Open Access Article
Open Access ArticleHigh-silica, heulandite-type zeolites prepared by direct synthesis and topotactic condensation†
Joel E.
Schmidt
a,
Dan
Xie
 b and
Mark E.
Davis
*a
b and
Mark E.
Davis
*a
aChemical Engineering, California Institute of Technology, Pasadena, CA 91125, USA. E-mail: mdavis@cheme.caltech.edu
bChevron Energy Technology Company, Richmond, CA 94802, USA
First published on 27th May 2015
Abstract
There are both natural minerals and synthetic zeolites that possess the HEU framework topology. These materials have a limited compositional range (Si/Al < 6), and the natural zeolites often contain a large amount of impurities such as Fe3+. The preparation of impurity-free HEU-type zeolites with higher Si/Al ratio could open many areas of application, particularly in catalysis. Here, we report the first high-silica HEU-type zeolite that can be prepared via two different procedures. In the first method high-silica HEU (denoted CIT-8) is prepared using a topotactic condensation mechanism (layered precursor denoted CIT-8P); CIT-8P is obtained from a low-water synthesis in fluoride media. CIT-8 prepared in this manner has a product Si/Al ratio of 9.8 ± 0.7 and a micropore volume of 0.10 cm3 g−1 (measured by nitrogen adsorption). The variable temperature powder X-ray diffraction shows that CIT-8 forms via topotactic condensation from CIT-8P along the b axis. Additionally, high-silica heulandite can be synthesized directly from a hydroxide-mediated reaction mixture (denoted CIT-8H), and has a Si/Al ratio of 6.4 ± 0.3 and a micropore volume of 0.10 cm3 g−1. Both synthesis methods produce zeolites that expand the compositional range of HEU-type zeolites. These synthetic methods allow for the addition of other heteroatoms, and titanium-containing CIT-8 is prepared as an illustrative example.
1. Introduction
Natural zeolites form under hydrothermal processes, normally near volcanic deposits, and according to the International Mineralogical Association definition, there were known to be 93 different mineral species belonging to 44 different framework types as of 2014.1 Approximately 2.5 million metric tons of natural zeolites are consumed per year, compared to 1.7–2 million metric tons of synthetic zeolites.2 Natural zeolites are employed in applications such as separations, impurity removal (such as ammonia), and as additives in paper, consumer products and animal feed.3 Many natural zeolites are known to have synthetic analogs that can be produced at different compositions than the natural material. Additionally, natural zeolites often have large amounts of impurities, making them unsuitable for many applications. Natural zeolites commonly occur at compositions (typically low Si/Al ratio, e.g., below 5) that are not thermally or hydrothermally stable, further rendering them unusable in demanding process conditions, such as catalytic applications. Therefore, discovery of synthetic methods to produce analogs of natural zeolites that result in desired frameworks free of impurities and exhibiting robust thermal and hydrothermal stability (typically Si/Al ratio higher than 5) are highly desired. One recent example of this approach is that of the synthetic analogue of the mineral boggsite (BOG4), recently prepared as ITQ-47.5 The BOG framework has a 2-dimensional system of intersecting 10- and 12-membered rings (MRs) and was prepared synthetically using an organic structure directing agent based on phosphazenes, as a silicoborate framework material instead of the aluminosilicate material that is the rare natural zeolite. ITQ-47 has been evaluated for catalytic applications including the production of cumene by the alkylation of benzene with propylene where it shows better product selectivities to cumene than *BEA, the current industrial standard material for this reaction.5Zeolites with the HEU framework exist as both natural minerals as well as their synthetic analogs. The natural materials can be excavated from quarries at low cost, and are used in applications including building materials, paper fillers, livestock feed supplements, water purification, dehydration and gas separations.3 The HEU