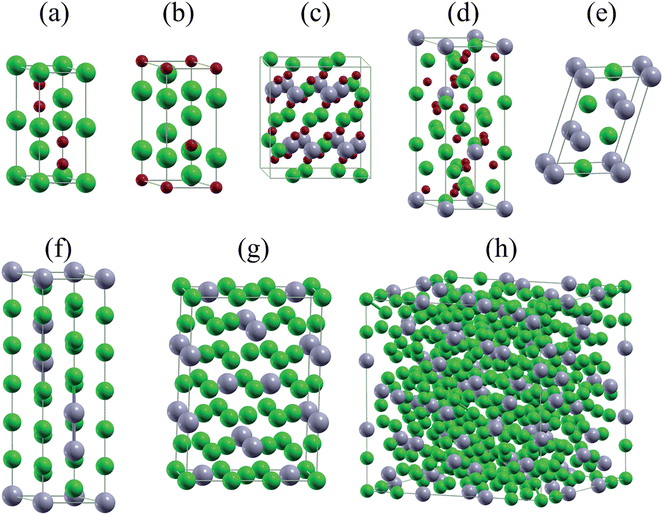 | ||
| Fig. 3 Unit cells of (a) Li2O2, (b) LiO2, (c) Li2SnO3, (d) Li8SnO6, (e) LiSn, (f) Li13Sn5, (g) Li7Sn2, and (h) Li17Sn4. Green, gray, and red balls represent Li, Sn, and O atoms. | ||
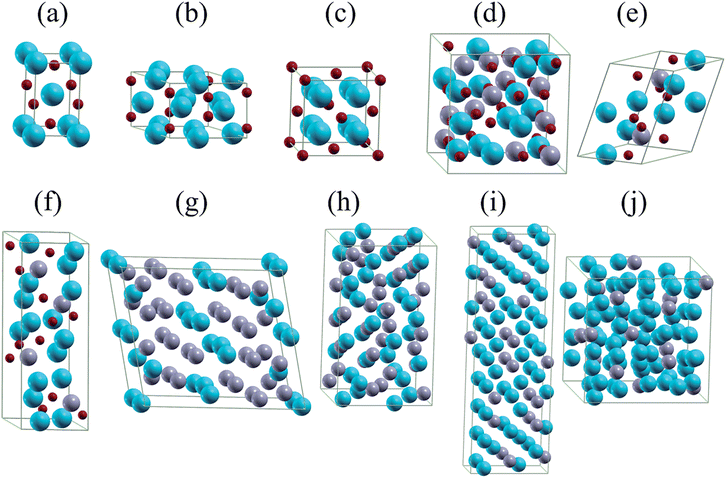 | ||
| Fig. 4 Unit cells of (a) NaO2, (b) Na2O2, (c) Na2O, (d) Na4Sn3O8, (e) Na4SnO4, (f) Na4SnO3, (g) NaSn2, (h) NaSn, (i) Na9Sn4, and (j) Na15Sn4. Cyan, gray, and red balls represent Na, Sn, and O atoms. | ||
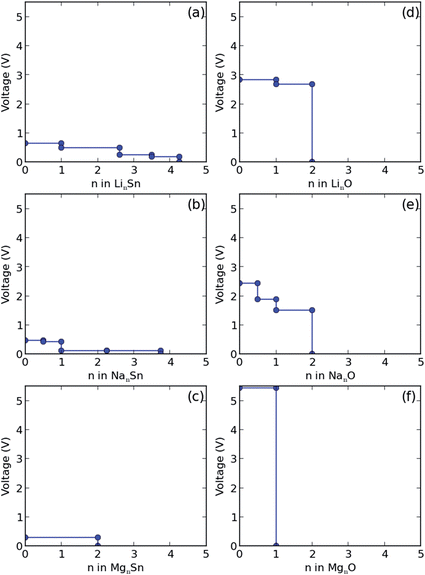
According to the phase diagram in Fig. 2(a), the final lithiation products are Li2O and Li17Sn4. The voltage required for the initial lithiation from SnO2 to Li2SnO3 ranges from 1.93 to 1.16 eV for different byproducts, see Table 1. The values are obtained as negative ratios of the change in G and the number of alkali atoms transferred.43 It is known that Sn nanoparticles form during the first lithiation,15 while LixSn forms without Sn nanoparticles using LiCoO2 as the cathode and a liquid-based electrolyte.18 The different lithiation products can be attributed to the different experimental setups. The voltage for the second lithiation from Li2SnO3 to Li8SnO6 is lower as compared to that for the initial lithiation. For lithiation to Li2O, the voltage ranges from 0.82 to 1.12 eV. We found that the final product is Li17Sn4. During delithiation, Li2O is assumed to be transformed into Li2O2 and Li17Sn4 into Sn. While a transformation from Li17Sn4 to Sn has already been reported,15 transformation from Li2O to Li2O2 has not been observed yet. The absence of Li2O2 is explained by the fact that it will oxidize LixSn and Sn to transfer to Li2O. Therefore, Li2O is always present. The intermediate phases in later cycles (Li7Sn2, Li13Sn5, and LiSn) are different from those during the first lithiation. The voltage profile of LinSn shown in Fig. 5(a) is fully consistent with a previous report.43 Moreover, the reversibility of the Li–Sn alloying process agrees with the cycling behavior observed in ref. 6 for SnO2 anodes and the formation of Li2O explains that experimentally the capacity of the battery is reduced after the first cycle.6,8
| Reaction | Voltage (eV) |
|---|---|
| 4Li + 3SnO2 → 2Li2SnO3 + Sn | 1.93 |
| 3Li + 3SnO2 → 2Li2SnO3 + LiSn | 1.68 |
| 33Li + 15SnO2 → 10Li2SnO3 + Li13Sn5 | 1.39 |
