 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Low-activated Li-ion mobility and metal to semiconductor transition in CdP2@Li phases†
N.
Eckstein
a,
I.
Krüger
a,
F.
Bachhuber
b,
R.
Weihrich
b,
J. E.
Barquera-Lozada
c,
L.
van Wüllen
c and
Tom
Nilges
*a
aProfessur für Synthese und Charakterisierung innovativer Materialien, TU München, Lichtenbergstraße 4, 85747 Garching, Germany. E-mail: tom.nilges@lrz.tum.de; Fax: +49 89 289 13762; Tel: +49 89 289 13110
bFakultät für Chemie und Pharmazie, Universität Regensburg, Universitätsstraße 31, 93040 Regensburg, Germany. E-mail: richard.weihrich@chemie.uni-r.de; Fax: +49 941 943 4983; Tel: +49 941 943 4552
cProfessur für Chemische Physik funktioneller Materialien am Lehrstuhl CPM, Universität Augsburg, Universitätsstr. 1, 86159 Augsburg, Germany. E-mail: leo.van.wuellen@physik.uni-augsburg.de; Fax: +49 821 598 3227; Tel: +49 821 598 3356
First published on 17th February 2015
Abstract
Solids with high ion mobility are of broad interest for energy storage applications. New systems featuring low-activated ion mobility are important to improve the performance in such systems. Herein we report on a model system dealing with such improved properties. Li0.2CdP2 was synthesized from the elements, lithium as structure stabilizer and CdI2 as reaction promoters in sealed silica ampoules at 823 K. It crystallizes tetragonal, in space group I4122 (α-CdAs2 structure type), with lattice parameters a = 7.6691(8) Å, c = 4.4467(4) Å and V = 261.53(4) Å3. After 24 h of storage in air lithium ions can be removed in a spontaneous delithiation reaction resulting in Li(OH)·H2O formation on the surface of the crystals. Formed α′-CdP2 adopts the α-CdAs2 structure type. Both compounds consist of isolated cadmium atoms and helical 1∞[P−]-chains generating empty channels suitable to accommodate Li ions. The heavy atom structure was determined by X-ray diffraction methods while a full model including lithium was derived from a combined solid state NMR and quantum chemical calculation approach. An low activation barrier range in the order of 0.1 to 0.2 eV was determined by NMR spectroscopy pointing towards an extraordinary high Li mobility in Li0.2CdP2. Of course a Cd-based solid will have certain disadvantages like toxicity and mass for storage applications but substitution of Cd by suitable lighter elements can solve this issue.
Introduction
Phosphorus is an element with intriguing structure chemistry.1 In the case of Cd the reactivity is reasonable and binaries like CdP2,2–4 Cd3P2,5,6 CdP4,7 or Cd7P10 (ref. 8) exist. Cd is a prominent element used in photo-semiconductors like CdTe9 or Cd3Pn2 (with Pn = P, As)10,11 quantum dots for solar cell applications. While solar energy conversion applications are well established features for Cd pnictides and Cd chalcogenides due to their high electron mobility, the knowledge of ion mobility in Cd pnictides is rather small. Mixed ion and electron conducting compounds are potential candidates for batteries while pure ion conductors are also important as solid state electrolytes.12,13 Prominent solid ion conductors like garnet-type compounds show conductivities of 10−3 S cm−1 at room temperature and activation energies of 0.3 eV.14CdP2 exists in two different modifications. At room temperature, α-CdP2 crystallizes orthorhombic, in space group Pna21 (ref. 2–4) while a high temperature phase β-CdP2 occurs above 693 K, adopting the space group P41212.3 The prominent structural feature in both cases is a helical 1∞[P−]-chain coordinated to cadmium ions. A helical arsenic chain was detected in the compound α-CdAs2, featuring the space group I4122.15 An examination of the solid solution CdP2–CdAss reveals a continuous series up to CdAs2−xPx with x = 1.8, where all representatives crystallize tetragonal, thus adopting the α-CdAs2 structure type.16 It has been reported in ref. 16 that a cadmium polyphosphide in the α-CdAs2 structure type could not be realized. Unfortunately, this α-CdAs2 structure offers open channels ready for a coordination of mobile ions whereas such channels are not present in the known polymorphs.
Metal halides are prominent compounds to act as reaction promoters for the synthesis of kinetically stabilized materials and compounds adopting uncommon structure features.17 This feature, called mineralizer principle from now on, can also be used to selectively synthesize element allotropes of phosphorus.18 Recently, fibrous red phosphorus has been prepared by using CuCl2 (ref. 19) and orthorhombic black phosphorus can be realized by a SnI4/Sn combination.20–22 This synthesis principle was used to synthesize the present compounds.
Herein we report on the synthesis, reactivity, quantum chemical characterization and ion mobility of Li0.2CdP2 and α′-CdP2, featuring the end member of the solid solution CdAs2−xPx with x = 2.0. The mineralizer principle has successfully been adopted using CdI2 as mineralizer agent.