framework contains a pore channel system with openings consisting of 10 MRs in the [001] direction that are 3.1 × 7.5 Å as well as 8 MRs with dimensions of 3.6 × 4.6 Å in the [001] direction. Additionally, there is another set of 8 MRs along the [100] direction with dimensions of 2.8 × 4.7 Å.6 Materials with the HEU framework are divided into two distinct classes based on Si/Al ratio. Those with Si/Al of less than 4 are known as heulandite and those with Si/Al greater than 4 are known as clinoptilolite, or silica-rich heulandite. The key difference in these materials is those with Si/Al of less than 4 are not thermally stable to calcination above 350 °C (though the literature contains some ambiguity about the definition).7 Natural heulandites are often encountered containing Ba, Ca, Na, K and Sr in addition to a significant amount of iron as Fe3+ (up to 1.5% of the tetrahedra).3
A synthetic method to produce a high-silica heulandite was first reported by removing aluminum from natural clinoptilolite to make a material with Si/Al ≥ 5.5, known as LZ-219.8 Later, methods were developed to produce synthetic heulandites across a range of Si/Al = 2.5–6 using various sources of silica and alumina and a wide range of inorganic cations (Ca, K, Li, Na, Ca, Sr) under hydrothermal synthesis conditions.7,9–12 These materials have been considered for applications such as gas cleaning and separations, ion exchange, isomerization of 1-butene and xylene, methanol dehydration, acetylene hydration and the separation of nitrogen from natural gas.7,13 In any of these applications, the ability to tailor the framework composition is important for material properties such as exchange capacity, thermal and hydrothermal stability as well as pore accessibility.
Herein, we report methods to prepare high-silica heulandite. High-silica heulandite is prepared via topotactic condensation of a layered aluminosilicate material (layered material denoted CIT-8P, condensation product denoted CIT-8) formed using an organic structure directing agent (OSDA). High-silica heulandite can also be prepared by direct synthesis in hydroxide media using an OSDA that is found to be occluded in the product (material prepared in hydroxide media denoted CIT-8H).
2. Experimental
2.1 Organic structure directing agent
The diquaternary OSDAs used in this work (shown in Fig. 1) were synthesized by reacting 200 mmol of 1,2,4,5-tetramethylimidazole (TCI Chemicals) with 100 mmol of dibromoalkane (Aldrich) at reflux in methanol overnight. The solvent was then removed using rotary evaporation and the product washed with ether. The product was verified using 13C NMR in D2O with methanol added as an internal standard and the characterization results for the diquats are given in Table 1. The product was ion exchanged to hydroxide form using Dowex Marathon A exchange resin and the final product concentration was determined using a Mettler-Toledo DL22 autotitrator using 0.01 M HCl as the titrant.| Dibromoalkane | 13C-NMR (125 MHz, D2O) |
|---|---|
| 1,3-Dibromopropane | 7.93, 7.98, 9.96, 29.02, 31.70, 41.81, 124.62, 126.42, 142.05 |
| 1,4-Dibromobutane | 7.56, 9.35, 21.21, 25.88, 30.17, 44.14, 124.60, 126.00, 141.89 |
| 1,5-Dibromopentane | 7.76, 7.82, 9.61, 22.82, 28.58, 31.42, 44.72, 124.84, 126.03, 141.95 |
| 1,6-Dibromohexane | 7.88, 7.95, 9.72, 25.42, 28.88, 31.51, 44.97, 124.96, 126.08, 142.01 |
2.2 Microporous materials synthesis
13C, 27Al and 29Si solid-state NMR were performed using a Bruker DSX-500 spectrometer (11.7 T) and a Bruker 4 mm MAS probe. The spectral operating frequencies were 500.2 MHz, 125.721 MHz, 130.287 MHz and 99.325 MHz for 1H, 13C, 27Al and 29Si nuclei, respectively. Spectra were referenced to external standards as follows: tetramethylsilane (TMS) for 1H and 29Si, adamantane for 13C as a secondary external standard relative to tetramethylsilane and 1.0 M Al(NO3)3 aqueous solution for 27Al. Samples were spun at 14 kHz for 1H and 27Al MAS NMR and 8 kHz for 13C and 29Si MAS and CPMAS NMR experiments. For detection of 27Al signal, a short 0.5 μs – π/18 pulse was used before FID was recorded in order to make quantitative comparison among resonances.
