Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metal oxide arrays from block copolymer thin film templates†
Michael K.
Mayeda
a,
Jeffery
Hayat
b,
Thomas H.
Epps
III
*a and
Jochen
Lauterbach
*c
aDepartment of Chemical & Biomolecular Engineering, University of Delaware, Newark, DE 19716, USA. E-mail: thepps@udel.edu; Fax: +1 302 831 1048; Tel: +1 302 831 0215
bDepartment of Chemistry & Biochemistry, University of South Carolina, Columbia, SC 29201, USA
cDepartment of Chemical Engineering, University of South Carolina, Columbia, SC 29201, USA. E-mail: lauteraj@cec.sc.edu; Fax: +1 803 777 8292; Tel: +1 803 777 7904
First published on 2nd March 2015
Abstract
We present a simple, though uncommonly used, method to produce versatile, well-ordered, nanoscale arrays of metal oxides such as MgO, Al2O3, TiO2, MnO2, Fe2O3, Co3O4, NiO, CuO, ZnO, ZrO2, RuO2, SnO2, or Ce2O3 by decoupling metal oxide precursor incorporation from block copolymer (BCP) template formation. In this work, neat BCP thin films were cast and annealed, using standard techniques, to generate templates. The templates were immersed in a precursor solution and formed metal–polymer complexes in one polymer domain. Finally, the organics were removed in an oxidative environment to leave the templated metal oxides. As a concrete example of the method's applicability, we show that the templating method produced ordered TiO2 arrays that exhibited a 13% increase in photocatalytic activity over TiO2 produced by EISA. Furthermore, the addition of gold nanoparticles further improved photocatalytic activity by 43% on our templated TiO2, whereas gold nanoparticles on EISA TiO2 exhibited no improvement. The simplicity and modularity of the templating method makes it amenable to additional applications in catalysis, optics, and sensors.
Introduction
The development of Mobil's zeolites in the 1960s sparked an avalanche of research on the design of structured materials with nanometer-scale features (>1 nm) for applications in catalysis and separations.1 Innovative uses of structure directing agents can yield nanomaterials with improved catalytic activity. Whereas small-molecule surfactants have proven to be excellent subnanometer structure directing agents,2 block copolymers (BCPs) offer a macromolecular analog with tunable molecular weights, block choices, and compositions.3,4 When implemented in thin film environments (<100 nm in thickness), BCPs can serve as ideal organic templates, whose nanostructure can be controlled through processing techniques.5–10 These films can be used to direct the nanoscale structure of inorganic materials for catalysts, sensors, and optics applications.11 Improved ordering of the thin film morphology, to ∼cm2 grain sizes, is important for applications in competition (or in concert) with lithography and could be achieved by implementing complementary techniques that employ topographical patterning or external fields.8,12–16BCP templated metal oxide arrays facilitate a variety of exciting applications in magnetic bit patterned media, seed-mediated nanotube growth, energy harvesting, and heterogeneous catalysis.17–22 The vast majority of previous investigations involving BCP templating follow two routes: (1) segregating preformed nanoparticles or (2) using in situ sol–gel methods. The former approach benefits from advances in nanoparticle synthesis, which allows researchers to tune particle size, shape, crystallinity, and composition.23 Additionally, templating preformed nanoparticles is a convenient route to ensure that the inorganic material retains the desired properties of interest. Previous work by Epps and coworkers templated pre-synthesized gold nanoparticles in poly(styrene-b-isoprene-b-styrene) thin films by tuning the particles' surface energies using ligand exchanges.24 Kramer and coworkers also have investigated gold nanoparticle segregation in poly(styrene-b-2 vinylpyridine) (PS-b-P2VP) BCPs by manipulating ligand chemistry, density, and molecular weight.25–28 Several reviews succinctly describe efforts to incorporate particles with varying composition, size, and shape into BCPs.29–32 In all cases, preformed nanoparticle miscibility and templating generally were dominated by the particle-polymer entropic (particle size and shape) and enthalpic (ligand chemistry) interactions.
