Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A low cost azomethine-based hole transporting material for perovskite photovoltaics†
M. L.
Petrus
ab,
T.
Bein
a,
T. J.
Dingemans
*b and
P.
Docampo
*a
aDepartment of Chemistry, Center for NanoScience (CeNS), University of Munich (LMU), Butenandtstr. 11, 81377 Munich, Germany. E-mail: pablo.docampo@cup.lmu.de
bDelft University of Technology, Faculty of Aerospace Engineering, Kluyverweg 1, 2629 HS Delft, The Netherlands. E-mail: t.j.dingemans@tudelft.nl
First published on 11th May 2015
Abstract
Most hole transporting materials (HTMs) prepared for perovskite solar cell applications are synthesized via cross-coupling reactions that require expensive transition metal catalysts, inert reaction conditions and extensive product purification; making large-scale production cost-prohibitive. Here, we describe the synthesis of a simple azomethine-based conjugated small-molecule (EDOT-OMeTPA) which is easily prepared in a cost effective Schiff base condensation reaction, with water being the only by-product. As the hole transporter in planar CH3NH3PbI3 perovskite solar cells, efficiencies exceeding 11% were reached. This result is comparable to state-of-the-art materials such as Spiro-OMeTAD on a like-to-like comparison, while cost estimations show that the material cost is about one order of magnitude lower for EDOT-OMeTPA, resulting in a negligible cost-per-peak-Watt contribution of 0.004 $ W−1. In addition, the high synthetic accessibility of EDOT-OMeTPA also reduces the toxic chemical waste and therefore greatly reduces its environmental impact. Our results pave the way towards low-cost, environmentally friendly and efficient HTMs.
The efficiency of methylammonium lead iodide (MAPI) perovskite-based solar cells has increased rapidly in the last five years and is already comparable with current commercial technologies.1–4 Record efficiencies exceeding 20% have been reported and further improvements are expected.5 Although the cost of the perovskite material itself is relatively low, most of the state-of-the-art devices incorporate an expensive organic hole transporting material (HTM), termed Spiro-OMeTAD (Fig. 1).6,7 While several other organic HTMs have been reported,8–12 they generally show a lower performance and/or are expensive to synthesize.
 | ||
| Fig. 1 Chemical structures of EDOT-OMeTPA (with the azomethine bond in red), and the reference molecules: Spiro-OMeTAD and H101.13 | ||
An interesting approach towards the synthesis of low-cost, small-molecule HTMs was recently introduced by Li et al.13 They reported a 3,4-ethylenedioxythiophene based HTM with efficiencies up to 13.2%, which is comparable to Spiro-OMeTAD. However, the small-molecule presented by Li et al. (H101, Fig. 1) and most other novel HTM are synthesized via cross-coupling reactions that require transition metal catalysts, inert reaction conditions and extensive product purification; making large-scale production cost-prohibitive.
Schiff base condensation chemistry offers a less complicated route towards conjugated materials,14–16 since the reaction can be performed at near ambient conditions and water is the only by-product, making product purification very straightforward.17–19 The azomethine bond (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–, also known as Schiff-base) is isoelectronic to the vinyl bond and possesses similar optoelectronic and thermal properties. Azomethine-based conjugated materials have already been successfully applied as donor materials in organic photovoltaics.20–25
N–, also known as Schiff-base) is isoelectronic to the vinyl bond and possesses similar optoelectronic and thermal properties. Azomethine-based conjugated materials have already been successfully applied as donor materials in organic photovoltaics.20–25
Here we demonstrate the synthesis and characterisation of a novel azomethine-based small-molecule (EDOT-OMeTPA, Fig. 1) based on triphenylamine (TPA) and 3,4-ethylenedioxythiophene (EDOT) moieties. When employed in perovskite solar cells, we show that the obtained performance is comparable to that of state-of-the-art materials such as Spiro-OMeTAD in a like-to-like comparison. Our results show a simple route towards obtaining easily up-scalable hole transporters with reduced cost and reduced environmental impact compared to state-of-the-art HTMs.