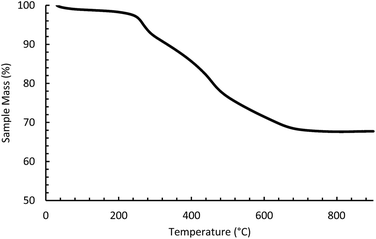
Thermogravimetric analysis measurements were performed on Netzsch STA 449C Jupiter. Samples were heated in air to 900 °C at a rate of 1 °C min−1.
Nitrogen physical adsorption isotherms were performed at 77 K using a Quantachrome Autosorb iQ and the micropore volume was determined using the t-plot method.14
Powder X-ray diffraction data (PXRD) data were collected on a Rigaku MiniFlex II with Cu Kα radiation.
Variable temperature PXRD patterns were collected from 30 °C to 580 °C at increments of 50 °C under ambient conditions, using a PANalytical Empyrean powder diffractometer (Cu Kα radiation) equipped with an Anton Paar HTK 1200N high-temperature chamber. The sample was stabilized at each measurement temperature for 15 min before starting each measurement. The temperature ramp between two consecutive temperatures was 5 °C min−1.
Scanning electron micrographs (SEM) were recorded on a Hitachi S-570 instrument. EDS spectra were acquired with an Oxford X-Max SDD X-ray Energy Dispersive Spectrometer system on a ZEISS 1550 VP FESEM, equipped with in-lens SE.
Diffuse reflectance UV-visible (DRUV) spectra were recorded using a Cary 3G spectrophotometer equipped with a diffuse reflectance cell; zeolite samples were calcined prior to data collection.
Three-dimensional electron diffraction data were collected using the rotation electron diffraction (RED) technique.15,16 The RED software was installed on a JEOL 2010 microscope operating at 200 kV, and data were collected over a tilt range of ±50° with a tilt step of 0.50°, the exposure time is 3 seconds per tilt step.
A general synthesis procedure for the microporous materials can be found below. In all situations where a rotating oven was used, the samples were spun at 43 rpm.
| Linker length | Si/Al = 15 H2O/SiO2 = 4 | Si/Al = 20 H2O/SiO2 = 4 | Si/Al = 20 H2O/SiO2 = 7 | Si/Al = 30 H2O/SiO2 = 4 | Si/Al = 50 H2O/SiO2 = 4 | Si/Al = 50 H2O/SiO2 = 7 |
|---|---|---|---|---|---|---|
| 3 | PREFER | CIT-8P + PREFER | CIT-7 | Dense | ||
| 4 | CIT-8P | CIT-8P, CIT-7 | CIT-7 | CIT-8P + CIT-7 | CIT-7 + CIT-8P | CIT-7 |
| 5 | CIT-8P | CIT-8P | CIT-7+ CIT-8P | IWV | ||
| 6 | CIT-8P | *BEA | Unknown | STF |
| Gel Si/Al | Gel Na/Si | Gel ROH/Si | Gel H2O/Si | Temp (°C) | Seeds | Time (days) | Product |
|---|---|---|---|---|---|---|---|
| 4.15 | 0.25 | 0.16 | 30 | 160 | None | 51 | CIT-8H |
| 5 | 0.25 | 0.16 | 30 | 160 | None | 43 | CIT-8H |
| 5 | 0.25 | 0.16 | 30 | 160 | CIT-7 | 35 | CIT-7 |
| 5 | 0.25 | 0.16 | 30 | 160 | CIT-8H | 24 | CIT-8H |
| 7.5 | 0.25 | 0.16 | 30 | 160 | CIT-8H | 20 | CIT-7 |
| 10 | 0.25 | 0.16 | 30 | 160 | CIT-8H | 20 | CIT-7 |
2.3 Calcination and ammonium exchange
All products were calcined in breathing grade air. The material was heated to 150 °C at 1 °C min−1, held for three hours, then heated to 580 °C at 1 °C min−1 and held for six hours to assure complete combustion of the organic. After calcination, materials prepared in hydroxide media were exchanged to ammonium form using 1 M NH4NO3 (100 mL of solution per gram of catalyst) at 95 °C with stirring for three hours, this was done a total of three times per sample. After ammonium exchange the materials were washed with water and dried and then calcined to proton form using the standard method.3. Results and discussion
3.1 Fluoride-mediated syntheses
The diquaternary OSDAs investigated in this work were first screened over a range of aluminum and water contents and the results are reported in Table 2. Several different products were produced using these diquats depending on the water and aluminum content. CIT-8P was found as a product in these screening reactions along with *BEA, CIT-7, IWV, STF and STW. This competition demonstrates the weak nature of the structure direction exhibited by these diquats, and is similar to the behavior observed in our recent work reporting CIT-7.17 Additionally, many of these products have been reported before with other diquaternary OSDAs in fluoride media, such as *BEA and STF.18 CIT-8P forms in reactions with relatively high amounts of aluminum (Si/Al = 15, 20), and decreasing the aluminum content generally led to the formation of CIT-7, IWV or STF. Seeding these higher-silica syntheses with CIT-8P materials was not successful in eliminating the formation of competing phases. Therefore, CIT-8P could only be formed from a limited range of synthesis compositions. Synthesis times to form CIT-8P were around 12 days without seeds added, and around 5 days when they were added (some variation in these times was observed in different synthesis trials). Phase competition was also observed, and once a pure-phase sample of CIT-8P was obtained, seeds were used in all subsequent reactions to help favor the formation of CIT-8P.The PXRD of CIT-8P is shown in Fig. 2 along with the product that results from calcination (identified as a HEU-type material). A framework also formed by topotactic condensation similar to HEU, RRO, has been previously reported as RUB-39 for the layered precursor and RUB-41 for the calcined framework (RRO).19,20 RUB-41 is formed from heulandite-type layers and has a similar PXRD pattern as HEU. However, careful analysis of the diffraction patterns for the two materials shows distinct reflections for each of the materials. For HEU, there are strong reflections near 17° and 22° 2θ that are not present in RRO. The calcined material had a micropore volume of 0.10 cm3 g−1 from nitrogen adsorption (Fig. 4). SEM images of the as-made and calcined material are shown in Fig. 9. Both the as-made and calcined materials exhibit a plate-like morphology, similar to what has been observed in other materials prepared via topotactic condensation. EDS analysis of the calcined material found a Si/Al ratio of 9.8 ± 0.7. Since the starting gel had a Si/Al = 20, a large portion of the silica initially present was not incorporated in the final product. It is evident from 13C CPMAS NMR that the organic is occluded intact in as-made CIT-8P (Fig. 5). The state of the aluminium in CIT-8P and CIT-8 was studied using 27Al NMR and the spectra are shown in Fig. 3. In CIT-8P, only tetrahedral aluminium was observed. Calcination caused the formation of some octahedral aluminium (near 0 ppm). Deconvolution of the spectra shows that the calcined material contains ca. 21% octahedral aluminium.
 | ||
| Fig. 5 13C liquid NMR of the diquat with a 5-carbon chain (lower) compared to the 13C CPMAS NMR of as-made CIT-8P (upper). | ||
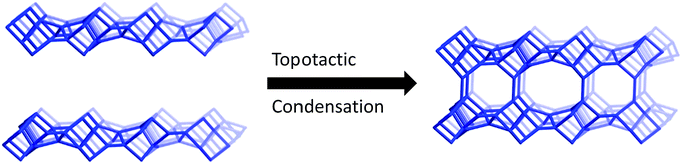
3.2 Mechanism of topotactic condensation
Topotactic condensations can occur with layered materials that contain terminal silanol groups (coordination of Si(OSi)3(OH), Q3 Si). With calcination, these terminal silanol groups condense, releasing water and forming Si–O–Si bonds. In this process, a 2-dimensional material is converted into a 3-dimensional framework material. The presence of silanol groups in CIT-8P was investigated by using 29Si MAS and CPMAS NMR of the as-made and calcined materials. 29Si CPMAS NMR was used on the as-made material in addition to MAS NMR to confirm the resonances (due poor signal to noise ratio with MAS NMR often found prior to calcination). The 29Si NMR spectra are shown in Fig. 7. In the calcined material, three resonances can be found at −113.8, −108.2 and −102.7 ppm. In accordance with the resonances reported for other heulandite materials, the peak at −113.8 ppm is assigned to Si(OSi)4 coordination environment, and the peaks at −108.2 and −102.7 ppm are assigned to Si(OAl)(OSi)3 coordination environments.21–24 The amount of Si(OAl)2(OSi)2 coordination present is very small, and deconvolution determined that the amount of silicon in this coordination is insignificant (see ESI†). When the 29Si MAS NMR of the calcined material was deconvoluted, the Si/Al ratio was found to be 9.8, in good agreement with the ratio found using EDS, further supporting the peak assignments (see ESI†). Both the as-made and calcined materials contain a resonance near −113.5 ppm that is attributed to Si(OSi)4, Q4 coordination environment as well as a resonance near −109 ppm that is attributed to Si(OAl)(OSi)3 coordination environment. The resonance in the as-made material near −103 ppm that largely disappears upon calcination is attributed to silanol groups (Si(OSi)3(OH)), or Q3 Si species that are abundant on the surface of the layered material as well as Si(OAl)(OSi)3 coordinated Si.25–27 The resonance at −103 ppm decreases significantly after calcination as the topotactic condensation (vide infra) causes the materials to loose surface silanols and form Si–O–Si bonds. Integration of the resonances in the as-made material from the 29Si MAS NMR shows that ca. 30% of the Si is in a Q3 environment. For the ideal case of a pure-silica layered precursor, 22.22% of the Si atoms should be in this environment (see ESI†); however, the presence of aluminum also complicates the spectrum, so finding a result close to the ideal case supports the assignments. All of the results from 29Si NMR are consistent with a topotactic condensation process occurring as Q3 Si disappears upon calcination. | ||
| Fig. 7 29Si NMRs of CIT-8P by CPMAS (lower), MAS NMR (middle) compared to the calcined material (upper). | ||
CIT-8P was investigated by variable temperature PXRD (Fig. 10) in order to elucidate the structural changes caused by the condensation. Two main events are observed in the variable temperature PXRD. The first is that the low angle peak starts to shift to a higher angle around 280 °C. When the PXRD data are combined with the TGA results (Fig. 6), it is apparent that the observed PXRD peak shift coincides with the first mass loss. The second main event observed in the variable temperature PXRD is a dramatic change between 430 and 480 °C, where the low angle peak either disappears or quickly shifts to become the main peak in HEU. In the same temperature range, the TGA results show a mass loss as the final HEU framework is formed.
 | ||
| Fig. 10 Variable temperature PXRD of CIT-8P forming CIT-8. The low angle peak observed in higher traces is an instrument artifact due to the high temperature chamber. | ||
The structural changes caused by the condensation reaction were elucidated by monitoring the changes of the PXRD patterns from as-made material transformed via calcination. It is clear from the variable-temperature PXRD that peak positions for h0l reflections remain during heating, while the peak positions for the hkl (k ≠ 0) reflections are shifted to higher 2θ angles (i.e., lower d-spacing). This result indicates that the condensation occurs along the b-axis of the HEU structure and that the a and c axes are intact in the layered material. The as-made material was further studied using the RED technique. Three-dimensional RED tomography data (see Fig. 11) shows diffraction streaks between diffraction spots along b-axis clearly, without a significant indication of diffraction streaks along a- and c-axes. These data indicate that in the as-made material there is some disorder in the b-direction, but no disorder in the a- and c-directions, consistent with ordered inorganic layers separated by a disordered organic layer. As the material condenses along the b-axis, both the 10- and 8 MRs are formed (see graphical depiction shown in Fig. 8). In the as-made material, the low angle peak corresponds to an interplanar spacing of ∼12.8 Å. As the organic begins to degrade in the material (evident by TGA), the spacing is shrunk by ∼3.9 Å until the final HEU framework is formed by topotactic condensation.
3.3 Titanium aluminosilicate HEU
In order to prepare a material with both Brønsted and Lewis acidity, the fluoride preparation was modified by adding titanium, with the final gel molar ratio: 1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05Al
0.05Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02Ti
0.02Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5ROH
0.5ROH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5HF
0.5HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4H2O. This synthesis composition also produced the layered material CIT-8P, evident by PXRD, and the HEU framework after calcination. After calcination, the state of the titanium was studied using UV-VIS spectroscopy, and the results are shown in Fig. 12 compared to the aluminosilicate material. The band observed near 220 nm is consistent with tetrahedral titanium, indicating framework incorporation, demonstrating the synthesis of titanium aluminosilicate HEU.
4H2O. This synthesis composition also produced the layered material CIT-8P, evident by PXRD, and the HEU framework after calcination. After calcination, the state of the titanium was studied using UV-VIS spectroscopy, and the results are shown in Fig. 12 compared to the aluminosilicate material. The band observed near 220 nm is consistent with tetrahedral titanium, indicating framework incorporation, demonstrating the synthesis of titanium aluminosilicate HEU.
3.4 Hydroxide-mediated reactions
The diquat containing a 4-carbon chain was used in hydroxide-mediated syntheses in an attempt to produce HEU-type zeolites. Various Si/Al ratios were tested, both with and without seeds, and the conditions and experimental results are shown in Table 3. It was found that HEU-type zeolites would not form above a gel ratio of Si/Al = 5, even when seeds were present; CIT-7 was found as a product instead. At a gel ratio of both Si/Al = 4.15 and 5, CIT-8H was found to form with and without seeds. Adding CIT-8H seeds at a gel Si/Al = 5 significantly lowered the synthesis time from greater than 40 days to only 24 days. When seeds of CIT-7 were added at this gel ratio, CIT-7 forms instead. The CIT-8H formed from a gel with Si/Al = 5 (product Si/Al ratio of 6.4 ± 0.3) was found to be stable in proton form and have a micropore volume of 0.10 cm3 g−1 (Fig. 4). From TGA analysis of the as-made material there was 8.4 wt% organic occluded that was found to be intact by 13C CPMAS NMR. SEM images of the calcined material are shown in Fig. 9 and the material exhibits a plate like morphology, similar to that observed for the material prepared via topotactic condensation. The 27Al MAS NMR of the material in proton form (Fig. 13) showed that all of the aluminium was in tetrahedral coordination, indicating framework incorporation. The material prepared in hydroxide media exhibits good thermal stability as it was stable to calcination, ammonium exchange and a subsequent calcination to convert it to proton form.4. Conclusions
Diquaternary imidazolium compounds have been found to lead to a wide range of microporous material frameworks depending on the inorganic conditions. The synthesis of high-silica HEU-type zeolite (CIT-8) can occur via a topotactic condensation from a layered precursor (CIT-8P) as well as via direct synthesis in hydroxide media (CIT-8H). CIT-8 has a significantly higher Si/Al ratio than previously reported materials with the HEU framework. The ability to prepare HEU-type zeolites at a higher silica composition and in the absence of trace impurities commonly encountered in natural materials opens up possibilities for use in new applications, such as catalysis. The inclusion of additional heteroatoms in the HEU framework, with Ti4+ as an example, demonstrates flexibility in the inorganic elements included in the inorganic framework and opens up this framework to additional applications, such as combined Brønsted and Lewis acidity.Acknowledgements
We would like to thank Thomas Rea (Chevron Energy Technology Company) for collecting the RED data and Dr Stacey Zones (Chevron Energy Technology Company) for insightful discussions. Also, we would like to thank Dr Sonjong Hwang (Caltech) for assistance with solid-state NMR collection and interpretation. Chevron Energy Technology Company provided funding for this work. J.E.S. would like to thank the NDSEG for their support through a fellowship. PQ Corporation is thanked for providing us with sodium silicate.Notes and references
- C. Colella and W. S. Wise, Microporous Mesoporous Mater., 2014, 189, 4–10 CrossRef CAS PubMed
.
- B. Yilmaz and U. Müller, Top. Catal., 2009, 52, 888–895 CrossRef CAS PubMed
.
-
G. Gottardi and E. Galli, Natural zeolites, Springer-Verlag, 1985 Search PubMed
.
- Three-letter framework type codes (boldface capital letters) for all zeolites mentioned in the text are given in parentheses.
- R. Simancas, D. Dari, N. Velamazán, M. T. Navarro, A. Cantín, J. L. Jordá, G. Sastre, A. Corma and F. Rey, Science, 2010, 330, 1219–1222 CrossRef CAS PubMed
.
- C. Baerlocher and L. B. Mccusker, Database of Zeolite Structures, http://www.iza-structure.org/databases/ Search PubMed.
- D. Zhao, K. Cleare, C. Oliver, C. Ingram, D. Cook, R. Szostak and L. Kevan, Microporous Mesoporous Mater., 1998, 21, 371–379 CrossRef CAS
.
-
F. Related, A. Data and P. E. J. Meros, US Pat. 4,503,023, 1985
.
- O. Chiyoda and M. E. Davis, Microporous Mesoporous Mater., 1999, 32, 257–264 CrossRef CAS
.
- S. Satokawa and K. Itabashi, Microporous Mater., 1997, 8, 49–55 CrossRef CAS
.
- C. D. Williams, Chem. Commun., 1997, 2113–2114 RSC
.
- D. Zhao, L. Kevan and R. Szostak, Zeolites, 1997, 19, 366–369 CrossRef CAS
.
- A. Jayaraman, A. J. Hernandez-maldonado, R. T. Yang, D. Chinn, C. L. Munson and D. H. Mohr, Chem. Eng. Sci., 2004, 59, 2407–2417 CrossRef CAS PubMed
.
- P. E. Hathaway and M. E. Davis, Catal. Lett., 1990, 5, 333–347 CrossRef CAS
.
- D. Zhang, P. Oleynikov, S. Hovmöller and X. Zou, Z. Kristallogr., 2010, 225, 94–102 CrossRef CAS
.
- W. Wan, J. Sun, J. Su, S. Hovmöller and X. Zou, J. Appl. Crystallogr., 2013, 46, 1863–1873 CAS
.
- J. E. Schmidt, D. Xie, T. Rea and M. E. Davis, Chem. Sci., 2015, 6, 1728–1734 RSC
.
- A. Jackowski, S. I. Zones, S.-J. Hwang and A. W. Burton, J. Am. Chem. Soc., 2009, 131, 1092–1100 CrossRef CAS PubMed
.
- Y. X. Wang, H. Gies, B. Marler and U. Müller, Chem. Mater., 2005, 17, 43–49 CrossRef CAS
.
- H. Gies, U. Müller, B. Yilmaz, T. Tatsumi, B. Xie, F. Xiao, X. Bao, W. Zhang and D. De Vos, Chem. Mater., 2011, 23, 2545–2554 CrossRef CAS
.
- A. Godelitsas, D. Charistos, A. Tsipis, C. Tsipis, A. Filippidis, C. Triantafyllidis, G. Manos and D. Siapkas, Chemistry, 2001, 7, 3705–3721 CrossRef CAS
.
- A. Godelitsas, D. Charistos, C. Tsipis, P. Misaelides, A. Filippidis and M. Schindler, Microporous Mesoporous Mater., 2003, 61, 69–77 CrossRef CAS
.
- R. L. Ward and H. L. McKague, J. Phys. Chem., 1994, 98, 1232–1237 CrossRef CAS
.
- E. Lippmaa, M. Magi, A. Samoson, M. Tarmak and G. Engelbardtlb, J. Am. Chem. Soc., 1981, 103, 4992–4996 CrossRef CAS
.
- B. Yang, H.-H. Wu and P. Wu, J. Phys. Chem. C, 2014, 140929194213007 Search PubMed
.
- J. Ruan, P. Wu, B. Slater, Z. Zhao, L. Wu and O. Terasaki, Chem. Mater., 2009, 21, 2904–2911 CrossRef CAS
.
- P. Wu, J. Ruan, L. Wang, L. Wu, Y. Wang, Y. Liu, W. Fan, M. He, O. Terasaki and T. Tatsumi, J. Am. Chem. Soc., 2008, 130, 8178–8187 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Deconvolution of 29Si NMR spectrum and surface silanol calculation. See DOI: 10.1039/c5ta02354h |
| This journal is © The Royal Society of Chemistry 2015 |