In situ sol–gel methods use a sacrificial BCP to arrange metal salt precursors into nanoscale features and subsequently reduce, oxidize, or calcine the composite to simultaneously form the templated material and remove the polymer.33,34 This versatile method can be used to create many types of industrially relevant crystals, powders, and films. Within sol–gel techniques, evaporation induced self-assembly (EISA) is a popular method for templating inorganic materials.2 In short, the polymer and metal precursor are combined in a single solution and a thin film is coated onto a substrate. The evaporation of solvent creates a concentration gradient that acts as an ordering front that produces the nanoscale features. Features can be tuned by adjusting the polymer molecular weight,35 sol–gel concentration,33,36,37 and coating procedure.38 Researchers have used the EISA-templating method to produce arrays of gold, cobalt, and cobalt oxide, but ordering remains poor (grain size < 1 μm2).39–43 Poor ordering likely was due to polymer/metal inter- and intra-molecular interactions that inhibit polymer chain mobility and prevent BCP ordering.44 To maximize BCP chain mobility, Boyen and coworkers modified the EISA method by complexing Fe and Nb salts with homopolymer P2VP (h2PVP) prior to blending with the PS-b-P2VP solution.45 Thin films of salt/h2VP/PS-b-P2VP produced well-ordered arrays with ≈1 μm2 grain sizes. However, this modification to the traditional EISA method was limited to metal loading ratios of molmetal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol2VP-monomer ≤ 0.2 to avoid macrophase separation. On the other hand, Russell and co-workers employed a preformed BCP template to create well-ordered Au/Ag arrays for surface plasmon resonance studies.46 Morris and coworkers have used the method to produce metal oxides with superparamagnetic and ferroelectric properties.47–49 Although the method was proven,50 researchers have yet to use it for catalysis applications.
mol2VP-monomer ≤ 0.2 to avoid macrophase separation. On the other hand, Russell and co-workers employed a preformed BCP template to create well-ordered Au/Ag arrays for surface plasmon resonance studies.46 Morris and coworkers have used the method to produce metal oxides with superparamagnetic and ferroelectric properties.47–49 Although the method was proven,50 researchers have yet to use it for catalysis applications.
We use the preformed BCP template method, hereafter referred to as spincoat-pattern-immerse-complex-etch (SPICE) to produce thin films of well-ordered arrays that were otherwise difficult to achieve via the EISA/dip-coating approach. We build upon previous investigations46,50 by creating well-ordered hexagonally packed arrays of MgO, Al2O3, TiO2, MnO2, Fe2O3, Co3O4, NiO, CuO, ZnO, ZrO2, RuO2, SnO2, and Ce2O3. Furthermore, we demonstrate an improvement in photocatalytic activity of SPICE TiO2 and Au/TiO2 over EISA TiO2. In addition to photocatalysis, we anticipate this method will be critical to developing advanced materials for sensors, environmental catalysis, and lithography applications.
Experimental
Materials
All materials were used as received. Poly(styrene-b-ethylene oxide) (PS-b-PEO; Đ = 1.04,![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) N,styrene = 16 kg mol−1,
N,styrene = 16 kg mol−1, ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) N,ethylene oxide = 5 kg mol−1) was purchased from Polymer Source, Inc. Iron(III) nitrate nonahydrate (ACS reagent) was purchased from Sigma-Aldrich. Titanium(IV) tetraisopropoxide (98+%) and poly(acrylic acid) (25 wt% aqueous solution,
N,ethylene oxide = 5 kg mol−1) was purchased from Polymer Source, Inc. Iron(III) nitrate nonahydrate (ACS reagent) was purchased from Sigma-Aldrich. Titanium(IV) tetraisopropoxide (98+%) and poly(acrylic acid) (25 wt% aqueous solution, ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) W = 240 kg mol−1) were purchased from Acros Organics. Aluminum(III) nitrate nonahydrate (98%), cerium(III) nitrate hexahydrate (REacton®, 99.5%), cobalt(II) nitrate hexahydrate (ACS reagent), copper(II) nitrate hemipentahydrate (98%), magnesium(II) nitrate hexahydrate (98%), manganese(II) nitrate hydrate (99.98%), nickel(II) nitrate hexahydrate (98%), tin(II) chloride dihydrate (ACS reagent), zinc(II) nitrate hydrate (99%), zirconium(IV) dichloride oxide octahydrate (98%), and methylene blue (MB) (high purity biological stain) were purchased from Alfa Aesar. ACS grade toluene, tetrahydrofuran, ethanol, and 2-propanol were purchased from BDH. Deionized water was obtained from a Millipore Milli-Q Direct 8 system. Hydrochloric acid (37% in water, technical) was purchased from Fisher Scientific. Silicon and silicon oxide (500 nm on silicon) wafers were purchased from Wafer World. Both types of wafers were rinsed with toluene, dried with a nitrogen stream, and cleaned in a Jelight Model 42 UVO-cleaner for 30 min prior to polymer spincoating.