| 11Li + 6SnO2 → 4Li2SnO3 + Li7Sn2 | 1.26 |
| 35Li + 12SnO2 → 8Li2SnO3 + Li17Sn4 | 1.16 |
| 4Li + 2Li2SnO3 → Li8SnO6 + Sn | 1.36 |
| 3Li + 2Li2SnO3 → Li8SnO6 + LiSn | 1.22 |
| 33Li + 10Li2SnO3 → 5Li8SnO6 + Li13Sn5 | 1.05 |
| 15Li + 4Li2SnO3 → 2Li8SnO6 + Li7Sn2 | 0.95 |
| 33Li + 8Li2SnO3 → 4Li8SnO6 + Li17Sn4 | 0.88 |
| 3Li + 5Li8SnO6 → 15Li2O2 + Li13Sn5 | −16.06 |
| 3Li + 2Li8SnO6 → 6Li2O2 + Li7Sn2 | −6.27 |
| 9Li + 4Li8SnO6 → 12Li2O2 + Li17Sn4 | −4.12 |
| 5Li + Li8SnO6 → 6Li2O + LiSn | 1.12 |
| 33Li + 5Li8SnO6 → 30Li2O + Li13Sn5 | 0.97 |
| 5Li + 2Li8SnO6 → 12Li2O + Li7Sn2 | 0.89 |
| 33Li + 4Li8SnO6 → 24Li2O + Li17Sn4 | 0.82 |
For the first sodiation we found the products Na2O and Na15Sn4, as identified earlier.17 According to Fig. 2(b), there are three intermediate phases (Na4Sn3O8, Na4SnO4, and Na4SnO3), which have not been observed so far, see the structures in Fig. 4. The voltage required for the first sodiation turns out to be lower than that for the first lithiation (Tables 1 and 2). The lower voltages indicate that sodiation is slower than lithiation, as found earlier for SnO2,16–18 which is supported by the higher transition barrier of Na as compared to that of Li. Fig. 2(b) shows that the desodiation products are Na2O and Sn, as previously identified by TEM.17 There are Na2O2 as well as Na9Sn4, NaSn, and NaSn2 intermediate phases and the voltage profile of NanSn in Fig. 5(b) is consistent with that reported in the literature.1
| Reaction | Voltage (eV) |
|---|---|
| 4Na + 4SnO2 → Na4Sn3O8 + Sn | 1.45 |
| 9Na + 8SnO2 → 2Na4Sn3O8 + NaSn2 | 1.34 |
| 5Na + 4SnO2 → Na4Sn3O8 + NaSn | 1.25 |
| 25Na + 16SnO2 → 4Na4Sn3O8 + Na9Sn4 | 1.02 |
| 31Na + 16SnO2 → 4Na4Sn3O8 + Na15Sn4 | 0.85 |
| 4Na + Na4Sn3O8 → 2Na4SnO4 + Sn | 0.65 |
| 9Na + 2Na4Sn3O8 → 4Na4SnO4 + NaSn2 | 0.63 |
| 5Na + Na4Sn3O8 → 2Na4SnO4 + NaSn | 0.61 |
| 25Na + 4Na4Sn3O8 → 8Na4SnO4 + Na9Sn4 | 0.52 |
| 31Na + 4Na4Sn3O8 → 8Na4SnO4 + Na15Sn4 | 0.44 |
| 23Na + 4Na4SnO3 → 12Na2O + Na15Sn4 | 0.08 |
| 11Na + 4Na4SnO3 → 6Na2O2 + Na15Sn4 | −1.47 |
| 5Na + 4Na4SnO3 → 6NaO2 + Na15Sn4 | −5.50 |
| 17Na + 4Na4SnO3 → 12Na2O + Na9Sn4 | 0.08 |
| 5Na + 4Na4SnO3 → 6Na2O2 + Na9Sn4 | −3.36 |
| 3Na + Na4SnO3 → 3Na2O + NaSn | 0.05 |
| 5Na + 2Na4SnO3 → 6Na2O + NaSn2 | −0.03 |
| 2Na + Na4SnO3 → 3Na2O + Sn | −0.15 |