Experimental section
Synthesis
Li0.2CdP2 was synthesized by reacting Li (Rockwood lithium, 99.9%), Cd (Chempur, 99.9999%) and red P (Chempur, 99.999+%) in a molar ratio of 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.875
0.875![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in evacuated graphitized silica ampoules. CdI2 (25 mg), acting as mineralizer, was added to a total amount of 1 g starting materials. The mixture was heated up to 823 K within 12 h, kept for 7 days at this temperature, and slowly cooled down to room temperature within 12 h afterwards. Li0.2CdP2 was obtained as a black powder. Impurities of cadmium or iodide were never detected the final products.
2 in evacuated graphitized silica ampoules. CdI2 (25 mg), acting as mineralizer, was added to a total amount of 1 g starting materials. The mixture was heated up to 823 K within 12 h, kept for 7 days at this temperature, and slowly cooled down to room temperature within 12 h afterwards. Li0.2CdP2 was obtained as a black powder. Impurities of cadmium or iodide were never detected the final products.
Li0.2CdP2 can also be prepared from the elements in ideal ratios (Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cd
Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 0.2
P = 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) without a mineralizer, resulting in a microcrystalline black powder, but only with sparse crystals. The exchange of the mineralizer from CdI2 to another mineralizer like CuCl2 resulted in completely different products. Cd3P2 and crystals of LiP15 have been identified after phase analysis. Impurities of copper or chlorine were neither detected in Cd3P2 nor in LiP15 fractions.
2) without a mineralizer, resulting in a microcrystalline black powder, but only with sparse crystals. The exchange of the mineralizer from CdI2 to another mineralizer like CuCl2 resulted in completely different products. Cd3P2 and crystals of LiP15 have been identified after phase analysis. Impurities of copper or chlorine were neither detected in Cd3P2 nor in LiP15 fractions.
α′-CdP2 can be prepared from Li0.2CdP2 by a de-lithiation process upon exposing it to moist air. A white powder is formed on top of the needle-shaped Li0.2CdP2 crystals, after 24 h of exposure. After dissolution of the white powder in water Li2CO3 was found as main phase after removal of the solvent, substantiating the Li extraction from Li0.2CdP2. A full de-lithiation takes place after four weeks (Results and discussion section).
EDS and ICP analyses
Semi-quantitative EDS analysis was performed using a SEM 5900LV spectrometer (JEOL) scanning electron microscope equipped with an INCA energy dispersive microanalysis system (Oxford Instruments). An acceleration voltage of 30 kV was used for the measurements. Another system we used was a desktop scanning microscope JCM 6000 NeoScop™ (JEOL) equipped with an energy dispersive X-ray unit (JED 2200 JEOL). An acceleration voltage of 15 kV was applied for the measurements. The ICP analysis were performed using an Agilent Technologies (Santa Clara, CA) model 725 Series ICP OES instrument, equipped with an Echelle optics (model Vista/7XX ES), a monochromator (model Variant Liberty) and a CCD sensor (model Vista Pro 720/725_ES, resolution < 0.01 nm). Data analysis was done by the program ICP Expert II for Agilent 725 ES ICP OES.X-ray single crystal diffraction
Li0.2CdP2 was stored and treated in an argon filled glovebox, while α′-CdP2 was kept in air for 7 days and subsequently washed with water to remove Li(OH)·H2O on the surface. Single crystals of Li0.2CdP2 were sealed in a 0.3 mm glass capillary prior to the measurement. Data were collected on a Bruker APEX II diffractometer equipped with a D8 goniometer (MoKα radiation, λ = 0.71069 Å, graphite monochromator, rotating anode source) at 123 K. The frames were integrated with the Bruker SAINT software package23 using a narrow frame algorithm. Numerical absorption correction was done employing the SADABS routine.24 A suitable single crystal of α′-CdP2 was fixed on a glass capillary, and positioned on the goniometer head of an Oxford Xcalibur3 diffractometer (Sapphire 3 CCD detector; MoKα radiation, λ = 0.71073 Å, graphite monochromator). The Oxford CrysAlis RED software was used for data processing, including an empirical absorption correction with ABSPACK.25The space group I4122 was assigned on the basis of systematic absent reflections and the statistical analysis of the intensity distributions. The structure was solved using the superflip routine26 implemented in the Jana2006 program suite.27
X-ray powder diffraction
For the X-ray powder diffractometer analysis the sample kept under argon (Li0.2CdP2) and the sample on air (α′-CdP2) were ground and sealed in glass capillaries. Powder XRD data were collected with a STOE STADI P X-ray powder diffractometer equipped with a DECTRIS Mythen 1K Detector using CuKα1 radiation (λ = 1.54051 Å, curved Ge (111) monochromator). An external calibration was performed with Si (a = 5.43088 Å). Data were collected from 10.03° to 119.755° 2θ. The XRD pattern was fitted using the Rietveld routine implemented in the program Jana2006.27Electronic structure calculations