W = 240 kg mol−1) were purchased from Acros Organics. Aluminum(III) nitrate nonahydrate (98%), cerium(III) nitrate hexahydrate (REacton®, 99.5%), cobalt(II) nitrate hexahydrate (ACS reagent), copper(II) nitrate hemipentahydrate (98%), magnesium(II) nitrate hexahydrate (98%), manganese(II) nitrate hydrate (99.98%), nickel(II) nitrate hexahydrate (98%), tin(II) chloride dihydrate (ACS reagent), zinc(II) nitrate hydrate (99%), zirconium(IV) dichloride oxide octahydrate (98%), and methylene blue (MB) (high purity biological stain) were purchased from Alfa Aesar. ACS grade toluene, tetrahydrofuran, ethanol, and 2-propanol were purchased from BDH. Deionized water was obtained from a Millipore Milli-Q Direct 8 system. Hydrochloric acid (37% in water, technical) was purchased from Fisher Scientific. Silicon and silicon oxide (500 nm on silicon) wafers were purchased from Wafer World. Both types of wafers were rinsed with toluene, dried with a nitrogen stream, and cleaned in a Jelight Model 42 UVO-cleaner for 30 min prior to polymer spincoating.
Templating procedure
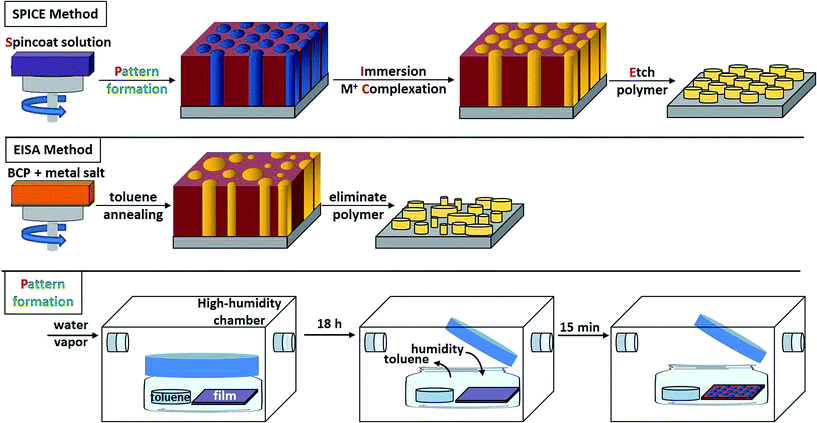
The SPICE method is depicted in Scheme 1. Each of the sub-processes are explained in detail; however, it should be noted that sub-processes can be substituted (e.g., thermal annealing instead of solvent vapor annealing, dip-coating instead of spincoating, calcination instead of ozone etching, etc.).Film characterization
Atomic force microscopy (AFM) images were recorded with a Bruker Multimode Nanoscope V system. AppNano ACL tips (190 kHz resonant frequency, 58 N m−1 spring constant) were used in tapping mode. Image processing and FFTs of micrographs were performed with ImageJ.X-ray photoelectron spectroscopy (XPS) measurements were performed on a Kratos Axis Ultra DLD instrument equipped with a monochromated Al Kα X-ray source and a hemispherical analyzer. All analyzed metal oxide arrays were deposited on silicon oxide wafers. Binding energies were calibrated using Si 2p at 103.3 eV.
Transmission electron microscopy (TEM) images were taken with a Hitachi H8000 operated at 150 kV. PS-b-PEO films containing titania precursor were peeled and mounted onto copper mesh grids according to a method previously described in the literature.53 The composite film was prepared for peeling by depositing a thin layer of carbon (<10 nm) using a Hitachi carbon evaporator. A droplet (<1 mL) of 25 wt% poly(acrylic acid) (PAA) was drop-cast and allowed to dry overnight at atmospheric conditions. A razor blade was used to remove the resulting solid, which adhered to the polymer composite film. The PAA was dissolved in a large volume of water. The resulting floating films were mounted onto copper mesh grids or silicon nitride membrane window grids and allowed to air dry for at least 15 min before loading into the TEM.
Photocatalysis experiments
We demonstrate the SPICE method through the improved photocatalytic degradation of MB, which is an industrial pollutant commonly found in textile waste streams.20 In this work, an aqueous solution of 1 × 10−5 M MB was stirred for 2 days and then stored in a dark environment for later use. Wafer fragments (∼2 cm2) and 3.3 mL of MB solution were placed in Brandtech cuvettes (220–900 nm transparency). The surface areas of the samples were hard to measure when the wafer fragments were irregularly shaped. Therefore, degradation rates were normalized by the weights of the wafer fragments. Irradiation was accomplished with a ScienceTech SF300A solar simulator coupled with a ScienceTech 500–300 power supply (350 W average output). Wafer fragments were removed from the cuvettes prior to UV-vis measurements, which were recorded with a Shimadzu UV2450 instrument. The 665 nm peak was monitored during time-lapse studies; peak area integrations from 550–740 nm were calculated. Photocatalytic experiments were reproduced three times for each sample.Results and discussion
In heterogeneous catalysis, ordered and narrowly disperse features made by bottom-up processes are critical for applications requiring maximum active surface area. Thus, grain size and narrow structural dispersity inherent in BCP films is an important aspect of the SPICE method. In our case, perpendicularly-oriented and hexagonally-packed domains of PEO cylinders in PS-b-PEO films (30 nm thick) were obtained by toluene vapor annealing in a high-humidity chamber, as depicted in Scheme 1.51The average radius of the PEO domains in a toluene/water annealed neat polymer film was 8.7 ± 0.8 nm (>2500 measurements) (Fig. 1b). The inset fast Fourier transform (FFT) exhibits 4th order peaks, which corroborate the excellent order and large grain sizes achieved by high-humidity annealing. Grain sizes of toluene/water vapor annealed PS-b-PEO films were greater than 4 μm2. The PS-b-PEO films were used to template SPICE TiO2 (Fig. 1d) and Au on SPICE TiO2 (Fig. 1e) samples. On the other hand, films that were annealed by only toluene or tetrahydrofuran vapor contained grains that were less than 1 μm2 (Fig. 1a). Additionally, EISA-templated TiO2 exhibited large size distributions and poor order (Fig. 1c). These results highlighted the importance of using high-humidity during the annealing step.
Some metal precursors, such as the titanium alkoxide and tin chloride used in this study, are susceptible to hydrolysis and cannot be processed in humid or aqueous environments.54 By separating template formation and metal precursor inclusion into two decoupled steps, the SPICE method can take advantage of annealing techniques (using high humidity in this case) that otherwise would be precluded by the presence of the metal precursor. To further extend this idea, the authors envision bolstering the SPICE method with supplementary annealing techniques that employ external fields, shear fields, or patterned substrates. Achieving wafer-sized areas of templated material is industrially relevant for magnetic storage media and energy harvesting.55,56
Metal oxides were templated into the annealed PS-b-PEO films by a simple immersion process. BCP films were submerged in a metal precursor solution to allow the metal ions to selectively complex with the ethylene oxide monomer units. A typical loading ratio of 0.38 was achieved, which is almost twice that achieved by Boyen and coworkers (calculations are available in ESI†).45 Metal oxide formation and polymer removal were simultaneously achieved using an ultraviolet ozone (UVO) oven. The versatility of the SPICE procedure was exemplified by templating commonly used metal oxides: MgO, Al2O3, TiO2, MnO2, Fe2O3, Co3O4, NiO, CuO, ZnO, ZrO2, RuO2, SnO2, and Ce2O3. Empirical formulas for the metal oxides were determined using high resolution X-ray photoelectron spectroscopy (XPS) scans to identify the oxidation states of the metals.57Fig. 1a–e show AFM images of selected metal oxide arrays as well as corresponding FFTs in the insets. Height images of the remaining metal oxides (Fig. S1†) and XPS peak positions of all metal oxides (Table S2†) can be found in the ESI.† Hexagonally-packed metal oxide dots are noted in Fig. 1b, d, e, and S1;† all corresponding FFTs reveal reflections that are characteristic of hexagonal packing. 2nd order reflections are noted for Co3O4, Fe2O3, MgO, MnO2, ZnO, SnO2, and TiO2 samples. After polymer removal, the height of the templated materials ranged from 1–7 nm. For TiO2, a 30 nm thick BCP film template produced an average oxide dot height of 5.4 ± 1.4 nm. The height reduction was attributed to the significant mass loss during polymer template removal and was used to calculate the average oxide loading. The average radius of a templated dot was 8.5 ± 1.9 nm, which closely matches the initial PEO domain size after solvent annealing (8.7 ± 0.8 nm).46 The similarity in PEO domain size and the SPICE dot size suggests that templated metal oxide size can be controlled by tuning the polymer molecular weight and volume fraction.58 Highly ordered PS-b-PEO films led to highly ordered metal oxide dots, supporting the efficacy and simplicity of the SPICE templating method.
The EISA-templating method was applied to the same polymer and titania precursor solution for the purpose of comparison. Because the titania precursor was highly sensitive to water, toluene/water solvent vapor undesirably hydrolyzed the titania precursor in the EISA film (Fig. 2a). Thus, only toluene vapor was used to anneal EISA films. Despite, our best efforts to anneal the EISA composite with toluene vapor, the resulting titania were poorly ordered and disperse in diameter (Fig. 2b). On the other hand, the SPICE method allowed the use of high-humidity annealing conditions and produced highly-ordered PEO domains, which preferentially absorbed the titania precursor (Fig. 2c). TEM images illustrate the improvement in order and dispersity of the SPICE method.
The EISA method produced an average TiO2 dot radius of 9.8 ± 4.4 nm. Decreased morphology control was evidenced by the line scan in Fig. 1f, AFM image (Fig. 1c), and TEM image (Fig. 2b). Dispersion calculations are available in ESI.† The FFT of EISA micrographs showed no spots or rings, indicating the absence of any significant ordering. Poor ordering of annealed EISA films was attributed to hydrolytic oligomerization of titania and reduced polymer chain mobility caused by coordination bonds between the titania precursor and PEO domains.37,59 Huh and coworkers studied the effect of Cd coordination with poly(4-vinylpyridine) (P4VP) and found that increasing the salt loading increased gelation and decreased ordering of their Cd/PS-b-P4VP system.44 Similarly, it is expected that PEO–metal complexes hinder polymer chain mobility during solvent vapor annealing. By decoupling the polymer-ordering from the metal-coordinating, the SPICE method produced narrow size distributions and highly ordered arrays.
It has been established that Au or Ag nanoparticles can be used to improve the photocatalytic efficiency of TiO2.60–62 As a further validation of our synthesis approach, a toluene solution of gold nanoparticles (3.5 ± 1.0 nm diameter) was spincoated onto SPICE- and EISA-templated TiO2. The resulting Au/TiO2 catalysts contained approximately 5 mol% Au as determined by XPS survey scans (not shown). SPICE-templated TiO2 arrays maintained their high degree of ordering and dispersity after Au addition (Fig. 1e).
Photocatalytic studies were used to demonstrate the increased activity of SPICE TiO2 compared to EISA TiO2. Aqueous solutions of MB were photocatalyzed, under irradiation, with EISA TiO2, SPICE TiO2, and SPICE-templated and EISA-templated TiO2 coated with gold nanoparticles (Fig. 3). Cuvettes with no catalyst (Blank) and MB stored in the dark (Dark) were used as controls. More specifically, the Blank cuvette indicates how much MB degradation occurs due to irradiation alone. UV-vis spectra were collected every two hours, and peak areas were integrated between 550–740 nm (Fig. S2†). MB photodegradation was presumed to follow first order kinetics as indicated by the following expression:20
 | (1) |
 | ||
| Fig. 3 Plot of the natural log of MB concentrations against irradiation time. Error bars on data points represent standard deviations of the mean across three reproducibility studies. The slopes represent rate constants (k), which are reported in Table 1. The value of kDark was taken to be the error in the rate constants. | ||
The value of kDark was attributed to instrumental drift and was used as the error associated with the rate constants. SPICE TiO2 showed a 13% increase in photocatalytic activity over EISA TiO2 during MB degradation experiments. The addition of AuNPs to the SPICE TiO2 samples (Au/TiO2) further improved the photocatalytic activity by 43%. AuNPs did not improve the catalytic activity of the EISA TiO2.
Photocatalytic activity has been shown to depend on catalyst crystallinity, surface hydroxyl concentration, and surface area.63 Because the EISA and SPICE methods share the same materials and polymer removal process, it is expected that crystallinity and surface hydroxyl groups remain similar. Both SPICE and EISA processes were carried out at room temperature except for the UVO treatment, which remained below 60 °C. Therefore, the anatase phase is expected for both synthetic processes.64 The improvement in photocatalytic efficiency was attributed to increased surface area, as the SPICE-templated TiO2 surface area was increased by minimizing the TiO2 size distribution (Fig. S3†). Exposed surface area was estimated to be 25% larger for SPICE TiO2 over EISA TiO2; uncertainty in the measurement stems from the variations in EISA dot sizes. In addition to an improvement over EISA TiO2, our experiments demonstrated a 27% activity gain between SPICE Au/TiO2 over SPICE TiO2. On the other hand, our EISA Au/TiO2 exhibited no improvement over EISA TiO2. Numerous previous studies have demonstrated improvements to EISA TiO2 photocatalysts by adding metal nanoparticles. Catalysts were optimized by changes in synthetic techniques and metal type and loading. In some cases, AuNP addition caused losses in photocatalytic activity.60,65 Amongst our experiments, the simple addition of AuNPs to EISA TiO2 yielded no change, which indicated that optimization is required. On the other hand, AuNPs that were simply spincoated onto the SPICE TiO2 yielded an improvement of 27%. The improvement in efficiency indicates that the SPICE TiO2 can be more easily optimized and augmented than EISA TiO2.61,62