Instead of Li2O, Li–O2 cell experiments found Li2O2 as the product by Fourier transform infrared spectroscopy and surface enhanced Raman spectroscopy.44–46 Though there are reports about the formation of lithium superoxide (LiO2) in the cell, LiO2 is unstable and will disproportionate into Li2O2 and O2.46 Therefore, only Li2O2 and Li2O appear in Fig. 3(a). According to the phase diagram in Fig. 2(b), there are three sodium oxide polymorphs (NaO2, NaO, and Na2O), for which the structures are displayed in Fig. 4(a)–(c). In situ TEM of sodiation of SnO2 nanowires found Na2O as the product,17 whereas crystalline sodium superoxide (NaO2) was reported recently for a Na–O2 cell.47 The different alkali oxide products after intercalation can be attributed to the oxygen poor environment in the TEM experiments (high vacuum) and the oxygen rich environment in the Li/Na–O2 cell. Voltage profiles of the Li–O2 and Na–O2 cells are shown in Fig. 5(d) and (e), respectively. The voltage for the formation of Li2O2 (2.8 eV) is consistent with the experimental value of 2.6 eV,46,47 whereas that for the formation of NaO2 (2.4 eV) exceeds the experimental value of 1.8 eV substantially.47
Being an additional parameter of the ion battery performance, a large volume expansion indicates the high capacity of the anode but at the same time induces much damage. Since by ion intercalation into SnO2 the volume will change, we studied the ratio Vproducts/VSnO2, where Vproducts is the total volume of the products and VSnO2 is that of SnO2. We obtained values of 3.8/6.1 for Li/Na, which suggests a higher capacity for Na than for Li. For the extraction process, the result is 1.9/2.0 for Li/Na and thus more than 1, which indicates irreversibility for both Na and Li. The volume expansion after the first sodiation of a SnO2 nanowire is 4.7,19 which is less than the theoretical value, and 2.6 after the first desodiation, which exceeds the theoretical value of 2.0. This discrepancy points to incomplete sodiation and desodiation in the experiment. Moreover, the volume expansion decreases for further sodiation and increases for further desodiation, which suggests the formation of intermediate structures that hinder the cycling and eventually result in failure of batteries. The formation of such structures also could be the origin of observed cracking.19 During a full cycle, the volume changes by a factor of 2.0 for Li and a larger factor of 3.0 for Na.