The small-molecule design was motivated by our previous work on conjugated azomethines.17 In order to increase the solubility of the small-molecule and to align the energy level with the perovskite material, a cyclic ether and methoxy groups were introduced. In addition, the functionalization makes this small-molecule a direct analogue of its fully aromatic counterpart H101.13 This gives us the opportunity to study the effect of the azomethine bond on the optoelectronic and thermal properties and device performance.
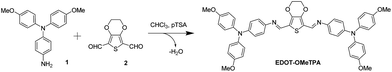
EDOT-OMeTPA was prepared by condensing 4-amino-4′,4′′-dimethoxytriphenylamine (1) with commercially available 2,3-dihydrothieno[3,4-b][1,4]dioxine-5,7-dicarboxaldehyde (2) in the presence of a catalytic amount of para-toluenesulfonic acid (Fig. 2). The product was purified in a simple washing step and obtained in a high yield. During the workup the product was washed with triethylamine to prevent the presence of protonated species which can negatively impact the final device performance.17 Experimental details and characterization can be found in the ESI.† As a result of this uncomplicated chemistry, this reaction can be performed on a bench top without the need for advanced equipment (only a beaker and stirrer are required), making the chemistry accessible to a wide range of researchers, rather than confining it to specialized organic chemists.
The thermal properties were studied by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) measurements. EDOT-OMeTPA possesses a high thermal stability, with a 5% weight loss at 359 °C (Fig. S1†), which can be attributed to the all-aromatic backbone. On the first heating cycle, DSC measurements show a Tm only at 241 °C and this Tm appears to be solvent induced. After the first heating cycle, the material is fully amorphous and shows no Tm but a Tg only at 105 °C. Consecutive heating cycles confirm the amorphous nature of this material. The Tg of EDOT-OMeTPA is well above the general device operating temperature (Fig. S2, Table S1†),26 which is considered essential for the stability of the hole transporting layer and the photovoltaic device. Therefore, an increase in Tg of 32 °C of EDOT-OMeTPA over H101 (Tg = 73 °C)13 should be considered as a very important improvement. In comparison, Spiro-OMeTAD exhibits a high Tg, which is well above the operating temperature of photovoltaic devices (125 °C).27
UV-Vis absorption spectra of EDOT-OMeTPA (Fig. 3, Table S1†) show absorption maxima at 495 nm in solution and at 505 nm in the film. The absorption onset was found at 625 nm, resulting in an optical bandgap (Eg) of 2.0 eV. The rather large bathochromic shift of EDOT-OMeTPA compared to H101 can be attributed to the extended conjugation, which is a direct consequence of the electron withdrawing azomethine bond28 and/or the more planar conformation of the azomethine-based backbone compared to the aromatic backbone.
A good energy alignment between the hole transporter and the perovskite is essential to maximize the charge transfer processes. In order to study the energy level alignment of EDOT-OMeTPA with state-of-the-art MAPI perovskite, the electronic properties were determined using cyclic voltammetry. The onset of the oxidation potential was found at +0.17 V (vs. Fc+/Fc), which is approximately 0.15 V higher than Spiro-OMeTAD (Fig. 3 and S3 and Table S1†). The HOMO energy level was estimated according to literature (assuming that the energy level of Fc+/Fc is −5.1 eV)29 to be around 5.28 eV for EDOT-OMeTPA, resulting in an excellent alignment with the MAPI valence band with only a 0.15 eV loss. The energy level of EDOT-OMeTPA is approximately 0.19 eV deeper compared to its analogue H101, which is the result of the electron-withdrawing azomethine bond, and thus higher open circuit voltages can be expected when integrated into solar cells.28
Photovoltaic devices were prepared by spin coating the perovskite layer using the solvent engineering process as was described by Jeon et al.30 In our devices, the perovskite layer is approximately 300 nm thick and is known to be relatively smooth with a root mean square roughness of 8.3 nm.30 On top, the different hole transporting materials were spin coated from a chlorobenzene solution. Due to the limited solubility of the compound, relatively low concentrations (10 mg mL−1) were used for spin coating EDOT-OMeTPA in order to obtain good films. These concentrations result in films with a thickness of approximately 40 nm, significantly thinner than generally used for Spiro-OMeTAD (200–400 nm). We note here that spin coating films from similar low-concentration Spiro-OMeTAD solutions result in poor film quality and severe shunting (details in ESI†). Photovoltaic devices prepared with EDOT-OMeTPA show efficiencies exceeding 11.0%, which is comparable to optimized Spiro-OMeTAD (11.9%) and H101 (10.9%) (Fig. 4). The obtained Voc of EDOT-OMeTPA is comparable to H101 and about 50 mV lower compared to Spiro-OMeTAD, with all materials resulting in similar short circuit currents of approximately 19 mA cm−2. In contrast to H101, doping with the cobalt dopant FK102 did not increase the efficiency for our devices under the tested conditions (see ESI†).13 Furthermore, the devices comprising EDOT-OMeTPA as the HTM show a good stability over 1000 hours with very little degradation in the PCE (∼10%) when stored at ambient conditions i.e. room temperature and a relative humidity of 30% (Fig. S5†).
 | ||
| Fig. 4 J–V curves of solar cells based on the different hole transporting materials. The inset shows the characteristics for the best devices. | ||
The efficiencies for EDOT-OMeTPA show a larger spread than Spiro-OMeTAD (Table S2†). Here, it is likely that a significant number of pinholes can exist in the thinner EDOT-OMeTPA layers which would result in device shunting paths.31,32 We believe that optimisation of the chemical structure, for example to increase the solubility which could assist in the formation of thicker films, and the processing conditions would further increase the efficiency and reproducibility; making azomethine-based materials promising alternatives for HTMs that are currently available.
Although metal halide perovskite materials show great potential for low-cost solar cells, currently employed state-of-the-art hole transporting materials would add around $40 per square meter to the photovoltaic devices (see ESI† for estimation). We have already shown that with EDOT-OMeTPA it is possible to prepare efficient devices with significantly thinner layers, resulting in a first reduction of the cost of almost an order of magnitude.
Furthermore, condensation chemistry offers a simpler and therefore more cost-effective route to conjugated materials compared to (palladium catalysed) cross-coupling reactions. We performed a materials cost estimation for the synthesis of EDOT-OMeTPA and the reference HTMs, Spiro-OMeTAD and H101, following the procedure established by Osedach et al. (flow charts and details of the cost estimation can be found in the ESI†).33 We estimated that the material cost for EDOT-OMeTPA (∼10 $ g−1) is about an order of magnitude lower compared to the other HTMs (Table 1 and S4†). However, we note here that as a result of the limited amount of reported synthetic protocols towards H101, we believe that the material cost of H101 can be significantly reduced by optimisation of the starting material synthesis. The difference in cost between materials prepared via cross-coupled reactions and condensation reactions mainly originates from the cost for purification; in particular column chromatography has a large impact on the cost (Table S4†). Column chromatography is generally necessary to purify products from palladium cross-coupling reactions, since this type of reaction suffers from impurities as a result of homocoupling and halogen-dance, which are difficult to remove via other purification methods.34 The low cost of EDOT-OMeTPA combined with the thinner layer could reduce the cost of the HTM layer in photovoltaic devices by approximately two orders of magnitude. This results in an estimated cost-per-peak-Watt contribution of 0.004 $ W−1, which could be considered negligible (details in ESI†).33
| HTM | Chemical wastea,b (kg g−1) | Material costc ($ g−1) | Commercial price ($ g−1) |
|---|---|---|---|
| a Total estimated weight of halogenated solvents is given in parentheses. b Total estimated weight of chemical waste for the synthesis of 1 gram of product. c Total estimated material cost for the synthesis of 1 gram of product. | |||
| EDOT-OMeTPA | 0.7 (0.1) | 10 | n/a |
| Spiro-OMeTAD | 3.6 (1.0) | 92 | 170–475 |
| H101 | 4.0 (2.3) | 111 | 250 |
As a result of the intensive purification that is required for Spiro-OMeTAD and H101, the amount of chemical waste is significantly higher than for EDOT-OMeTPA, which not only has a large impact on the cost, but also on the environment. Specifically, the quantity of halogenated solvents, known to be highly toxic, used for the synthesis of Spiro-OMeTAD and H101 (1.0 and 2.3 kg per gram of product respectively) is very high compared to the synthesis of EDOT-OMeTPA (0.1 kg g−1). This not only makes the azomethine-based hole transporting material a more cost-effective option but also has a reduced environmental impact compared to state-of-the-art materials.
Conclusions
In conclusion, an azomethine-based hole transporting material, EDOT-OMeTPA, was prepared via a simple condensation reaction. The azomethine-bond in EDOT-OMeTPA increases the glass transition temperature compared to its vinyl analogue (H101) by 32 °C, while it lowers the HOMO energy level (5.28 eV compared to 5.09 eV for H101), which results in a better alignment with the valence band of the perovskite material. Planar (CH3NH3PbI3) perovskite-based solar cells with EDOT-OMeTPA as the hole transporting layer show power conversion efficiencies exceeding 11%. This efficiency is comparable to state-of-the-art Spiro-OMeTAD on a like-to-like comparison, while significant improvements can be expected after further optimization of the chemical structure and processing conditions. Furthermore, we show that Schiff base condensation chemistry offers a cost-effective and more environmentally friendly route towards up-scalable hole-transporting materials, leading to a reduction of materials costs of approximately two orders of magnitude compared to state-of-the-art materials, resulting in a negligible contribution of 0.004 $ W−1. Our results demonstrate that Schiff-base condensation chemistry is a viable route to achieving cost-effective hole transporting materials, thus bringing perovskite solar cells one step closer to commercial realization.Notes and references
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- J.-H. Im, C.-R. Lee, J.-W. Lee, S.-W. Park and N.-G. Park, Nanoscale, 2011, 3, 4088–4093 RSC.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–648 CrossRef CAS PubMed.
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316–319 CrossRef CAS PubMed.
- M. A. Green, K. Emery, Y. Hishikawa, W. Warta and E. D. Dunlop, Progress in Photovoltaics: Research and Applications, 2015, 23, 1–9 CrossRef PubMed.
- L. Yang, B. Xu, D. Bi, H. Tian, G. Boschloo, L. Sun, A. Hagfeldt and E. M. J. Johansson, J. Am. Chem. Soc., 2013, 135, 7378–7385 CrossRef CAS PubMed.
- U. Bach, D. Lupo, P. Comte, J. E. Moser, F. Weissörtel, J. Salbeck, H. Spreitzer and M. Grätzel, Nature, 1998, 395, 583–585 CrossRef CAS.
- N. J. Jeon, J. Lee, J. H. Noh, M. K. Nazeeruddin and S. Il Seok, J. Am. Chem. Soc., 2013, 135, 19087–19090 CrossRef CAS PubMed.
- K. Neumann and M. Thelekkat, RSC Adv., 2014, 4, 43550–43559 RSC.
- B. Xu, E. Sheibani, P. Liu, J. Zhang, H. Tian, N. Vlachopoulos, G. Boschloo, L. Kloo, A. Hagfeldt and L. Sun, Adv. Mater., 2014, 6629–6634 CrossRef CAS PubMed.
- P. Qin, S. Paek, M. I. Dar, N. Pellet, J. Ko, M. Grätzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2014, 136, 8516–8519 CrossRef CAS PubMed.
- T. Krishnamoorthy, F. Kunwu, P. P. Boix, H. Li, T. M. Koh, W. L. Leong, S. Powar, A. Grimsdale, M. Grätzel, N. Mathews and S. G. Mhaisalkar, J. Mater. Chem. A, 2014, 2, 6305 CAS.
- H. Li, K. Fu, A. Hagfeldt, M. Grätzel, S. G. Mhaisalkar and A. C. Grimsdale, Angew. Chem., Int. Ed., 2014, 53, 4085–4088 CrossRef CAS PubMed.
- M. E. Mullholland, D. Navarathne, M. L. Petrus, T. J. Dingemans and W. G. Skene, J. Mater. Chem. C, 2014, 2, 9099–9108 RSC.
- L. Sicard, D. Navarathne, T. Skalski and W. G. Skene, Adv. Funct. Mater., 2013, 23, 3549–3559 CrossRef CAS PubMed.
- C. Yang and S. A. Jenekhe, Chem. Mater., 1991, 3, 878–887 CrossRef CAS.
- M. L. Petrus, R. K. M. Bouwer, U. Lafont, S. Athanasopoulos, N. C. Greenham and T. J. Dingemans, J. Mater. Chem. A, 2014, 2, 9474–9477 CAS.
- H. Niu, J. Cai, P. Zhao, C. Wang, X. Bai and W. Wang, Dyes Pigm., 2013, 96, 158–169 CrossRef CAS PubMed.
- D. Janeliunas, P. van Rijn, J. Boekhoven, C. B. Minkenberg, J. H. van Esch and R. Eelkema, Angew. Chem., Int. Ed. Engl., 2013, 52, 1998–2001 CrossRef CAS PubMed.
- M. L. Petrus, F. S. F. Morgenstern, A. Sadhanala, R. H. Friend, N. C. Greenham and T. J. Dingemans, Chem. Mater., 2015 DOI:10.1021/acs.chemmater.5b00313.
- J. C. Hindson, B. Ulgut, R. H. Friend, N. C. Greenham, B. Norder, A. Kotlewski and T. J. Dingemans, J. Mater. Chem., 2010, 20, 937–944 RSC.
- A. Bolduc, S. Barik, M. Lenze, K. Meerholz and W. G. Skene, J. Mater. Chem. A, 2014, 2, 15620–15626 CAS.
- A. Iwan, B. Boharewicz, K. Parafiniuk, I. Tazbir, L. Gorecki, A. Sikora, M. Filapek and E. Schab-Balcerzak, Synth. Met., 2014, 195, 341–349 CrossRef CAS PubMed.
- C. Moussallem, O. Segut, F. Gohier, M. Allain and P. Frere, ACS Sustainable Chem. Eng., 2014, 2, 1043–1048 CrossRef.
- M. L. Petrus, R. K. M. Bouwer, U. Lafont, D. H. K. Murthy, R. J. P. Kist, M. L. Böhm, Y. Olivier, T. J. Savenije, L. D. A. Siebbeles, N. C. Greenham and T. J. Dingemans, Polym. Chem., 2013, 4, 4182–4191 RSC.
- M. C. Alonso García and J. L. Balenzategui, Renewable Energy, 2004, 29, 1997–2010 CrossRef PubMed.
- T. Leijtens, I. Ding, T. Giovenzana, J. T. Bloking, M. D. Mcgehee and A. Sellinger, ACS Nano, 2012, 6, 1455–1462 CrossRef CAS PubMed.
- A. Bolduc, A. Al Ouahabi, C. Mallet and W. G. Skene, J. Org. Chem., 2013, 78, 9258–9269 CrossRef CAS PubMed.
- C. M. Cardona, W. Li, A. E. Kaifer, D. Stockdale and G. C. Bazan, Adv. Mater., 2011, 23, 2367–2371 CrossRef CAS PubMed.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. Il Seok, Nat. Mater., 2014, 13, 897–903 CrossRef CAS PubMed.
- G. E. Eperon, V. M. Burlakov, P. Docampo, A. Goriely and H. J. Snaith, Adv. Funct. Mater., 2014, 24, 151–157 CrossRef CAS PubMed.
- P. Docampo and H. J. Snaith, Nanotechnology, 2011, 22, 225403 CrossRef PubMed.
- T. P. Osedach, T. L. Andrew and V. Bulović, Energy Environ. Sci., 2013, 6, 711–718 CAS.
- K. H. Hendriks, W. Li, G. H. L. Heintges, G. W. P. van Pruissen, M. M. Wienk and R. A. J. Janssen, J. Am. Chem. Soc., 2014, 136, 11128–11133 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterisation, device preparation, material cost estimation and flow charts. See DOI: 10.1039/c5ta03046c |
| This journal is © The Royal Society of Chemistry 2015 |