Conclusions
In conclusion, the SPICE templating method (spincoat-pattern-immersion-complex-etch) was used to produce arrays of a variety of metal oxides. The method decoupled metal–PEO complexation from BCP ordering and thus enabled both exceptionally well-ordered arrays and simple sol–gel templating. A variety of metal oxides were templated; all exhibited the same high degree of ordering as their sacrificial BCP thin film template. In particular, TiO2 arrays exhibited a high degree of ordering and narrow size dispersion, which could not be achieved using standard EISA methods. Finally, MB photodegradation studies showed that the SPICE TiO2 (1) showed a 13% increase in photocatalytic activity over EISA TiO2 and (2) could be augmented easily with gold nanoparticles to further improve photocatalytic activity by 27%, whereas the EISA Au/TiO2 showed no such improvements. The SPICE method opens exciting pathways for thin films of ordered metal oxides in sensors, magnetic bit storage media, and energy harvesting.Acknowledgements
This work was partially supported by the National Science Foundation (NSF) through DMR-1207041 to T.H.E. and the South Carolina SmartState™ center for Strategic Approaches to the Generation of Electricity (SAGE). M.K.M. thanks the NSF Solar Hydrogen IGERT Program (no. DGE-0549399) for funding. J.H. acknowledges the Global Research Collaboration Program of Semiconductor Research Corporation (Task ID 2222.001). We acknowledge Prof. Chuanbing Tang for use of the atomic force microscope and Prof. Miao Yu for use of the solar simulator.References
- G. J. d. A. A. Soler-Illia, C. Sanchez, B. Lebeau and J. Patarin, Chem. Rev., 2002, 102, 4093–4138 CrossRef PubMed.
- C. J. Brinker, MRS Bull., 2004, 29, 631–640 CrossRef CAS.
- F. S. Bates, M. A. Hillmyer, T. P. Lodge, C. M. Bates, K. T. Delaney and G. H. Fredrickson, Science, 2012, 336, 434–440 CrossRef CAS PubMed.
- F. S. Bates and G. H. Fredrickson, Phys. Today, 1999, 52, 32–38 CrossRef CAS PubMed.
- M. Rawolle, M. A. Niedermeier, G. Kaune, J. Perlich, P. Lellig, M. Memesa, Y. J. Cheng, J. S. Gutmann and P. Muller-Buschbaum, Chem. Soc. Rev., 2012, 41, 5131–5142 RSC.
- K. Koo, H. Ahn, S.-W. Kim, D. Y. Ryu and T. P. Russell, Soft Matter, 2013, 9, 9059–9071 RSC.
- C. Sinturel, M. Vayer, M. Morris and M. A. Hillmyer, Macromolecules, 2013, 46, 5399–5415 CrossRef CAS.
- M. Luo and T. H. Epps III, Macromolecules, 2013, 46, 7567–7579 CrossRef CAS.
- J. N. L. Albert, W. S. Young, R. L. Lewis, T. D. Bogart, J. R. Smith and T. H. Epps III, ACS Nano, 2012, 6, 459–466 CrossRef CAS PubMed.
- J. E. Seppala, R. L. Lewis and T. H. Epps III, ACS Nano, 2012, 6, 9855–9862 CrossRef CAS PubMed.
- R. A. Segalman, Mater. Sci. Eng., R, 2005, 48, 191–226 CrossRef PubMed.
- C. Park, J. Yoon and E. L. Thomas, Polymer, 2003, 44, 6725–6760 CrossRef CAS PubMed.
- C. M. Bates, M. J. Maher, D. W. Janes, C. J. Ellison and C. G. Willson, Macromolecules, 2014, 47, 2–12 CrossRef CAS.
- D. J. C. Herr, J. Mater. Res., 2011, 26, 122–139 CrossRef CAS.
- D. E. Angelescu, J. H. Waller, D. H. Adamson, P. Deshpande, S. Y. Chou, R. A. Register and P. M. Chaikin, Adv. Mater., 2004, 16, 1736–1740 CrossRef CAS.
- S. O. Kim, H. H. Solak, M. P. Stoykovich, N. J. Ferrier, J. J. de Pablo and P. F. Nealey, Nature, 2003, 424, 411–414 CrossRef CAS PubMed.
- X. Quan, S. Yang, X. Ruan and H. Zhao, Environ. Sci. Technol., 2005, 39, 3770–3775 CrossRef CAS.
- H. F. Zhuang, C. J. Lin, Y. K. Lai, L. Sun and J. Li, Environ. Sci. Technol., 2007, 41, 4735–4740 CrossRef CAS.
- Y. Li, T. Sasaki, Y. Shimizu and N. Koshizaki, J. Am. Chem. Soc., 2008, 130, 14755–14762 CrossRef CAS PubMed.
- A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard and J.-M. Herrmann, Appl. Catal., B, 2001, 31, 145–157 CrossRef CAS.
- A. Syoufian and K. Nakashima, J. Colloid Interface Sci., 2008, 317, 507–512 CrossRef CAS PubMed.
- H. Acharya, J. Sung, I. Bae, T. Kim, D. H. Kim and C. Park, Chem.–Eur. J., 2012, 18, 14695–14701 CrossRef CAS PubMed.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- M. K. Mayeda, W.-F. Kuan, W.-S. Young, J. A. Lauterbach and T. H. Epps III, Chem. Mater., 2012, 24, 2627–2634 CrossRef CAS.
- B. J. Kim, J. Bang, C. J. Hawker, J. J. Chiu, D. J. Pine, S. G. Jang, S.-M. Yang and E. J. Kramer, Langmuir, 2007, 23, 12693–12703 CrossRef CAS PubMed.
- B. J. Kim, G. H. Fredrickson and E. J. Kramer, Macromolecules, 2007, 41, 436–447 CrossRef.
- J. J. Chiu, B. J. Kim, E. J. Kramer and D. J. Pine, J. Am. Chem. Soc., 2005, 127, 5036–5037 CrossRef CAS PubMed.
- B. J. Kim, J. J. Chiu, G. R. Yi, D. J. Pine and E. J. Kramer, Adv. Mater., 2005, 17, 2618–2622 CrossRef CAS.
- A. C. Balazs, T. Emrick and T. P. Russell, Science, 2006, 314, 1107–1110 CrossRef CAS PubMed.
- A. Haryono and W. H. Binder, Small, 2006, 2, 600–611 CrossRef CAS PubMed.
- M. R. Bockstaller, R. A. Mickiewicz and E. L. Thomas, Adv. Mater., 2005, 17, 1331–1349 CrossRef CAS.
- J. Kao, K. Thorkelsson, P. Bai, B. J. Rancatore and T. Xu, Chem. Soc. Rev., 2013, 42, 2654–2678 RSC.
- Z. C. Sun, D. H. Kim, M. Wolkenhauer, G. G. Bumbu, W. Knoll and J. S. Gutmann, ChemPhysChem, 2006, 7, 370–378 CrossRef CAS PubMed.
- M. C. Orilall and U. Wiesner, Chem. Soc. Rev., 2011, 40, 520–535 RSC.
- E. Ortel, A. Fischer, L. Chuenchom, J. Polte, F. Emmerling, B. Smarsly and R. Kraehnert, Small, 2012, 8, 298–309 CrossRef CAS PubMed.
- V. N. Urade, L. Bollmann, J. D. Kowalski, M. P. Tate and H. W. Hillhouse, Langmuir, 2007, 23, 4268–4278 CrossRef CAS PubMed.
- Y. J. Cheng and J. S. Gutmann, J. Am. Chem. Soc., 2006, 128, 4658–4674 CrossRef CAS PubMed.
- D. Grosso, J. Mater. Chem., 2011, 21, 17033–17038 RSC.
- G. Kastle, H. G. Boyen, F. Weigl, G. Lengl, T. Herzog, P. Ziemann, S. Riethmuller, O. Mayer, C. Hartmann, J. P. Spatz, M. Moller, M. Ozawa, F. Banhart, M. G. Garnier and P. Oelhafen, Adv. Funct. Mater., 2003, 13, 853–861 CrossRef.
- H. G. Boyen, G. Kastle, K. Zurn, T. Herzog, F. Weigl, P. Ziemann, O. Mayer, C. Jerome, M. Moller, J. P. Spatz, M. G. Garnier and P. Oelhafen, Adv. Funct. Mater., 2003, 13, 359–364 CrossRef CAS.
- J. Polleux, M. Rasp, I. Louban, N. Plath, A. Feldhoff and J. P. Spatz, ACS Nano, 2011, 5, 6355–6364 CrossRef CAS PubMed.
- J. P. Spatz, S. Mossmer, C. Hartmann, M. Moller, T. Herzog, M. Krieger, H. G. Boyen, P. Ziemann and B. Kabius, Langmuir, 2000, 16, 407–415 CrossRef CAS.
- R. E. Palmer, S. Pratontep and H. G. Boyen, Nat. Mater., 2003, 2, 443–448 CrossRef CAS PubMed.
- D. H. Lee, S. H. Han, W. Joo, J. K. Kim and J. Huh, Macromolecules, 2008, 41, 2577–2583 CrossRef CAS.
- L. Shan, S. Punniyakoti, M. J. Van Bael, K. Temst, M. K. Van Bael, X. Ke, S. Bals, G. Van Tendeloo, M. D'Olieslaeger, P. Wagner, K. Haenen and H.-G. Boyen, J. Mater. Chem. C, 2014, 2, 701–707 RSC.
- P. A. Mistark, S. Park, S. E. Yalcin, D. H. Lee, O. Yavuzcetin, M. T. Tuominen, T. P. Russell and M. Achermann, ACS Nano, 2009, 3, 3987–3992 CrossRef CAS PubMed.
- T. Ghoshal, T. Maity, J. F. Godsell, S. Roy and M. A. Morris, Adv. Mater., 2012, 24, 2390–2397 CrossRef CAS PubMed.
- T. Ghoshal, T. Maity, R. Senthamaraikannan, M. T. Shaw, P. Carolan, J. D. Holmes, S. Roy and M. A. Morris, Sci. Rep., 2013, 3, 1–8 Search PubMed.
- J. Varghese, T. Ghoshal, N. Deepak, C. O’Regan, R. W. Whatmore, M. A. Morris and J. D. Holmes, Chem. Mater., 2013, 25, 1458–1463 CrossRef CAS.
- (a) H. Cho, H. Park, T. P. Russell and S. Park, J. Mater. Chem., 2010, 20, 5047–5051 RSC; (b) L. L. Wu, B. Leng and A. Bisht, Appl. Phys. A, 2014, 116, 893–900 CrossRef CAS; (c) J. Q. Lu, J. Phys. Chem. C, 2008, 112, 10344–10351 CrossRef CAS; (d) T. Ghoshal, M. T. Shaw, C. T. Bolger, J. D. Holmes and M. A. Morris, J. Mater. Chem., 2012, 22, 12083–12089 RSC; (e) M. Roulet, M. Vayer and C. Sinturel, Eur. Polym. J., 2013, 49, 3897–3903 CrossRef CAS PubMed.
- S. H. Kim, M. J. Misner, T. Xu, M. Kimura and T. P. Russell, Adv. Mater., 2004, 16, 226–231 CrossRef CAS.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801–802 RSC.
- T. H. Epps III, D. M. DeLongchamp, M. J. Fasolka, D. A. Fischer and E. L. Jablonski, Langmuir, 2007, 23, 3355–3362 CrossRef PubMed.
- M. Rawolle, M. A. Ruderer, S. M. Prams, Q. Zhong, D. Magerl, J. Perlich, S. V. Roth, P. Lellig, J. S. Gutmann and P. Muller-Buschbaum, Small, 2011, 7, 884–891 CrossRef CAS PubMed.
- B. D. Terris and T. Thomson, J. Phys. D: Appl. Phys., 2005, 38, R199–R222 CrossRef CAS.
- K. Q. Peng and S. T. Lee, Adv. Mater., 2011, 23, 198–215 CrossRef CAS PubMed.
- NIST X-ray Photoelectron Spectroscopy Database, Version 4.1, National Institute of Standards and Technology, Gaithersburg, 2012, http://srdata.nist.gov/xps/.
- P. Yang, D. Zhao, D. I. Margolese, B. F. Chmelka and G. D. Stucky, Chem. Mater., 1999, 11, 2813–2826 CrossRef CAS.
- J. Peng, X. Li, D. H. Kim and W. Knoll, Macromol. Rapid Commun., 2007, 28, 2055–2061 CrossRef CAS.
- I. M. Arabatzis, T. Stergiopoulos, D. Andreeva, S. Kitova, S. G. Neophytides and P. Falaras, J. Catal., 2003, 220, 127–135 CrossRef CAS.
- A. Primo, A. Corma and H. Garcia, Phys. Chem. Chem. Phys., 2011, 13, 886–910 RSC.
- E. Kowalska, O. O. P. Mahaney, R. Abe and B. Ohtani, Phys. Chem. Chem. Phys., 2010, 12, 2344–2355 RSC.
- K. Nagaveni, G. Sivalingam, M. S. Hegde and G. Madras, Appl. Catal., B, 2004, 48, 83–93 CrossRef CAS PubMed.
- A. A. Gribb and J. F. Banfield, Am. Mineral., 1997, 82, 717–728 CAS.
- E. Kowalska, M. Janczarek, L. Rosa, S. Juodkazis and B. Ohtani, Catal. Today, 2014, 230, 131–137 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: AFM images of MgO, Al2O3, MnO2, Co3O4, NiO, CuO, ZnO, ZrO2, RuO2, SnO2, and Ce2O3 arrays; XPS peak positions of template oxides; titania loading calculations; time lapse UV-vis spectra of methylene blue photodegradation using SPICE TiO2; and TiO2 dispersion calculations. See DOI: 10.1039/c5ta00117j |
| This journal is © The Royal Society of Chemistry 2015 |