According to Fig. 2(c), there is only one intermediate phase for magnesiation (Mg2SnO4), see the structure in Fig. 6, and the products are MgO and Mg2Sn. Because there are no other magnesium oxide phases, it is difficult to transform MgO, see the MgnO voltage profile in Fig. 5(f). By comparing Table 3 with Tables 1 and 2 we find that the voltage required for the first magnesiation is much higher than in the cases of lithiation and sodiation. The value of 5.5 eV is much higher than that found for lithiation and sodiation, which implies that SnO2 is not a suitable anode for magnesium ion batteries. Only one magnesium tin alloy (Mg2Sn) appears in Fig. 2(c). However, the MgnSn voltage is 0.3 eV and thus much lower than the values found for LinSn and NanSn. Therefore, it is expected that the products of demagnesiation are Sn and MgO. We obtained theoretical volume expansions of 3.1 and 2.0 for magnesiation and demagnesiation, respectively.
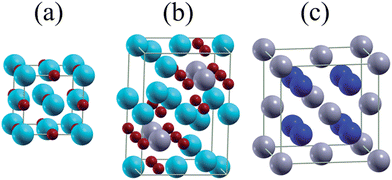 | ||
| Fig. 6 Unit cells of (a) MgO, (b) Mg2SnO4, and (c) Mg2Sn. Blue, gray, and red balls represent Mg, Sn, and O atoms. | ||
| Reaction | Voltage (eV) |
|---|---|
| 2Mg + 2SnO2 → Mg2SnO4 + Sn | 3.04 |
| 4Mg + 2SnO2 → Mg2SnO4 + Mg2Sn | 1.66 |
| 2Mg + Mg2SnO4 → 4MgO + Sn | 2.87 |
| 4Mg + Mg2SnO4 → 4MgO + Mg2Sn | 1.58 |
While our present study focuses on the phase transitions in the SnO2 electrode during alkali ion intercalation, typically also the interface between the electrolyte and the electrodes (both cathode and anode) plays an important role in the performance of batteries. For example, it has been demonstrated theoretically that the voltage depends on the surface charge density,48,49 which can explain differences between experimental values and the voltages calculated for bulk electrodes. Important challenges for the first principles modeling in this area have been reviewed very recently in ref. 50.
Conclusions
Based on the results taken from the Materials Project database of high throughput first principles calculations, we have established the Sn, O, and Li/Na/Mg phase diagram for batteries based on SnO2. For the first intercalation, we predict intermediate phases (Li2SnO3 and Li8SnO6 for lithiation, Na4Sn3O8, Na4SnO4, and Na4SnO3 for sodiation, and Mg2SnO4 for magnesiation) which so far have not been verified experimentally. After full intercalation SnO2 is transformed into tin alloys (Li17Sn4, Na15Sn4, and Mg2Sn) and oxides (Li2O, Na2O, and MgO) and after full extraction the products are Sn and oxides (Li2O2, NaO2, and MgO). The volume expansion after Li/Na/Mg intercalation is predicted as 3.8/6.1/3.1 and that after extraction as 1.9/2.1/2.0. Different oxide products found in recent experiments on Li/Na air batteries and in in situ TEM on nanowires are attributed to different oxygen chemical potentials in the two experiments. Comparison of different reaction paths allows us to predict that an applied voltage experimentally will have a crucial effect on the final products, which is an important insight because voltage dependent investigations are still strongly limited today. We have demonstrated that the results of high throughput calculations can be used for predicting the phase transitions in batteries and thus pave the way to provide instructions for the development of novel battery concepts.Acknowledgements
Research reported in this publication was supported by the King Abdullah University of Science and Technology (KAUST).References
- V. L. Chevrier and G. Ceder, Challenges for Na-Ion Negative Electrodes, J. Electrochem. Soc., 2011, 158, A1011–A1014 CrossRef CAS PubMed.
- V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-Gonzalez and T. Rojo, Na-Ion Batteries, Recent Advances and Present Challenges to Become Low Cost Energy Storage Systems, Energy Environ. Sci., 2012, 5, 5884–5901 CAS.
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Research Development on Sodium-Ion Batteries, Chem. Rev., 2014, 114, 11636–11682 CrossRef CAS PubMed.
- H. D. Yoo, I. Shterenberg, Y. Gofer, G. Gershinsky, N. Pour and D. Aurbach, Mg Rechargeable Batteries: an On-Going Challenge, Energy Environ. Sci., 2013, 6, 2265–2279 CAS.
