Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improved oxide-ion conductivity of NdBaInO4 by Sr doping†
Kotaro
Fujii
a,
Masahiro
Shiraiwa
a,
Yuichi
Esaki
b,
Masatomo
Yashima
*a,
Su Jae
Kim
c and
Seongsu
Lee
c
aDepartment of Chemistry and Materials Science, Graduate School of Science and Engineering, Tokyo Institute of Technology, 2-12-1-W4-17, O-okayama, Meguro-ku, Tokyo 152-8551, Japan
bDepartment of Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology, 2-12-1-W4-17, O-okayama, Meguro-ku, Tokyo 152-8551, Japan
cNeutron Science Division, Research Reactor Utilization Department, Korea Atomic Energy Research Institute, 1045 Daedeok-daero, Yuseong-gu, Daejeon 305-353, Korea
First published on 20th April 2015
Abstract
The oxide-ion conductivity of NdBaInO4 has been increased by Sr doping. Nd0.9Sr0.1BaInO3.95 showed the highest electrical conductivity among Nd1−xSrxBaInO4−x/2 (x = 0.0, 0.1, 0.2, and 0.3). The oxide-ion conductivity σion of Nd0.9Sr0.1BaInO3.95 (σion = 7.7 × 10−4 S cm−1) is about 20 times higher than that of NdBaInO4 (σion = 3.6 × 10−5 S cm−1) at 858 °C, and the activation energy of oxide-ion conduction is a little lower for Nd0.9Sr0.1BaInO3.95 (0.795(10) eV) than that for NdBaInO4 (0.91(4) eV). The structure analysis based on neutron powder diffraction data revealed that the Sr exists at the Nd site and oxygen vacancies are observed in Nd0.9Sr0.1BaInO3.95. This result indicates that the increase of the oxide-ion conductivity is mainly due to the increase of the carrier concentration. The bond valence-based energy landscape indicated two-dimensional oxide-ion diffusion in the (Nd,Sr)2O3 unit on the bc-plane and a decrease of the energy barrier by the substitution of Nd with Sr cations.
Introduction
Oxide-ion conductors, which include pure ionic conductors and mixed oxide-ion and electronic conductors, attract significant interest because of their varied uses in oxygen separation membranes and cathodes for solid-oxide fuel cells (SOFCs).1 The oxide-ion conductivity is strongly dependent on the crystal structure and particularly the defects. At present, several structures, such as fluorites,2,3 perovskites,2,4 K2NiF4,2,5 mellilites,2,6 and apatites,2,7 are known to show high oxide-ion conductivities. Further development of oxide-ion conductors involves investigating materials with new types of structures. Recently, we have discovered a new structure family of oxide-ion conductors based on NdBaInO4, a monoclinic P21/c perovskite-related phase with a layered structure.8 In this study, we have successfully improved the oxide-ion conductivity of NdBaInO4 by Sr doping at the Nd site, which aims to increase the concentration of oxygen vacancies (i.e., carriers for the oxide-ion conduction) and to lower the activation energy by exchanging Nd3+ with the larger Sr2+ cation. This study reports on the electrical conductivity and the crystal structure of Sr-doped NdBaInO4. The electrical conductivity of NdBaInO4 was also investigated again for comparison.Experimental section
Synthesis and characterization of the chemical composition
Nd1−xSrxBaInO4−x/2 (x = 0.0, 0.1, 0.2, 0.3, and 0.4) compounds were synthesized by solid-state reactions. Nd2O3 (99.95% purity) and BaCO3 (99.9% purity) from Kanto Chemical Co. Inc., SrCO3 and In2O3 (both 99.9% purity) from Kojundo Chemical Lab. Co., Ltd. were accurately weighed in 1−x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cation molar ratios, and they were mixed and ground using a planetary ball mill (Fritsch, P7) for 30 min. The mixtures were calcined at 1000 °C for 8 h in air for decarbonization. Then, the calcined mixtures were milled again for 30 min and uniaxially pressed into pellets at about 50 MPa. These pellets were sintered in air at 1400 °C for 24 h.
1 cation molar ratios, and they were mixed and ground using a planetary ball mill (Fritsch, P7) for 30 min. The mixtures were calcined at 1000 °C for 8 h in air for decarbonization. Then, the calcined mixtures were milled again for 30 min and uniaxially pressed into pellets at about 50 MPa. These pellets were sintered in air at 1400 °C for 24 h.
The cation ratio of Nd0.9Sr0.1BaInO3.95 was confirmed by inductively coupled plasma optical emission spectrometry (ICP-OES) as Nd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sr
Sr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba
Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In = 0.919(8)
In = 0.919(8)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0996(9)
0.0996(9)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.992(3)
0.992(3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.989(9), which agreed with the average chemical composition of the starting mixture, Nd
0.989(9), which agreed with the average chemical composition of the starting mixture, Nd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sr
Sr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba
Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In = 0.9
In = 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 within 3σ. Here, the σ is the standard deviation of the measured chemical composition and the number in the parenthesis is the last digit of σ.
1 within 3σ. Here, the σ is the standard deviation of the measured chemical composition and the number in the parenthesis is the last digit of σ.
Thermogravimetric analyses (TGA) of NdBaInO4 and Nd0.9Sr0.1BaInO3.95 in Ar flow (50 mL min−1) were conducted using a Bruker-AXS TG-DTA2020SA instrument with heating and cooling rates of 10 °C min−1. The TG measurements were repeated three times to confirm the reproducibility and minimize artefacts from adsorbed species such as water.
Electrical conductivity measurements
The electrical conductivities of Nd1−xSrxBaInO4−x/2 (x = 0.0, 0.1, 0.2, and 0.3) were measured using a DC 4-probe method using sintered pellets (ca. 4.4 mm ϕ × 30 mm with densities in the range of 90–95% of theoretical density) with Pt electrodes over the temperature range from 400 °C to 1200 °C in air. The oxygen partial pressure P(O2) dependence of the electrical conductivities of NdBaInO4 and Nd0.9Sr0.1BaInO3.95 was measured at 858 °C using N2/H2, N2/CO2, and N2/O2 gas mixtures. The P(O2) was monitored by an oxygen sensor that was set close to the sample. The oxide-ion conductivities of NdBaInO4 and Nd0.9Sr0.1BaInO3.95 were measured from 610 °C to 1100 °C under P(O2) = 3.6 ± 2.6 × 10−17 atm for NdBaInO4 and P(O2) = 8.8 ± 6.2 × 10−14 atm for Nd0.9Sr0.1BaInO3.95.Neutron and synchrotron X-ray diffraction measurements
Synchrotron X-ray powder diffraction (XRPD) measurements were conducted using a Debye–Scherrer camera with an imaging plate on beam line BL19B2 at SPring-8 (27 °C; wavelength = 0.399662(2) Å).9 Room temperature time-of-flight (TOF) neutron powder diffraction (NPD) measurements (24 °C) were performed using the iMATERIA diffractometer of the J-PARC facility, Tokai, Japan.10 High-temperature angle dispersive-type NPD measurements (800 °C using a vacuum furnace; wavelength = 1.83432(4) Å) were performed using a neutron powder diffractometer HRPD installed at HANARO reactor, KAERI, Korea.11Results and discussion
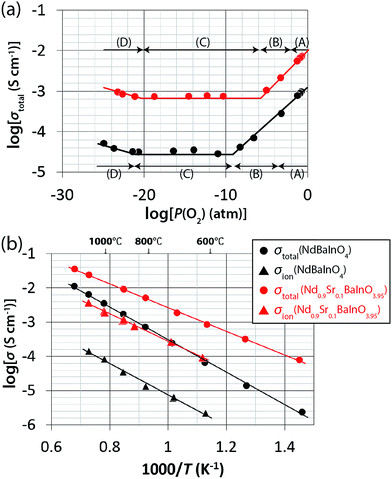
XRPD patterns of Nd1−xSrxBaInO4−x/2 (x = 0.0, 0.1, 0.2, 0.3, and 0.4) identified the final products to be the monoclinic P21/c NdBaInO4 phase, except x = 0.4, which showed a different XRPD pattern with additional peaks, indicating possible saturation of the dopant within the NdBaInO4 structure (Fig. 1a). We found that the total electrical conductivity of Nd0.9Sr0.1BaInO3.95 is higher than that of NdBaInO4, Nd0.8Sr0.2BaInO3.9, and Nd0.7Sr0.3BaInO3.85 (Fig. 1b). Therefore, we focused on the Nd0.9Sr0.1BaInO3.95 composition for further detailed studies.Fig. 2a shows the P(O2) dependence of the total electrical conductivity σtotal of NdBaInO4 and Nd0.9Sr0.1BaInO3.95 at 858 °C. With decreasing P(O2), the σtotal decreased in the high P(O2) range (region [A] and [B] in Fig. 2a), was constant in the intermediate P(O2) range (region [C] in Fig. 2a) and increased in the low P(O2) range (region [D] in Fig. 2a). The slope of log(σtotal) versus log(P(O2)) of NdBaInO4 in the P(O2) range from 5.9 × 10−4 to 2.0 × 10−1 atm is 0.215(2) and of Nd0.9Sr0.1BaInO3.95 in the P(O2) range from 5.7 × 10−3 to 2.0 × 10−1 atm is 0.216(6), which indicates that these materials show p-type conductivity in region [A], and mixed oxide-ion and hole conduction in region [B]. The constant conductivities independent of P(O2) in region [C] indicate that both NdBaInO4 and Nd0.9Sr0.1BaInO3.95 materials show pure oxide-ion conduction.