The first-principles calculations were carried out within the framework of DFT with exchange-correlation functionals in the generalized gradient approximation (GGA)28,29 according to Perdew–Burke–Enzerhof (PBE)30 and in the local density approximation according to Perdew and Zunger (LDA).31 Full geometry optimizations were executed with the Vienna Ab initio Simulation Package (VASP).32,33 All atomic site parameters and cell constants were allowed to fully relax with the conjugant gradient algorithm. The interactions between the ions and the electrons are described by the projector-augmented-wave (PAW) method34 with a cut-off energy of 500 eV. All calculations were performed in several subsequent steps with increasing k-grid meshes (depending on the size of the unit cell) between 2 × 2 × 2 and 12 × 12 × 12 to reach sufficient accuracy. A structure optimization was considered to be converged with a difference in total energy of less than 1 × 10−6 eV and a maximum Hellmann–Feynman force of 1 × 10−4 eV Å−1. The final values of the total energies of the investigated systems were obtained with energy differences (between last and second to last step) of less than 1 × 10−3 eV per formula unit.Solid state NMR
Experiments were performed on a Bruker Advance III spectrometer operating at 7 T with resonance frequencies of 116.6 MHz, 121.5 MHz and 300.13 MHz for 7Li, 31P and 1H, respectively. A Bruker 4 mm WVT MAS probe was used for the experiments. The Li0.2CdP2 sample was handled in a glovebox and dry N2 was employed for spinning and cooling the sample. Static 7Li NMR spectra were recorded in the temperature range 117 K < T < 561 K employing single pulse excitation and the solid echo pulse sequence for data acquisition. For the 7Li T1-NMR measurements, the inversion recovery sequence was used. The temperature was calibrated employing the chemical shift of Pb(NO3)2 as a chemical shift thermometer.35,3631P MAS NMR and 7Li MAS NMR spectra were recorded employing spinning speeds of 8–12 kHz using the Bruker system. Additional spectra at higher spinning speeds (up to 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz) for 1H and 31P were acquired on a Varian DMR2 NMR spectrometer operating at 11, 7 T using a Varian T3 MAS 1.6 mm NMR probe. Spectra are referenced as usual employing TMS for 1H, 1 M LiCl for 7Li and 85% H3PO4 for 31P.
000 Hz) for 1H and 31P were acquired on a Varian DMR2 NMR spectrometer operating at 11, 7 T using a Varian T3 MAS 1.6 mm NMR probe. Spectra are referenced as usual employing TMS for 1H, 1 M LiCl for 7Li and 85% H3PO4 for 31P.
Differential scanning calorimetry (DSC)
Finely ground samples of phase pure Li0.2CdP2 (8.8 mg) and α′-CdP2 (9.0 mg) were transferred to aluminum crucibles and were measured with a rate of 10 K min−1 using a Netzsch DSC 200 F3 Maja machine. H2O, In and Zn were used as standards for temperature calibration. All measurements were performed under N2 atmosphere. No thermal effect was found in the temperature range from 123 K to 373 K for Li0.2CdP2. α′-CdP2 cannot be transferred to α-CdP2 or β-CdP2 up to 673 K.DC conductivity measurements
Total electric conductivities of Li0.2CdP2 and α′-CdP2 have been measured using a homemade tool, which is capable to measure pressurized powdered samples (200 MPa, up to 2 mm height and 6 mm diameter) with a scale for measuring the height of the compressed powder. With a potentiostat (VMP-3, Biologic), a voltage of up to 10 V is applied to the compressed powder and the resulting current is measured. This unconventional procedure was necessary because a stable and dense pellet for impedance measurements could not be made from powdered samples in both cases. Temperature dependent conductivity measurements on single crystals are currently underway.Results and discussion
CdP2 shows polymorphism with two known polymorphs, a low temperature α-CdP2 adopting the space group Pna21 (ref. 2–4) and a high temperature β-phase stable above 693 K, crystallizing in space group P41212.3 A brief overview of the two CdP2 structures is given in Fig. 1a and b.The main difference is the arrangement of 1∞[P−]-chains parallel and perpendicular towards each other featuring alternating P–P bond lengths. α-CdAs2 contains a 1∞[As−]-chain (see Fig. 1c) with no bond length alteration and a slightly different arrangement of the Cd substructure than observed in α-CdP2. Surprisingly, a α-CdAs2-type CdP2 polymorph does not exist till now – a solid solution α-CdAs2−xPx has only been realized up to x = 1.8.16 We therefore started systematic investigations to close this gap to x = 0.2 and call this new phase α′-CdP2 from now on.
All our attempts to prepare α′-CdP2, either from the elements directly or by the mineralizer principle using CdI2 or CuCl2, failed. CdP4, Cd3P2 and β-CdP2 were found instead. If lithium was added in small portions as starting material, Li0.2CdP2, adopting the α-CdAs2 structure type, was found.
Structural characterization and elemental analysis of Li0.2CdP2
Single crystals of Li0.2CdP2, separated from a batch with the nominal composition x = 0.125, have been used to perform a single crystal X-ray structure determination. An executed neutron scattering experiment of microcrystalline Li0.2CdP2 has also been performed, but due to the large neutron absorption cross section of σa = 2520b, it was not possible to derive any structural parameters from that data.Li0.2CdP2 crystallizes tetragonal, isostructural to α-CdAs2, in space group I4122, with Z = 4. The lattice parameters (a = 7.6691(8) Å, c = 4.4467(4) Å and V = 261.53(4) Å3; [c/a = 0.5798]) are taken from the powder analysis. Displacement parameters of Cd are slightly anisotropic, pointing towards a possible symmetry reduction. We found no evidences for a loss of the 41 symmetry which would directly lead to a bond length variation within the P substructure. Also a loss of centering was checked but could not be confirmed. The selected symmetry was crosschecked by X-ray powder phase analysis including Rietveld refinements (see ESI†).
The cadmium atoms are located on 4a position (0, 0, 0) while phosphorus occupies 8f (0.42546(9), 0.25, 0.125). Li could not be localized in this case. Even at 123 K we were not able to locate Li in the structure (see ESI†). This arrangement of heavy atoms leads to four channels per unit cell along the c-direction, of which two of them are filled with a helical 1∞[P−]-chain (Fig. 1d). The bond length observed within the helical 1∞[P−] chain is d(P–P) = 2.2040(6) Å, lying within the expected range for covalently bonded phosphorus (2.14–2.30 Å).1 The Cd–P bond length of 2.6043(4) Å represents a well suited value reported for many cadmium polyphosphides. Examples are α-CdP2 (d(Cd–P) = 2.495(15) to 2.627(15) Å)4 or Cd7P10 (d(Cd–P) = 2.49 to 2.85 Å).8 The distances between neighbored cadmium atoms are close to 4 Å. A section of the crystal structure of Li0.2CdP2 is given in Fig. 1d.
The composition and exact Li-content was determined from single crystals by ICP-OES resulting in Li0.20(3)Cd0.90(5)P2.00(3) (or Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cd
Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 6.43
P = 6.43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28.93
28.93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64.64 in at%). This finding is close to the expected composition but features a slightly reduced Cd content. An EDX analysis substantiated the 1
64.64 in at%). This finding is close to the expected composition but features a slightly reduced Cd content. An EDX analysis substantiated the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of the heavy atoms. As shown later on by quantum chemical calculations, NMR spectroscopy, and phase analysis the Li0.2Cd1.0P2 composition was substantiated. In the following, we use the idealized composition Li0.2CdP2 for further discussions.
2 ratio of the heavy atoms. As shown later on by quantum chemical calculations, NMR spectroscopy, and phase analysis the Li0.2Cd1.0P2 composition was substantiated. In the following, we use the idealized composition Li0.2CdP2 for further discussions.
Lithium mobility in Li0.2CdP2
Due to the high Li mobility (see later on) we tried to localize Li by other methods than scattering techniques. We therefore decided to do quantum chemical model calculations and temperature dependent solid state NMR spectroscopy to evaluate the most probable position within the structure and to check for Li mobility in the present system.A first hint for a high Li ion mobility is the reasonable reactivity of Li0.2CdP2 against humid air. If Li0.2CdP2 is stored in air for 24 h, a white powder can be observed on the surface being identified as Li(OH)·H2O. Details are given in the ESI.† Assuming simple exchange reactions the formation of Li(OH)·H2O is explainable.
On the crystal surface:
| 10 × 0.2Li+(Li0.2CdP2) + H2O (air) ⇆ 2LiOH + 2H+ |
| LiOH + H2O → Li(OH)·H2O |
| LiOH + H+ ⇆ Li2O + H2O |
After dissolution and removal of water on air:
| 2Li+ + 2OH− + CO2 (from air) ⇆ Li2CO3↓ + H2O |
Driving force for this de-lithiation reaction is the formation of a lithium compounds on the surface of the crystals. A possible proton exchange has been addressed by NMR spectroscopy. The proton content was determined as H0.050(5)CdP2 (see ESI†).
Structural characterization and elemental analysis of α′-CdP2
Carefully washed α′-CdP2 crystals were subject of a single crystal structure determination. The α-CdAs2 structure type has been confirmed (see ESI†) but slightly larger lattice parameters have been observed as for Li0.2CdP2. Lattice parameters of a = 7.6829(2) Å, c = 4.46036(11) Å and V = 263.279(12) Å3 [c/a = 0.581] have been derived from the scattering data, being 0.3% larger than for the lithiated compound. The effect is not drastic but outside the 3σ criteria for all lattice parameters. Bond length of d(P–P) of 2.2104(10) Å (d(P–P) = 2.2040(6) Å in Li0.2CdP2) and d(Cd–P) of 2.6102(3) Å (d(Cd–P) = 2.6043(4) Å in Li0.2CdP2) resulted.α′-CdP2 can be regarded as an electron precise compound with Cd2+ ions and two-bonded (2b)P−, resulting in Cd2+(P−)2 following the Zintl–Klemm37 concept. It represents the former unknown end member of a solid solution CdAs2−xPx (0 < x < 1.8),16 which was reported in 1989 (see Fig. 2). The differences in cell volume between Li0.2CdP2 and α′-CdP2 are not huge but α′-CdP2 fits to the Vegard line41 while LixCdP2 lies significantly below.
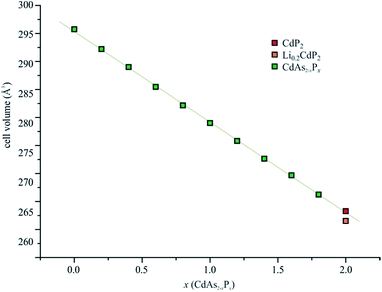 | ||
| Fig. 2 Cell volumes of the solid solution CdAs2−xPx derived from literature [ref. 16] for 0 < x < 1.8 and from single-crystal X-ray experiments for x = 2. A Vegard-like38 decrease of the cell volume is observed up to the maximum P content of x = 2. | ||
An ICP analysis of the carefully washed black crystals after full de-lithiation resulted in Cd0.89(3)P2.00(3). Li can be extracted quantitatively from Li0.2CdP2 by storage on air to form CdP2. Following the notation we called the new phase α′-CdP2 to illustrate the close relationship but also significant difference to α-CdP2.
Quantum chemical calculations
Band structure calculations and DOS representations were performed for Li0.2CdP2 and its de-lithiated form α′-CdP2. Li positions in Li0.2CdP2 were determined by a supercell approach and localized within the empty channels along the crystallographic c-axis. While α′-CdP2 is destabilized in relation to known α-CdP2 and β-CdP2, a substantial stabilization is found for a Li incorporation into the empty channels (see Fig. 3 and ESI†). Substitution of Cd by Li does not lead to a significant stabilization effect and can be ruled out.Li0.2CdP2 is metallic featuring a pseudo band gap 0.9 eV below the Fermi level (see Fig. 3). In contrast, α′-CdP2 is a semiconductor with a calculated band gap of 0.77 eV (LDA) and 0.71 eV (GGA). Total conductivity measurements of microcrystalline Li0.2CdP2 (10−5 S cm−1) and α′-CdP2 (10−9 S cm−1) exhibit a difference of four orders of magnitude at room temperature, nicely reflecting the different electronic structures. The present conductivity values from powdered samples cannot be more than a first hint due to the extreme sensitivity of the samples against mechanical stress. This feature causes disintegration of the structure upon grinding prior to the measurements. Temperature dependent conductivity measurements on single crystals are currently underway.
Obviously, the intercalation of lithium ions into the host structure leads to the closure of the band gap and the occurrence of a metal to semiconductor transition. Upon intercalation of Li into α′-CdP2, 0.2 electrons are located in the conduction band and the band gap vanishes. The integrated DOS (i-DOS) between the Fermi level and the pseudo gap of Li0.2CdP2 corresponds to 0.2 electrons, in good accordance with the expected value from the formula sum (black area in Fig. 3).
Solid state NMR spectroscopy
The 31P-MAS-NMR spectra for α′-CdP2 and Li0.2CdP2 are compiled in Fig. 4. In accordance with the crystal structure, we find a single phosphorous site in α′-CdP2 with an isotropic chemic shift of δiso = −172.6 ppm. From a simulation employing the DMFIT software,39 the chemical shift anisotropy (CSA) parameters were determined as δCS = 189.5 ppm and ηCS = 0.5. We note that the spinning sidebands on the high field side of the spectrum experience some broadening, especially at higher spinning speeds.We ascribe this to the combined action of the chemical shift anisotropy and extremely strong homo-nuclear dipolar coupling. The experimental data is found to be in good agreement with the results of quantum chemical calculations of the CSA parameters employing the WIEN2k software package,40 which produces δiso = −171 ppm, δCS = 210 ppm and ηCS = 0.46.
For Li0.2CdP2, on the other hand, clearly two different P sites with isotropic chemical shifts of −157.4 ppm and −167.4 ppm with relative ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 can be identified. The CSA parameters for both sites are δCS = 182 ppm and ηCS = 0.6. The virtual identical CSA parameters for P in Li0.2CdP2 and the de-lithiated α′-CdP2 sample confirm that the local 31P environment in both compounds is rather similar, as suggested by the XRD results. In addition the presence of two distinct P sites in Li0.2CdP2 indirectly confirms the presence of Li within the structure.
1 can be identified. The CSA parameters for both sites are δCS = 182 ppm and ηCS = 0.6. The virtual identical CSA parameters for P in Li0.2CdP2 and the de-lithiated α′-CdP2 sample confirm that the local 31P environment in both compounds is rather similar, as suggested by the XRD results. In addition the presence of two distinct P sites in Li0.2CdP2 indirectly confirms the presence of Li within the structure.
The static 7Li NMR spectra as a function of temperature in the temperature range 140 K ≤ T ≤ 295 K, acquired employing either a single pulse excitation or a solid echo pulse sequence, are collected in Fig. 5.
For a I = 3/2 nucleus, the overall static line width represents the magnitude of the quadrupole coupling constant CQ = eVZZQ/h, with Q denoting the quadrupolar moment, elementary charge and VZZ the principal component of the electric field gradient. The narrow line in the middle of the spectrum (central transition) is only affected by the homo- and hetero-nuclear dipolar interactions. Since both, the (first order) quadrupolar coupling and the dipole interaction scale with the second Legendrian 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos2
cos2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β − 1 with β denoting the angle between the direction of the magnetic field B0 and the principal axis of the respective interaction (quadrupolar or dipolar), the line shape will be influenced by dynamic processes, leading to an averaging of the interactions and hence a motional narrowing of the NMR line if the inverse of the corresponding correlation time exceeds the magnitude of the interaction.
β − 1 with β denoting the angle between the direction of the magnetic field B0 and the principal axis of the respective interaction (quadrupolar or dipolar), the line shape will be influenced by dynamic processes, leading to an averaging of the interactions and hence a motional narrowing of the NMR line if the inverse of the corresponding correlation time exceeds the magnitude of the interaction.
In the absence of any motional process on the other hand, the line width adopts a constant line width, which is usually referred to as the rigid lattice value. From the evolution of the line shape of the static 7Li NMR spectra with temperature we can clearly conclude considerable Li ion mobility in Li0.2CdP2. At T < 180 K, the spectrum is characterized by a broad signal (m = ±1/2 to m = ±3/2 transitions) from which a quadrupolar coupling constant of CQ = 95 kHz can be deduced. The width of the central transition at T = 140 K (4 kHz) indicates the presence of sizeable dipole coupling. Upon increasing the temperature, the overall width of the spectrum decreases to a value of 11 kHz at ambient temperature. Thus, the quadrupolar interaction is partially averaged out due to Li dynamics. For an isotropic diffusive motion, a vanishing EFG and hence zero quadrupolar coupling would be expected. The presence of a residual average quadrupolar coupling as observed here indicates a hopping process in which the Li ion traces well defined positions within the crystal structure, as observed e.g. in crystalline Li ion conductors.41–43 In this situation, which is often termed anisotropic diffusion, the effective EFG, averaged over these positions, does not average to zero, but to a small residual value.
The evolution of the width of the central line with temperature is plotted in Fig. 5b. At ambient temperature, a line width of 800 Hz is observed. The line width does not suffer from any broadening down to temperatures of 200 K, then a broadening sets in, resulting in a line width of 3300 Hz at T = 117 K. Surprisingly, the rigid lattice regime is not reached even at these low temperatures. Employing the empirical Waugh–Fedin relation, EA = 0.156 × Tonset, which relates the activation energy EA of the motional process to the onset temperature Tonset, at which the motional narrowing sets in, we can give an upper limit for the activation energy of approx. 20 kJ mol−1.
A more precise evaluation of the activation energy is possible from an analysis of the temperature dependence of the spin lattice relaxation time T1, which was measured using a standard inversion recovery pulse sequence. The T1 values were determined from measuring the magnetization built-up which was found to be exponential over the complete investigated temperature range. The data (cf.Fig. 6) exhibits a clear T1-minimum at approx. 310 K. The activation energy and the correlation time of the dynamic process were then obtained from a simulation of the data employing the relaxation model of Bloembergen, Purcell and Pound (BPP),49 resulting in EA = 15 kJ mol−1, τ0c = 3.1 × 10−12 s−1.
Since the low temperature side of the BPP curve is – especially in the case of disordered solids – often biased to lower values,50,51 the low temperature T1 data was not taken into account during the simulation. We emphasize that this value is among the lowest observed activation energies for crystalline Li ion conductors. A selection of Li ion conductors is shown in Table 1. Such a low value is in line with the best known Li ion conductors. With respect to the proposed structures for Li0.2CdP2, the 7Li NMR results clearly rule out the substitution model and favor the intercalation model.
| Compound | E A/eV (kJ mol−1) | Determination method | T/K | Lit. |
|---|---|---|---|---|
| a IS: Impedance Spectroscopy NMR: Nuclear Magnetic Resonance. | ||||
| LiFePO4 | 0.6 (57.9) | IS | RT | 44 |
| Li7PS6 | 0.16 (Li/LiClO4) (15.4) | IS | 313 | 45 |
| 0.33 (Pt) (31.8) | IS | 313 | 45 | |
| NASICON | 0.19 (18.3) | IS | 473–773 | 46 |
| LiCoO2 | 0.30 (29.0) | NMR | RT | 47 |
| LiNiO2 | 0.59 (56.9) | NMR | RT | 47 |
| Li7La3Zr2O12 | 0.32 (30.9) | IS | 291–323 | 48 |
| Li0.2CdP2 | 0.15 (15) | NMR | 117–561 | This work |
Conclusions
Metallic Li0.2CdP2 was prepared by a mineralizer assisted reaction from the elements and CdI2 as reaction promoter. Semiconducting α′-CdP2 with a band gap of ∼0.7 eV can be synthesized via a de-lithiation process upon a simple storage process on humid air. Li(OH)·H2O is formed on the surface of Li0.2CdP2 after 24 h and a complete de-lithiation to α′-CdP2 takes place within weeks. A combined approach using diffraction techniques, spectroscopy and quantum chemistry was necessary to determine the crystal structures. Li0.2CdP2 shows a reasonable Li-ion mobility which was examined and confirmed by solid state NMR spectroscopy. An activation barrier range of 0.15 eV has been derived from spin lattice relaxation time (T1) experiments, being at the low end of the activation energy spectrum known for the best solid Li-ion conducting materials. During the de-lithiation process, a metal to semiconductor transition occurs which has been examined and substantiated by quantum chemical calculations.Acknowledgements
NE and TN thank the state of Bavaria and the DFG for the funding of an X-ray powder diffractometer.References
- R. Pöttgen, W. Hönle and H. G. von Schnering, Encyclopedia of Inorganic Chemistry, ed. R. B. King, Wiley, Chichester, 2nd edn, 2005, vol. VIII, pp. 4255–4308 Search PubMed.
- J. Goodyear and G. A. Steigmann, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1969, 25, 2371 CrossRef CAS.
- J. Berak and Z. Pruchnik, Rocz. Chem., 1968, 42, 1403–1410 CAS.
- O. Olofsson and J. Gullman, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1970, 26, 1883–1884 CrossRef CAS.
- M. von Stackelberg and R. Paulus, Z. Phys. Chem., Abt. B, 1935, 28, 427–460 Search PubMed.
- K. Masumoto, S. Isomura and K. Sasaki, Phys. Status Solidi A, 1971, 6, 515–523 CrossRef CAS.
- M. von Stackelberg and R. Paulus, Z. Phys. Chem., Abt. B, 1935, 28, 427–460 Search PubMed.
- J. Kalicinska Karut, Z. Pruchink and K. Lukaszewicz, Rocz. Chem., 1971, 45, 1991–1992 CAS.
- X. Wu, Sol. Energy, 2004, 6, 803–814 CrossRef PubMed.
- S. Miao, S. G. Hickey, B. Rellinghaus, C. Waurisch and A. Eychmüller, J. Am. Chem. Soc., 2010, 132, 5613–5615 CrossRef CAS PubMed.
- D. K. Harris, P. M. Allen, H.-S. Han, B. J. Walker, J. Lee and M. G. Bawendi, J. Am. Chem. Soc., 2011, 133, 4676–4679 CrossRef CAS PubMed.
- J.-M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- A. R. West, J. Mater. Chem., 1991, 1, 157–162 RSC.
- V. Thangadurai, S. Narayanana and D. Pinzarua, Chem. Soc. Rev., 2014, 43, 4714–4727 RSC.
- L. Červinka and A. HrubČ, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1970, 26, 457–458 CrossRef.
- V. A. Rubtsov, E. M. Smolarenko, V. M. Trukhan, V. N. Yakimovich and L. K. Orlik, Phys. Status Solidi A, 1989, 115, K155–K158 CrossRef CAS.
- S. Lange, M. Bawohl, R. Weihrich and T. Nilges, Angew. Chem., 2008, 120, 5736–5739 ( Angew. Chem., Int. Ed. , 2008 , 47 , 5654–5657 ) CrossRef.
- F. Bachhuber, J. von Appen, R. Dronskowski, P. Schmidt, T. Nilges, A. Pfitzner and R. Weihrich, Angew. Chem., 2014, 126, 11813–11817 ( Angew. Chem., Int. Ed. , 2014 , 53 , 11629–11633 ) CrossRef.
- N. Eckstein, A. Hohmann, R. Weirich, T. Nilges and P. Schmidt, Z. Anorg. Allg. Chem., 2013, 639, 2741–2743 CrossRef CAS.
- S. Lange, P. Schmidt and T. Nilges, Inorg. Chem., 2007, 46, 4028–4035 CrossRef CAS PubMed.
- T. Nilges, M. Kersting and T. Pfeifer, J. Solid State Chem., 2008, 181, 1707–1711 CrossRef CAS PubMed.
- N. Eckstein, L.-A. Jantke, T. F. Fässler, J. Mink, M. Drees and T. Nilges, Eur. J. Inorg. Chem., 2014, 30, 5135–5144 CrossRef.
- Bruker SAINT, 7.68 A, Bruker AXS Inc., Madison, Wisconsin, USA, 2001 Search PubMed.
- Bruker SADABS, 2008/2, Bruker AXS Inc., Madison, Wisconsin, USA, 2001 Search PubMed.
- CrysAlis RED, Version 1.171.33.34d, Oxford Diffraction Ltd., 2009 Search PubMed.
- L. Palatinus and G. J. Chapuis, Appl. Crystallogr., 2007, 40, 786–790 CrossRef CAS.
- V. Petřiček, M. Dušek and L. Palatinus, JANA2006 The Crystallographic Computing System, Institute of Physics, Praha, Czech Republic, 2006 Search PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1997, 78, 1396 CrossRef CAS.
- J. P. Perdew and Y. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 13244–13249 CrossRef.
- J. P. Perdew and A. Zunger, Phys. Rev. B: Condens. Matter Mater. Phys., 1981, 23, 5048–5079 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251–14269 CrossRef CAS.
- G. Kresse and J. Furthmuller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Furthmuller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- T. Takahashi, H. Kawashima, H. Sugisawa and T. Baba, Solid State Nucl. Magn. Reson., 1999, 15, 119 CrossRef CAS.
- A. Bielecki and D. P. Burum, J. Magn. Reson., Ser. A, 1995, 116, 215 CrossRef CAS.
- E. Zintl, Angew. Chem., 1939, 52, 1 CrossRef CAS.
- L. Z. Vegard, Z. Phys., 1921, 5, 17–26 CrossRef CAS.
- D. Massiot, F. Fayon, M. Capron, I. King, S. Le Calvé, B. Alonso, J. O. Durand, B. Bujoli, Z. Gan and G. Hoatson, Magn. Reson. Chem., 2002, 40, 70–76 CrossRef CAS.
- P. Blaha, K. Schwarz, G. K. H. Madsen, D. Kvasnicka and J. Luitz, WIEN2K, An Augmented Plane Wave plus Local Orbitals Program for calculating Crystal Properties program suite, Technische Universiät, Wien, Austria, 2001 Search PubMed.
- L. van Wüllen, L. Hildebrandt and M. Jansen, Solid State Ionics, 2005, 176, 1449–1456 CrossRef PubMed.
- L. van Wüllen, N. Sofina, L. Hildebrandt, C. Mühle and M. Jansen, Solid State Ionics, 2006, 117, 1665–1672 CrossRef PubMed.
- L. van Wüllen, T. Echelmeyer, H.-W. Meyer and D. Wilmer, Phys. Chem. Chem. Phys., 2007, 9, 3298–3303 RSC.
- C. Delacourt, L. Laffont, R. Bouchet, C. Wurm, J.-B. Leriche, M. Morcrette, J.-M. Tarascon and C. Masquelier, J. Electrochem. Soc., 2005, 152, A913–A921 CrossRef CAS PubMed.
- H.-J. Deiseroth, J. Maier, K. Weichert, V. Nickel, S.-T. Kong and C. Reiner, Z. Anorg. Allg. Chem., 2011, 637, 1287–1294 CrossRef CAS.
- G. Jasinski, P. Jasinski, B. Chachulski and A. Nowakowski, Mater. Sci., 2006, 24, 261–268 CAS.
- K. Nakamura, H. Ohno, K. Okamura, Y. Michihiro, I. Nakabayashi and T. Kanashiro, Solid State Ionics, 2000, 135, 143–147 CrossRef CAS.
- R. Murugan, V. Thangadurai and W. Weppner, Angew. Chem., 2007, 119, 7925–7928 CrossRef.
- N. Bloembergen, E. M. Purcell and R. V. Pound, Phys. Rev., 1948, 73, 679–712 CrossRef CAS.
- D. Brinkmann, Magn. Reson. Rev., 1989, 14, 101 Search PubMed.
- R. Winter, K. Siegmund and P. Heitjans, J. Non-Cryst. Solids, 1997, 212, 215 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Cif files containing the heavy atom structures, details concerning the elemental and thermal analyses, conductivity measurements and details concerning the quantum chemical calculations. See DOI: 10.1039/c5ta00471c |
| This journal is © The Royal Society of Chemistry 2015 |