- J. Yang, M. Winter and J. O. Besenhard, Small Particle Size Multiphase Li-alloy Anodes for Lithium-ion-batteries, Solid State Ionics, 1996, 90, 281–287 CrossRef CAS.
- I. A. Courtney and J. R. Dahn, Electrochemical and In Situ X-ray Diffraction Studies of The Reaction of Lithium with Tin Oxide Composites, J. Electrochem. Soc., 1997, 144, 2045–2052 CrossRef CAS PubMed.
- Y. Idota, T. Kubota, A. Matsufuji, Y. Maekawa and T. Miyasaka, Tin-based Amorphous Oxide: A High-capacity Lithium-ion-storage Material, Science, 1997, 276, 1395–1397 CrossRef CAS.
- T. Brousse, R. Retoux, U. Herterich and D. M. Schleich, Thin-film Crystalline SnO2-lithium Electrodes, J. Electrochem. Soc., 1998, 145, 1–4 CrossRef CAS PubMed.
- C. Kim, M. Noh, M. Choi, J. Cho and B. Park, Critical Size of a Nano SnO2 Electrode for Li-secondary Battery, Chem. Mater., 2005, 17, 3297–3301 CrossRef CAS.
- Y. Wang, D. W. Su, C. Y. Wang and G. X. Wang, SnO2@MWCNT Nanocomposite as a High Capacity Anode Material for Sodium-Ion Batteries, Electrochem. Commun., 2013, 29, 8–11 CrossRef CAS PubMed.
- D. W. Su, H. J. Ahn and G. X. Wang, SnO2@Graphene Nanocomposites as Anode Materials for Na-ion Batteries with Superior Electrochemical Performance, Chem. Commun., 2013, 49, 3131–3133 RSC.
- T. Xiao, Y. W. Tang, Z. Y. Jia and S. L. Feng, Synthesis of SnO2/Mg2SnO4 Nanoparticles and Their Electrochemical Performance for Use in Li-ion Battery Electrodes, Electrochim. Acta, 2009, 54, 2396–2401 CrossRef CAS PubMed.
- Y. X. Wang, Y. G. Lim, M. S. Park, S. L. Chou, J. H. Kim and H. K. Liu, et al. Ultrafine SnO2 Nanoparticle Loading onto Reduced Graphene Oxide as Anodes for Sodium-Ion Batteries with Superior Rate and Cycling Performances, J. Mater. Chem. A, 2014, 2, 529–534 CAS.
- H. Zheng, J. B. Wang, J. Y. Huang, A. J. Cao and S. X. Mao, In Situ Visualization of Birth and Annihilation of Grain Boundaries in an Au Nanocrystal, Phys. Rev. Lett., 2012, 109, 225501 CrossRef.
- A. M. Nie, L. Y. Gan, Y. C. Cheng, H. Asayesh-Ardakani, Q. Q. Li and C. Z. Dong, et al. Atomic-Scale Observation of Lithiation Reaction Front in Nanoscale SnO2 Materials, ACS Nano, 2013, 7, 6203–6211 CrossRef CAS PubMed.
- J. Y. Huang, L. Zhong, C. M. Wang, J. P. Sullivan, W. Xu and L. Q. Zhang, et al. In Situ Observation of the Electrochemical Lithiation of a Single SnO2 Nanowire Electrode, Science, 2010, 330, 1515–1520 CrossRef CAS PubMed.
- M. Gu, A. Kushima, Y. Y. Shao, J. G. Zhang, J. Liu and N. D. Browning, et al. Probing the Failure Mechanism of SnO2 Nanowires for Sodium-Ion Batteries, Nano Lett., 2013, 13, 5203–5211 CrossRef CAS PubMed.
- C. M. Wang, W. Xu, J. Liu, J. G. Zhang, L. V. Saraf and B. W. Arey, et al. In Situ Transmission Electron Microscopy Observation of Microstructure and Phase Evolution in a SnO2 Nanowire during Lithium Intercalation, Nano Lett., 2011, 11, 1874–1880 CrossRef CAS PubMed.