Fig. 2b shows Arrhenius plots of the total electrical conductivity σtotal (circles in Fig. 2b) and oxide-ion conductivity σion (triangles in Fig. 2b) of NdBaInO4 (black) and Nd0.9Sr0.1BaInO3.95 (red). Over the entire temperature range, the total electrical conductivity σtotal and oxide-ion conductivity σion of Nd0.9Sr0.1BaInO3.95 are higher than those of NdBaInO4. For example, the σtotal and σion of Nd0.9Sr0.1BaInO3.95 at 858 °C were 7.3 × 10−3 S cm−1 and 7.7 × 10−4 S cm−1, respectively, are higher than those of NdBaInO4 1.0 × 10−3 S cm−1 and 3.6 × 10−5 S cm−1, respectively. The hole conductivities of Nd0.9Sr0.1BaInO3.95 and NdBaInO4 were calculated to be 6.5 × 10−3 and 9.6 × 10−4 S cm−1, respectively, at 858 °C. The activation energies of total, oxide-ion, and hole conductivities of Nd0.9Sr0.1BaInO3.95 were 0.685(7), 0.795(10), and 0.673 eV, which are lower than those of NdBaInO4 (0.952(13), 0.91(4), and 0.953 eV). Therefore, 10 mol% Sr doping into NdBaInO4 improves the oxide-ion conductivity and lowers its activation energy.
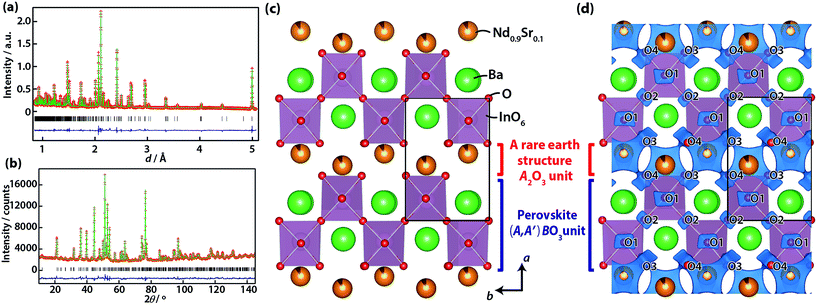
To investigate the structure changes in NdBaInO4 by 10 mol% Sr doping, Rietveld analysis was conducted for Nd0.9Sr0.1BaInO3.95 based on the synchrotron XRPD and NPD data using RIETAN-FP12 and Z-Code.13 Nd0.9Sr0.1BaInO3.95 is isostructural with NdBaInO4 (space group P21/c) and has seven independent sites at the general position, Nd, Ba, In, O1, O2, O3, and O4 (Table 2).8 The site preference of Sr was investigated in a preliminary analysis that gave the best reliable factors, Rwp and RB, in the case that Sr exists at the Nd site (Table S2 in (ESI†)). Here, Rwp is the weighted reliability factor of profile intensity and RB is the reliability factor based on integrated intensities. Therefore, the occupancy factors were fixed to g(Nd,Nd) = 0.9 and g(Sr,Nd) = 0.1 in the final refinement. Here, g(Y,X) represents the occupancy factor of atom Y at the X site. The refinement of the occupancy factors of the oxygen atoms using common values for all oxygen atoms yields 0.9842(10), which clearly indicates the existence of oxygen vacancies. The value agrees with the expected value of 0.9875 calculated from the charge balance. In the final refinement, the occupancy factors of oxygen atoms were fixed to 0.9875. The TGA of Nd0.9Sr0.1BaInO3.95 showed 0.18% weight loss between 50 and 800 °C, which corresponds to δ = 0.05 of Nd0.9Sr0.1BaInO3.95−δ (Fig. 3). Here, (0.05 + δ) is the amount of oxygen vacancies. Thus, the occupancy factors of oxygen atoms were fixed to 0.975 for the high-temperature (800 °C) data. The final Rietveld patterns are shown in Fig. 4a and b. The final refined atomic coordinates are shown in Table 1 for the TOF neutrons data and Table S1 in the ESI† for the synchrotron X-ray and the angle dispersive type neutron data.
| Source and facility | TOFa Neutron iMATERIA, J-PARC | Synchrotron BL19B2, SPring-8 | Neutron HRPD, HANARO | Ref. 8 |
|---|---|---|---|---|
| a TOF: Time-of-Flight. | ||||
| Chemical formula | Nd0.9Sr0.1BaInO3.95 | Nd0.9Sr0.1BaInO3.95 | Nd0.9Sr0.1BaInO3.90 | NdBaInO4.00 |
| Formula weight | 453.92 | 453.92 | 453.20 | 460.39 |
| Temperature / °C | 24 | 27 | 800 | 20 |
| Wavelength / Å | Time of flight (d = 0.494–5.223 Å) | 0.399662(2) | 1.83432(4) | |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c | P21/c | P21/c |
| a / Å | 9.106468(17) | 9.10285(12) | 9.2060(17) | 9.09538(3) |
| b / Å | 6.050490(11) | 6.04769(5) | 6.0999(11) | 6.04934(2) |
| c / Å | 8.268786(19) | 8.26670(9) | 8.2984(17) | 8.25620(2) |
| β / Å | 103.40613(14) | 103.3924(9) | 103.057(12) | 103.4041(3) |
| Unit-cell volume / Å3 | 443.184(2) | 442.716(8) | 453.95(15) | 441.89(2) |
| Z | 4 | 4 | 4 | 4 |
| Calculated density / Mg m−3 | 6.81 | 6.81 | 6.64 | 6.92 |
| R wp | 0.0458 | 0.0230 | 0.0362 | |
| R p | 0.0334 | 0.0150 | 0.0280 | — |
| Goodness of fit | 2.994 | 1.000 | 1.860 | — |
| R B | 0.0534 | 0.0139 | 0.0297 | — |
| R F | 0.0295 | 0.0117 | 0.0156 | — |
| Site label X | Atom Y | g(Y,X)a | x | y | z | U (Å2) |
|---|---|---|---|---|---|---|
| a g(Y,X): occupancy factor of atom Y at the X site. b Atomic displacement parameter. c Equivalent isotropic atomic displacement parameter. d U ij : anisotropic atomic displacement parameter. | ||||||
| Nd | Nd | 0.9 | 0.45269(5) | 0.74731(11) | 0.10734(6) | 0.00819 (Ueq)c |
| Sr | 0.1 | |||||
| Ba | Ba | 1 | 0.14825(7) | 0.25034(18) | 0.0328(11) | 0.00933 (Ueq) |
| In | In | 1 | 0.83211(9) | 0.2545(2) | 0.20649(14) | 0.0030(19) |
| O1 | O | 0.9875 | 0.18155(8) | 0.80285(9) | 0.04782(12) | 0.01422 (Ueq) |
| O2 | O | 0.9875 | 0.98669(11) | 0.98872(17) | 0.26951(12) | 0.00988 (Ueq) |
| O3 | O | 0.9875 | 0.38341(9) | 0.5429(13) | 0.32909(11) | 0.01730 (Ueq) |
| O4 | O | 0.9875 | 0.65046(8) | 0.50812(15) | 0.12937(11) | 0.01549 (Ueq) |
 | ||
| Fig. 3 TGA curve of Nd0.9Sr0.1BaInO3.95−δ measured in Ar. This figure shows the second cycle (first and third cycles are shown in the ESI†). The green dash lines indicate the δ of Nd0.9Sr0.1BaInO3.95−δ. The weight loss from 50 to 800 °C was 0.18 wt%, which corresponds to the increase in the oxygen vacancy content δ = +0.05. | ||
Comparing the unit-cell parameters between 24 °C and 800 °C, the a-, b- and c-axes increased and the β-angle decreased with increasing temperature. The average thermal expansion coefficients between 24 °C and 800 °C were found to be αa = 1.23(4) × 10−5 K−1, αb = 1.07(3) × 10−5 K−1, αc = 0.72(4) × 10−5 K−1, αβ = −3.73(17) × 10−5 K−1, and ![[small alpha, Greek, macron]](https://www.rsc.org/images/entities/char_e0c2.gif) = 1.06(2) × 10−5 K−1 (the definition of these coefficients are described in section D of the ESI†). These average thermal expansion coefficients of Nd0.9Sr0.1BaInO3.95 are similar to those of NdBaInO4 between 20 °C and 1000 °C (αa = 1.42(2) × 10−5 K−1, αb = 1.176(14) × 10−5 K−1, αc = 0.77(3) × 10−5 K−1, αβ = −3.81(4) × 10−5 K−1, and
= 1.06(2) × 10−5 K−1 (the definition of these coefficients are described in section D of the ESI†). These average thermal expansion coefficients of Nd0.9Sr0.1BaInO3.95 are similar to those of NdBaInO4 between 20 °C and 1000 °C (αa = 1.42(2) × 10−5 K−1, αb = 1.176(14) × 10−5 K−1, αc = 0.77(3) × 10−5 K−1, αβ = −3.81(4) × 10−5 K−1, and ![[small alpha, Greek, macron]](https://www.rsc.org/images/entities/char_e0c2.gif) = 1.176(15) × 10−5 K−1). There was an anisotropy observed in the thermal expansion. The average thermal expansion coefficients are similar for the a- and b-axes, whereas that of the c-axis is lower than the others. The average linear thermal expansion coefficients
= 1.176(15) × 10−5 K−1). There was an anisotropy observed in the thermal expansion. The average thermal expansion coefficients are similar for the a- and b-axes, whereas that of the c-axis is lower than the others. The average linear thermal expansion coefficients ![[small alpha, Greek, macron]](https://www.rsc.org/images/entities/char_e0c2.gif) of Nd0.9Sr0.1BaInO3.95 (1.06(2) × 10−5 K−1) and NdBaInO4 (1.176(15) × 10−5 K−1) are close to that of yttria stabilized zirconia (YSZ), which is favourable for using this material as a cathode in SOFC applications. The average thermal expansion coefficients of 3 and 8 mol% Y2O3–ZrO2 between 20 and 1000 °C were reported to be 1.08 × 10−5 and 1.05 × 10−5 K−1, respectively.14
of Nd0.9Sr0.1BaInO3.95 (1.06(2) × 10−5 K−1) and NdBaInO4 (1.176(15) × 10−5 K−1) are close to that of yttria stabilized zirconia (YSZ), which is favourable for using this material as a cathode in SOFC applications. The average thermal expansion coefficients of 3 and 8 mol% Y2O3–ZrO2 between 20 and 1000 °C were reported to be 1.08 × 10−5 and 1.05 × 10−5 K−1, respectively.14
The crystal structure of Nd0.9Sr0.1BaInO3.95 at 24 °C comprises the A rare earth structure A2O3 ((Nd,Sr)2O3) and the perovskite (A,A′)BO3 ((Nd,Sr)2/8Ba6/8InO3) units (Fig. 4c) which belongs to the same structural family as NdBaInO4.8 Here, A and A′ are relatively large cations and B is a smaller cation. The unit-cell volume at 24 °C of Nd0.9Sr0.1BaInO3.95 (443.184(2) Å3) is slightly larger than that of NdBaInO4 (441.8905(3) Å3). The larger volume is ascribed to the larger ionic radius15 of Sr2+ (1.21 Å for coordination number (CN) of 7) than that of Nd3+ (1.046 Å for CN = 7). The calculated bond valence sums (BVSs)16 from the bond lengths are 1.77 for Ba, 2.85 for (Nd0.9Sr0.1) and 2.99 for In sites in Nd0.9Sr0.1BaInO3.95. These values are consistent with their formal charges 2, 2.9, and 3, respectively, which indicates the validity of the refined crystal structure of Nd0.9Sr0.1BaInO3.95.
As described above, Nd0.9Sr0.1BaInO3.95 contains oxygen vacancies, while there are no significant oxygen vacancies within the 3σ of refined occupancy in NdBaInO4 at room temperature, where σ is the estimated standard deviation.8 Considering that Nd0.9Sr0.1BaInO3.95 has a much higher oxide-ion conductivity than NdBaInO4, the dominant carrier for the oxide-ion conduction in Nd0.9Sr0.1BaInO3.95 is the oxygen vacancy. The activation energy of the oxide-ion conduction is a little lower for Nd0.9Sr0.1BaInO3.95 (0.795(10) eV) than that for NdBaInO4 (0.91(4) eV). The lower activation energy of Nd0.9Sr0.1BaInO3.95 is attributable to the larger bottleneck size for the oxide-ion diffusion in Nd0.9Sr0.1BaInO3.95 compared with NdBaInO4. TGA of NdBaInO4, and Nd0.9Sr0.1BaInO3.95 showed little weight loss (around 0.02%) above 600 °C. Therefore, the effect of the carrier concentrations on the activation energy is thought to be negligible.
Diffusion pathways of oxide ions in the crystal structure of Nd0.9Sr0.1BaInO3.95 and NdBaInO4 were investigated by the bond valence based energy (BVE)17 using the program 3DBVSMAPPER18 based on the crystal structure at 800 °C. The blue surfaces in Fig. 4d represent the isosurfaces where the BVE for an oxide ion is +1.6 eV. In this landscape, the most stable position (at O4) was set to 0 eV. BVE isosurfaces around O1 and O2 sites are localized, while those around O3 and O4 sites spread in the A rare earth structure A2O3 ((Nd,Sr)2O3) unit and are connected with each other along both the b- and c-axes. The lowest energy path for oxide-ion conduction in Nd0.9Sr0.1BaInO3.95 was found to be along the b-axis with an energy barrier of 1.42 eV. The energy barriers of the path along the a- and c-axes were calculated to be 2.72 and 1.47 eV for Nd0.9Sr0.1BaInO3.95. The paths along the b- and c-axes have similar energy barriers and the path along the a-axis has significantly higher energy barriers than the others. Thus, the oxide-ion conduction in Nd0.9Sr0.1BaInO3.95 would be two dimensional.
BVE barriers of Nd0.9Sr0.1BaInO3.95 have lower values than those of NdBaInO4 along the oxide-ion diffusion paths. The energy barriers along the a-, b- and c-axes calculated based on the crystal structures at 24 °C are 1.47, 2.88, and 1.69 eV for Nd0.9Sr0.1BaInO3.95 and 1.72, 3.95, and 2.01 for NdBaInO4. These results are consistent with the experimental data that showed that Nd0.9Sr0.1BaInO3.95 has a lower activation energy of oxide-ion diffusion than NdBaInO4. The highest BVE point along the possible oxide-ion diffusion path is surrounded by two (Nd or (Nd,Sr)) and one Ba cations, which forms a cation triangle bottleneck. The areas of the triangles were calculated to be 6.889(5) Å2 for NdBaInO4 and 6.911(3) Å2 for Nd0.9Sr0.1BaInO3.95. Thus, the substitution of Nd with Sr increases this bottleneck area, and hence, lowers the activation energy of oxide-ion conduction.
Conclusions
The oxide-ion conductivity has been increased and the activation energy of oxide-ion conduction has been lowered by the substitution of Nd with Sr cations in NdBaInO4. Nd0.9Sr0.1BaInO3.95 showed the highest electrical conductivity among Nd1−xSrxBaInO4−x/2 (x = 0.0, 0.1, 0.2 and 0.3). The oxide-ion conductivity σion of Nd0.9Sr0.1BaInO3.95 was 7.7 × 10−4 S cm−1 at 858 °C, which is higher than that of NdBaInO4 (σion = 3.6 × 10−5 S cm−1 at 858 °C). The crystal structure of Nd0.9Sr0.1BaInO3.95 has been analysed, and we have confirmed that Sr exists at the Nd site. Nd0.9Sr0.1BaInO3.95 contains oxygen vacancies, which were not observed for NdBaInO4 at room temperature. Thus, the increase of the oxide-ion conductivity is mainly attributed to the increase of the carrier concentration. BVE calculations indicated two-dimensional oxide-ion diffusion in the A2O3 ((Nd,Sr)2O3) unit on the bc-plane and a decrease of the energy barrier by the substitution of Nd with Sr cations.Acknowledgements
We thank Dr K. Osaka for assistance with the synchrotron diffraction experiments and Prof. T. Ishigaki for assistance with the neutron diffraction experiments. The synchrotron radiation experiments were conducted on BL19B2 at SPring-8 (Proposal no. 2013B1718, 2014A1510, 2014B1660, and 2014B1922) and on BL-4B2 of the Photon Factory (Proposal no. 2013G216, 2013G053, and 2014G508). The neutron-diffraction experiments were conducted on the diffractometer iMATERIA at J-PARC (Proposal no. 2013A0136, 2013B0178, 2014A0011, and 2014B0114) and on the diffractometer HRPD at HANARO (Proposal no. NB-HRPD/2013-000026, 27, 2014-0071, and 72). We thank the Center for Materials Analysis at O-okayama of Tokyo Institute of Technology for the ICP-OES measurements. This study was partially supported by a Grant-in-Aid for Scientific Research (KAKENHI, no. 24850009, 24246107, 24226016, 25630365, 15H02291) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by the Murata Science Foundation. Neutron experiments at HANARO were partially supported by the National Research Foundation of Korea under Contract NRF-2012M2A2A6004261. Travel cost for HANARO neutron experiments was partially supported by the Institute for Solid State Physics, The University of Tokyo (proposal no. 12725, 13679, 14643 and 14657), Japan Atomic Energy Agency, Tokai, Japan.Notes and references
- (a) J. B. Goodenough, Nature, 2000, 404, 821 CrossRef CAS PubMed; (b) J. B. Goodenough, Annu. Rev. Mater. Res., 2003, 33, 91 CrossRef CAS; (c) V. Thangadurai and W. Weppner, Ionics, 2006, 12, 81 CrossRef CAS PubMed; (d) D. J. L. Brett, A. Atkinson, N. P. Brandon and S. J. Skinner, Chem. Soc. Rev., 2008, 37, 1568 RSC; (e) L. Malavasi, C. A. J. Fisher and M. S. Islam, Chem. Soc. Rev., 2010, 39, 4370 RSC; (f) S. Y. Istomin and E. V. Antipov, Russ. Chem. Rev., 2013, 82, 686 CrossRef PubMed; (g) A. B. Muñoz-García, A. M. Ritzmann, M. Pavone, J. A. Keith and E. A. Carter, Acc. Chem. Res., 2014, 47, 3340 CrossRef PubMed.
- (a) J. C. Boivin and G. Mairesse, Chem. Mater., 1998, 10, 2870 CrossRef CAS; (b) J. A. Kilner and M. Burriel, Annu. Rev. Mater. Res., 2014, 44, 365 CrossRef CAS PubMed.
- (a) M. Mogensen, D. Lybye, N. Bonanos, P. V. Hendriksen and F. W. Poulsen, Solid State Ionics, 2004, 174, 279 CrossRef CAS PubMed; (b) M. Yashima, Solid State Ionics, 2008, 179, 797 CrossRef CAS PubMed.
- (a) A. S. Bhalla, R. Guo and R. Roy, Mater. Res. Innovations, 2000, 4, 3 CrossRef CAS; (b) R. E. Schaak and T. E. Mallouk, Chem. Mater., 2002, 14, 1455 CrossRef CAS; (c) S. Stølen, E. Bakken and C. E. Mohn, Phys. Chem. Chem. Phys., 2006, 8, 428 RSC; (d) M. Yashima, J. Ceram. Soc. Jpn., 2009, 117, 1055 CrossRef CAS.
- (a) V. V. Kharton, A. P. Viskup, E. N. Naumovkh and F. M. B. Marques, J. Mater. Chem., 1999, 9, 2623 RSC; (b) S. Miyoshi, T. Furuno, O. Sangoanruang, H. Matsumoto and T. Ishihara, J. Electrochem. Soc., 2007, 154, B57 CrossRef CAS PubMed; (c) T. Ishihara, K. Nakashima, S. Okada, M. Enoki and H. Matsumoto, Solid State Ionics, 2008, 179, 1367 CrossRef CAS PubMed; (d) M. Yashima, M. Enoki, T. Wakita, R. Ali, Y. Matsushuta, F. Izumi and T. Ishihara, J. Am. Chem. Soc., 2008, 130, 2762 CrossRef CAS PubMed; (e) M. Yashima, N. Sirikanda and T. Ishihara, J. Am. Chem. Soc., 2010, 132, 2379 CrossRef PubMed; (f) M. Yashima, H. Yamada, S. Nuansaeng and T. Ishihara, Chem. Mater., 2012, 24, 4100 CrossRef CAS; (g) K. Kawamura, M. Yashima, K. Fujii, K. Omoto, K. Hibino, S. Yamada, J. R. Hester, M. Avdeev, P. Miao, S. Torii and T. Kamiyama, Inorg. Chem., 2015, 54, 3896 CrossRef CAS PubMed.
- X. Kuang, M. A. Green, H. Niu, P. Zajdel, C. Dicknson, J. B. Claridge, L. Jantsky and M. J. Rosseinsky, Nat. Mater., 2008, 7, 498 CrossRef CAS PubMed.
- (a) S. Nakayama and M. Sakamoto, J. Eur. Ceram. Soc., 1998, 18, 1413 CrossRef CAS; (b) M. Higuchi, Y. Masubuchi, S. Nakayama, S. Kikkawa and K. Kodaira, Solid State Ionics, 2004, 174, 73 CrossRef CAS PubMed; (c) R. Ali, M. Yashima, Y. Matsushita, H. Yoshioka, K. Ohoyama and F. Izumi, Chem. Mater., 2008, 20, 5203 CrossRef CAS.
- K. Fujii, Y. Esaki, K. Omoto, M. Yashima, A. Hoshikawa, T. Ishigaki and J. R. Hester, Chem. Mater., 2014, 26, 2488 CrossRef CAS.
- http://www.spring8.or.jp/wkg/BL19B2/instrument/lang-en/INS-0000000300/instrument_summary_view .
- T. Ishigaki, A. Hoshikawa, M. Yonemura, T. Morishima, T. Kamiyama, R. Oishi, T. Sakuma, Y. Tomota, M. Arai, M. Hayashi, K. Ebata, Y. Takano, H. Asano, Y. Takano and T. Kasao, Nucl. Instrum. Methods Phys. Res., Sect. A, 2009, 600, 189 CrossRef CAS PubMed.
- http://hanaro.kaeri.re.kr:444/NB/sub02/sub02.do .
- F. Izumi and K. Momma, Solid State Phenom., 2007, 130, 15 CrossRef CAS.
- (a) R. Oishi, M. Yonemura, Y. Nishimaki, S. Torii, A. Hoshikawa, T. Ishigaki, T. Morishima, K. Mori and T. Kamiyama, Nucl. Instrum. Methods Phys. Res., Sect. A, 2009, 600, 94 CrossRef CAS PubMed; (b) R. Oishi-Tomiyasu, M. Yonemura, T. Morishima, A. Hoshikawa, S. Torii, T. Ishigaki and T. Kamiyama, J. Appl. Crystallogr., 2012, 45, 299 CrossRef CAS.
- I. Yasuda and M. Hishinuma, Electrochemistry, 2000, 68, 526 CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751 CrossRef.
- R. E. Brese and M. O'Keeffe, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47, 192 CrossRef.
- (a) S. Adams, Solid State Ionics, 2000, 136, 1351 CrossRef; (b) S. Adams, Struct. Bonding, 2014, 158, 91 CrossRef; (c) M. Avdeev, M. Sale, S. Adams and R. P. Rao, Solid State Ionics, 2011, 225, 43 CrossRef PubMed.
- (a) M. Sale and M. Avdeev, J. Appl. Crystallogr., 2012, 45, 1054 CrossRef CAS; (b) M. Yashima, N. Kubo, K. Omoto, H. Fujimori, K. Fujii and K. Ohoyama, J. Phys. Chem. C, 2014, 118, 5180 CrossRef CAS; (c) M. Yashima, T. Sekikawa, D. Sato, H. Nakano and K. Omoto, Cryst. Growth Des., 2013, 13, 829 CrossRef CAS; (d) M. Yashima, Invited Review: Some recent developments in the atomic-scale characterization of structural and transport properties of ceria-based catalysts and ionic conductors, Catal. Today, 2015 DOI:10.1016/j.cattod.2015.03.034 , in press.
Footnote |
| † Electronic supplementary information (ESI) available: A document containing the crystallographic data of Nd0.9Sr0.1BaInO3.95, additional experimental information, and a crystallographic information file (CIF) of Nd0.9Sr0.1BaInO3.95. See DOI: 10.1039/c5ta01336d |
| This journal is © The Royal Society of Chemistry 2015 |