- M. Gu, Y. Li, X. L. Li, S. Y. Hu, X. W. Zhang and W. Xu, et al. In Situ TEM Study of Lithiation Behavior of Silicon Nanoparticles Attached to and Embedded in a Carbon Matrix, ACS Nano, 2012, 6, 8439–8447 CrossRef CAS PubMed.
- N. Liu, H. Wu, M. T. McDowell, Y. Yao, C. M. Wang and Y. Cui, A Yolk-Shell Design for Stabilized and Scalable Li-Ion Battery Alloy Anodes, Nano Lett., 2012, 12, 3315–3321 CrossRef CAS PubMed.
- X. H. Liu, Y. Liu, A. Kushima, S. L. Zhang, T. Zhu and J. Li, et al. In Situ TEM Experiments of Electrochemical Lithiation and Delithiation of Individual Nanostructures, Adv. Eng. Mater., 2012, 2, 722–741 CAS.
- X. H. Liu, J. W. Wang, S. Huang, F. F. Fan, X. Huang and Y. Liu, et al. In Situ Atomic-Scale Imaging of Electrochemical Lithiation in Silicon, Nat. Nanotechnol., 2012, 7, 749–756 CrossRef CAS PubMed.
- X. H. Liu, J. W. Wang, Y. Liu, H. Zheng, A. Kushima and S. Huang, et al. In situ Transmission Electron Microscopy of Electrochemical Lithiation, Delithiation and Deformation of Individual Graphene Nanoribbons, Carbon, 2012, 50, 3836–3844 CrossRef CAS PubMed.
- X. H. Liu, L. Zhong, S. Huang, S. X. Mao, T. Zhu and J. Y. Huang, Size-Dependent Fracture of Silicon Nanoparticles during Lithiation, ACS Nano, 2012, 6, 1522–1531 CrossRef CAS PubMed.
- H. Yang, S. Huang, X. Huang, F. F. Fan, W. T. Liang and X. H. Liu, et al. Orientation-Dependent Interfacial Mobility Governs the Anisotropic Swelling in Lithiated Silicon Nanowires, Nano Lett., 2012, 12, 1953–1958 CrossRef CAS PubMed.
- G. Bergerhoff, R. Hundt, R. Sievers and I. D. Brown, The Inorganic Crystal-Structure Database, J. Chem. Inf. Comput. Sci., 1983, 23, 66 CrossRef CAS.
- A. Jain, G. Hautier, C. J. Moore, S. P. Ong, C. C. Fischer and T. Mueller, et al. A High-Throughput Infrastructure For Density Functional Theory Calculations, Comput. Mater. Sci., 2011, 50, 2295–2310 CrossRef CAS PubMed.
- S. Curtarolo, W. Setyawan, S. D. Wang, J. K. Xue, K. S. Yang and R. H. Taylor, et al. AFLOWLIB.ORG: A Distributed Materials Properties Repository From High-Throughput Ab Initio Calculations, Comput. Mater. Sci., 2012, 58, 227–235 CrossRef CAS PubMed.
- S. Curtarolo, G. L. W. Hart, M. B. Nardelli, N. Mingo, S. Sanvito and O. Levy, The High-Throughput Highway to Computational Materials Design, Nat. Mater., 2013, 12, 191–201 CrossRef CAS PubMed.
- A. N. Kolmogorov and S. Curtarolo, Prediction of Different Crystal Structure Phases in Metal Borides: A Lithium Monoboride Analog to MgB2, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 180501 CrossRef.
- A. N. Kolmogorov, S. Shah, E. R. Margine, A. F. Bialon, T. Hammerschmidt and R. Drautz, New Superconducting and Semiconducting Fe-B Compounds Predicted with an Ab Initio Evolutionary Search, Phys. Rev. Lett., 2010, 105, 217003 CrossRef CAS.
- S. P. Ong, L. Wang, B. Kang and G. Ceder, Li-Fe-P-O2 Phase Diagram from First Principles Calculations, Chem. Mater., 2008, 20, 1798–1807 CrossRef CAS.
- S. P. Ong, A. Jain, G. Hautier, B. Kang and G. Ceder, Thermal Stabilities of Delithiated Olivine MPO4 (M = Fe, Mn) Cathodes Investigated Using First Principles Calculations, Electrochem. Commun., 2010, 12, 427–430 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- A. Jain, G. Hautier, S. P. Ong, C. Moore, C. C. Fischer and K. A. Persson, et al. Formation Enthalpies by Mixing GGA and GGA+U calculations, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 045115 CrossRef.
- K. Reuter and M. Scheffler, Composition, Structure, and Stability of RuO2(110) as a Function of Oxygen Pressure, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 65, 035406 CrossRef.
- K. Reuter and M. Scheffler, First-Principles Atomistic Thermodynamics for Oxidation Catalysis: Surface Phase Diagrams and Catalytically Interesting Regions, Phys. Rev. Lett., 2003, 90, 046103 CrossRef.
- N. G. Hörmann, A. Gross and P. Kaghazchi, Semiconductor-Metal Transition Induced by Nanoscale Stabilization, Phys. Chem. Chem. Phys., 2015, 17, 5569–5573 RSC.
- M. H. Hansen, L. A. Stern, L. G. Feng, J. Rossmeisl and X. L. Hu, Widely Available Active Sites on Ni2P for Electrochemical Hydrogen Evolution – Insights from First Principles Calculations, Phys. Chem. Chem. Phys., 2015, 17, 10823–10829 RSC.
- M. Nielsen, M. E. Bjorketun, M. H. Hansen and J. Rossmeisl, Towards First Principles Modeling of Electrochemical Electrode–Electrolyte Interfaces, Surf. Sci., 2015, 631, 2–7 CrossRef CAS PubMed.
- M. Garcia-Mota, A. Vojvodic, F. Abild-Pedersen and J. K. Norskov, Electronic Origin of the Surface Reactivity of Transition-Metal-Doped TiO2(110), J. Phys. Chem. C, 2013, 117, 460–465 CAS.
- F. Abild-Pedersen, J. Greeley, F. Studt, J. Rossmeisl, T. R. Munter and P. G. Moses, et al. Scaling Properties of Adsorption Energies for Hydrogen-Containing Molecules on Transition-Metal Surfaces, Phys. Rev. Lett., 2007, 99, 016105 CrossRef CAS.
- I. A. Courtney, J. S. Tse, O. Mao, J. Hafner and J. R. Dahn, Ab Initio Calculation of the Lithium–Tin Voltage Profile, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 15583–15588 CrossRef CAS.
- T. Zhang and H. S. Zhou, A Reversible Long-Life Lithium–Air Battery in Ambient Air, Nat. Commun., 2013, 4, 1817 CrossRef PubMed.
- Z. Q. Peng, S. A. Freunberger, Y. H. Chen and P. G. Bruce, A Reversible and Higher-Rate Li-O2 Battery, Science, 2012, 337, 563–566 CrossRef CAS PubMed.
- Z. Q. Peng, S. A. Freunberger, L. J. Hardwick, Y. H. Chen, V. Giordani and F. Barde, et al. Oxygen Reactions in a Non-Aqueous Li+ Electrolyte, Angew. Chem., Int. Ed., 2011, 50, 6351–6355 CrossRef CAS PubMed.
- P. Hartmann, C. L. Bender, M. Vracar, A. K. Durr, A. Garsuch and J. Janek, et al. A Rechargeable Room-Temperature Sodium Superoxide (NaO2) Battery, Nat. Mater., 2013, 12, 228–232 CrossRef CAS PubMed.
- K. Leung and C. M. Tenney, Toward First Principles Prediction of Voltage Dependences of Electrolyte/Electrolyte Interfacial Processes in Lithium Ion Batteries, J. Phys. Chem. C, 2013, 117, 24224–24235 CAS.
- K. Leung, Predicting the Voltage Dependence of Interfacial Electrochemical Processes at Lithium-Intercalated Graphite Edge Planes, Phys. Chem. Chem. Phys., 2015, 17, 1637–1643 RSC.
- N. G. Hörmann, M. Jäckle, F. Gossenberger, T. Roman, K. Forster-Tonigold and M. Naderian, et al. Some Challenges in the First-Principles Modeling of Structures and Processes in Electrochemical Energy Storage and Transfer, J. Power Sources, 2015, 275, 531–538 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |