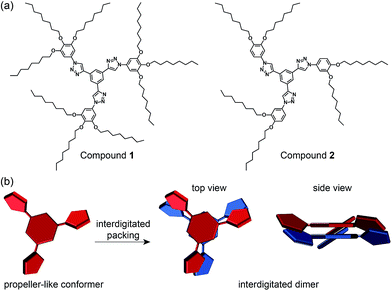
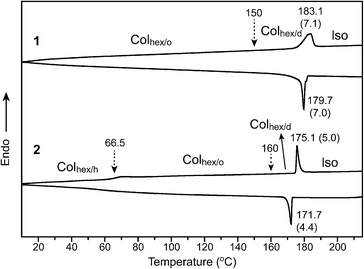
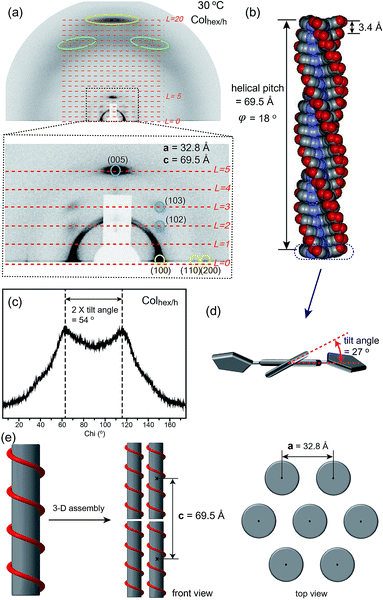
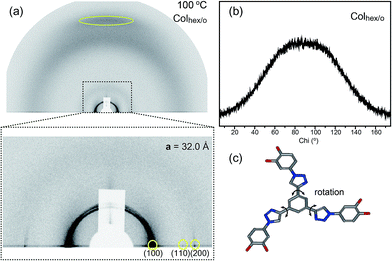
Sequential phase transformation of propeller-like C3-symmetric liquid crystals from a helical to ordered to disordered hexagonal columnar structure†
Soyoung
Park
and
Byoung-Ki
Cho
*
Department of Chemistry and Institute of Nanosensor and Biotechnology, Dankook University, Gyeonggi-Do 448-701, Korea. E-mail: chobk@dankook.ac.kr; Tel: +82 31 8005 3153
First published on 22nd October 2014
Abstract
In this paper, we report thermally induced intercolumnar phase transitions of C3-symmetric liquid crystals (LCs) bearing a triazole-based propeller-like aromatic mesogen. Since the constituting aromatic rings are conjugated through rotatable single bonds, the mesogenic shape is tuneable depending on the degree of conformational motion. Molecule 1 with ninefold octyl peripheries shows a hexagonal columnar liquid crystalline phase transition from ordered mesogenic stacking to disordered mesogenic stacking upon heating. On the other hand, molecule 2 with sixfold octyl peripheries displays a helical hexagonal columnar phase with the P6/mmm space group at ambient temperature as well as the ordered and disordered hexagonal columnar phases at higher temperatures. The intracolumnar helical order can be understood by an interdigitated stacking of the propeller-like mesogens along the columnar axis and the optimized space-filling. Notably, all the intercolumnar phase transformations in this study are revealed as second-order transitions. The thermodynamic nature agrees well with the fact that the conformational motions of the C3-symmetric aromatic mesogen change abruptly with each columnar transition.
Introduction
Columnar assemblies formed by aromatic building units have been paid a great deal of attention because they can be employed as a platform material for their application in photovoltaics and field effect transistors.1 It is well-known that discotic liquid crystals (DLCs) consisting of a flat aromatic disk and flexible chains organize into columnar phases via the orthogonal stacking of aromatic disks.2 Even though most columnar structures are derived from the classical two-dimensional (2-D) flat discogens, some molecules consisting of three-dimensional (3-D) aromatic cores such as bowl-like,3 tetrahedral,4 octahedral,5 star-shaped,6 or propeller-like mesogens7 are capable of self-assembling into columnar arrays via their unique stereoscopic stackings. Such unconventional 3-D aromatic stacks within a column sometimes induce interesting ferroelectric, charge-carrying, and thermochromic functions.8Depending on the unidirectional interactions and steric requirement, DLCs form helical arrangements within a column. As a result of the intracolumnar helical arrangement, the packing order of aromatic disks can improve, which sometimes increases the charge carrier mobility along the helical column.9 As ways to organize helical assemblies, non-covalent interactions,7b,10e.g., hydrogen-bonding, dipolar forces, and metal complexation, have been employed. In the molecular design of helically organizing DLCs, appropriate functional units should be incorporated with aromatic disks to induce helicity. As a steric element, chiral groups could be attached in the vicinity of a flat aromatic disk in order to promote the helical sense.11
Such a helical columnar structure is possibly derived by self-assembly of 3-D aromatic mesogens. As a strong mesogenic candidate, propeller-like molecules can be taken into consideration. Due to the symmetrically twisted shape, propeller-like molecules are inclined to produce an intracolumnar helical order, which can minimize void. Indeed, some LC examples with fixed propeller shapes were reported to organize into hexagonal columnar phases with helical orders, although they did not possess any chiral element at the periphery.7c–e Considering the 3-D molecular structure with the aromatic wings and central core, the mesogenic wing parts would be interdigitated to some extent along the columnar axis during the stepwise helical stacking.
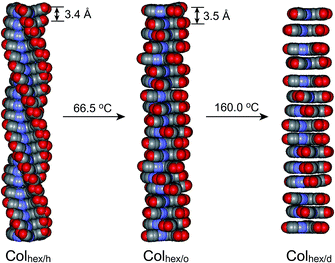
In this context, we envisioned that a propeller-like mesogen could be induced by the conformational variation of C3-symmetric molecules. As shown in Fig. 1a, we designed two LC molecules 1 and 2 with ninefold and sixfold octyl peripheries, respectively. The molecules are based on a C3-symmetric propeller-like mesogen which consists of three triazole-based aromatic wings and a benzenyl core. Since all the aromatic rings are conjugated through rotatable single bonds, we speculated that the mesogenic shape would be tuneable depending on the degree of the rotational motions of the dendritic wings which would change in response to temperature. Furthermore, we conjectured that the stacking mode of the shape-tuneable mesogen would be closely associated with the number of peripheral chains in terms of the space-filling requirement, and possibly a helical columnar phase could be derived from the C3-symmetric molecular propellers in cases when the dendritic wings could stack in an interdigitated mode (Fig. 1b).
Herein, we report a unique columnar phase sequence, i.e., a helical columnar (Colhex/h) to an ordered columnar (Colhex/o) to a disordered columnar (Colhex/d) phase, as a function of temperature. All the columnar phases display an identical 2-D hexagonal symmetry, but the structural difference occurs in the mesogenic packing order along the columnar axis. To the best of our knowledge, the sequential intercolumnar transition is reported for the first time in this study, although the fragmentary intercolumnar transitions, e.g., helical to ordered,9c,11c,12 ordered to disordered,13 and helical to disordered14 intercolumnar changes, were reported. In addition to the unique phase phenomenon, another remarkable feature is the thermodynamic nature of the intercolumnar transitions. Both Colhex/h-to-Colhex/o and Colhex/o-to-Colhex/d transformations turned out to be second-order transitions. In this paper, the unique phase transitional details are addressed.
Results and discussion
The design and synthesis of the C3-symmetric molecules utilized click chemistry.15 To prepare the molecules (1 and 2) through a click reaction, ethynyl and azido precursors were synthesized by following the procedures described previously.16 The target molecules were obtained by a click reaction of the obtained azido and alkyne precursors using CuSO4·5H2O and sodium ascorbate as catalysts (Scheme S1†). The molecules were characterized by 1H and 13C-NMR spectroscopy techniques, elemental analysis, and gel permeation chromatography (GPC). All the experimental data agree well with the molecular structures (Fig. S1 and S2†).The thermal behavior was investigated by differential scanning calorimetry (DSC). In Fig. 2, molecule 1 with ninefold octyl chains shows a reversible endothermic peak in the heating and cooling scans. Since the degree of supercooling (ΔT = 3.4 °C) and the corresponding enthalpy change (7.1 kJ mol−1) are small, the endothermic peak is considered to be a LC-to-liquid transition. Similar to 1, 2 with sixfold octyl peripheries exhibits an endothermic peak at 175.1 °C, which indicates the formation of an LC phase. However, a signal like a glass transition is observed at 66.5 °C. Considering the nature of the molecular components such as the aromatic and flexible octyl groups, the transition may be attributed to the conformational change of the aromatic mesogen.
To characterize their LC phases, we examined the optical textures of birefringent phases using polarized optical microscopy (POM). From the POM observations, both molecules 1 and 2 display a fan-like texture over the entire temperature range from 25 °C to the isotropization temperatures (Fig. S3†). This POM texture is typically observed from the columnar LC phases of conventional DLCs.17 Interestingly, the fan-like texture in the LC of 2 does not change all the way down to 30 °C, and the cover glass of the sandwich-type sample cell of 2 does not move at all when pushing it. These observations indicate that the transition at 66.5 °C is a second-order glass transition.
To demonstrate the microstructures of the birefringent phases, temperature-variable small- and wide angle X-ray scattering (SAXS and WAXS) experiments were performed. Fig. 3 represents the SAXS and WAXS data of 1. In the SAXS data, 1 reveals an identical reflection pattern over the entire temperature range of 25 °C–180 °C (Fig. 3a). Four reflections with a q-spacing ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √3
√3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √4
√4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √7 are observed, and they can be indexed to the (100), (110), (200), and (210) planes of a 2-D hexagonal lattice (Table 1). In contrast, the WAXS data alters upon heating. In the WAXS pattern at 30 °C, two broad reflections are observed (Fig. 3b and S4†). One reflection near 1.45 Å−1 corresponds to the mean distance of molten octyl chains, and the other reflection near 1.78 Å−1 is derived from the average packing distance (3.5 Å) between the aromatic segments. As the temperature elevates, the aromatic stacking reflection becomes gradually weaker, and finally disappears. Consequently, the gradual intercolumnar transition involves the loss of the long-range stacking correlation of the aromatic cores. The lower and higher temperature LC phases are assigned as Colhex/o and Colhex/d phases, respectively. Although the transition temperature of the gradual intercolumnar transformation is difficult to pinpoint, it may be determined through a Gaussian peak separation of the WAXS data. In the peak separation, the two peak positions are set near 1.45 and 1.79 Å−1, respectively. Up to 140 °C, the peak separation yields two noticeable peaks. But, the peak at 1.79 Å−1 cannot be assigned above 150 °C, which is approximately determined to be the transition temperature (Fig. S5†).
√7 are observed, and they can be indexed to the (100), (110), (200), and (210) planes of a 2-D hexagonal lattice (Table 1). In contrast, the WAXS data alters upon heating. In the WAXS pattern at 30 °C, two broad reflections are observed (Fig. 3b and S4†). One reflection near 1.45 Å−1 corresponds to the mean distance of molten octyl chains, and the other reflection near 1.78 Å−1 is derived from the average packing distance (3.5 Å) between the aromatic segments. As the temperature elevates, the aromatic stacking reflection becomes gradually weaker, and finally disappears. Consequently, the gradual intercolumnar transition involves the loss of the long-range stacking correlation of the aromatic cores. The lower and higher temperature LC phases are assigned as Colhex/o and Colhex/d phases, respectively. Although the transition temperature of the gradual intercolumnar transformation is difficult to pinpoint, it may be determined through a Gaussian peak separation of the WAXS data. In the peak separation, the two peak positions are set near 1.45 and 1.79 Å−1, respectively. Up to 140 °C, the peak separation yields two noticeable peaks. But, the peak at 1.79 Å−1 cannot be assigned above 150 °C, which is approximately determined to be the transition temperature (Fig. S5†).
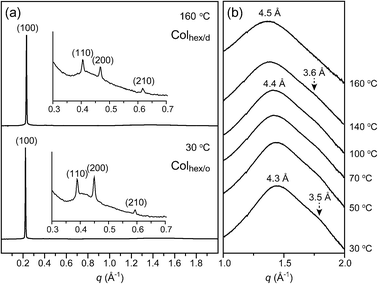 | ||
| Fig. 3 (a) SAXS data of the bulk sample of 1 at 30 °C and 160 °C and (b) WAXS data of the bulk sample of 1 at various temperatures. | ||
| d cal/Å | d exp/Å | (hkl) | Lattice parameters | |
|---|---|---|---|---|
| a d cal, calculated lattice spacing; dexp, experimental lattice spacing. | ||||
| 30 °C Colhex/o | 28.2 | 28.2 | (100) | a = 32.5 Å, 3.5 Å, (π–π stacking) |
| 16.3 | 16.3 | (110) | ||
| 14.1 | 14.1 | (200) | ||
| 10.7 | 10.7 | (210) | ||
| 140 °C Colhex/o | 27.3 | 27.3 | (100) | a = 31.5 Å, 3.6 Å, (π–π stacking) |
| 15.8 | 15.8 | (110) | ||
| 13.7 | 13.6 | (200) | ||
| 10.4 | 10.3 | (210) | ||
| 160 °C Colhex/d | 27.0 | 27.0 | (100) | a = 31.1 Å |
| 15.6 | 15.6 | (110) | ||
| 13.5 | 13.5 | (200) | ||
| 10.2 | 10.2 | (210) | ||
It should be noted that no peak is found at the Colhex/o-to-Colhex/d transition in the DSC data (Fig. 2). Therefore, this intercolumnar transition is a second-order transition rather than a first-order one. On the other hand, in rigid fused aromatic ring-based discotic LCs, similar intercolumnar transitions were reported to be first-order transitions previously.7a,12a–d,13 An endothermic peak appeared in the DSC data. On the basis of our previous results, we assume that the thermodynamic nature of such an intercolumnar transition is strongly dependent upon the mesogenic structure. For the propeller-like mesogens in this study, the packing correlation becomes diminished by the rotations of the bonds connecting aromatic rings, which results in the second-order transition. In contrast, for the fused aromatic mesogens like triphenylene or porphyrazine, a certain amount of energy has to be supplied to weaken the stronger interdisc π–π interactions, which results in the first-order transition. Consequently, the degree of the interdisc interactions associated with the aromatic structure should be taken into account to evaluate the thermodynamic properties.
Molecule 2 with the sixfold octyl peripheries displays a more unique phase sequence with temperature. In addition to the Colhex/o and Colhex/d LC phases, a 3-D helical columnar structure is formed below the Tg of 66.5 °C. Fig. 4 represents the bulk SAXS and WAXS data of 2. Similar to the intercolumnar transition of 1, the Colhex/o and Colhex/d phases of 2 show the 2-D hexagonal reflections at 100 °C and 160 °C, respectively. Additionally, the WAXS reflection corresponding to the aromatic packing disappears upon entering into the Colhex/d phase. However, a drastic change in the SAXS pattern occurs below the Tg. The SAXS data at 30 °C exhibits a complicated reflection pattern including the 2-D hexagonal peaks, and concomitantly the WAXS reflection becomes even intensified.
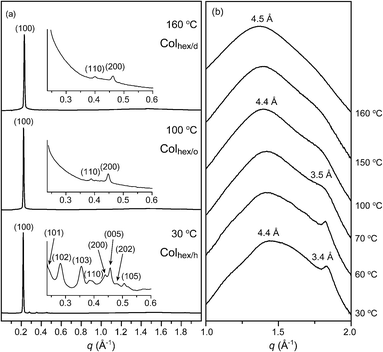 | ||
| Fig. 4 (a) SAXS data of the bulk sample of 2 at 30 °C, 100 °C and 160 °C and (b) WAXS data of the bulk sample of 2 at various temperatures. | ||
For the accurate structural analysis of the phase below the Tg, grazing incidence X-ray scattering (GIXS) experiments were performed with the surface-oriented sample. The aligned sample was prepared by casting the chloroform solutions (∼3 wt%) on 3-aminopropyltriethoxysilane (APS)-coated Si-wafers, and was slowly cooled to room temperature (RT) from the isotropic transition temperature.18Fig. 5a represents the spotty GIXS image of 2 at 30 °C. The hexagonal reflections corresponding to the (100), (110), and (200) planes are found at the equator, which indicates that columns stand on the substrate. On the other hand, two intense reflections are revealed along the meridian. The reflection in the wider angle corresponds to the stacking order of aromatic segments. The stacking distance is 3.4 Å. In particular, one that appears at q = 1.84 Å−1 strongly suggests an intracolumnar order which is a helical stacking between propeller-like mesogens. Furthermore, two off-axis reflections are observed in the small angle region. Based on the X-ray reflections, the meridian component can be divided into twenty equal distances. From the GIXS pattern, the helical parameter (φ, a mutual rotational angle between neighboring propellers) is calculated to be 18° (Fig. 5b).7a,9b In the wide angle region of the GIXS data, two symmetric reflections at q = 1.61 Å−1 are found (Fig. 5a). Since this helical phase forms below the Tg, the conformation of the aromatic mesogens is considered to be fixed. Thus, these reflections are attributed to the tilted aromatic wings with respect to the central benzenyl group. From the azimuthal plot of the reflections, the fixed tilt angle is measured to be 27° (Fig. 5c and d).7a,9b The tilted conformation of the aromatic wings may lead to an effective space-filling.
As shown in the magnified small angle region (the below part in Fig. 5a), the off-meridional reflections on the L = 2 and 3 layers can be assigned to the (102) and (103) planes, which demonstrates the correlation between intercolumnar helical units. Consequently, the assembled structure below the Tg can be interpreted as a 3-D helical columnar structure (Colhex/h) with the P6/mmm space group (Fig. 5e). The lattice parameters are estimated to be a = 32.8 Å and c = 69.5 Å. The complicated pattern of the bulk SAXS data at 30 °C in Fig. 4a also agrees well with the Colhex/h structure (Table 2).
| d cal/Å | d exp/Å | hkl | Lattice parameters | |
|---|---|---|---|---|
| a d cal, calculated lattice spacing; dexp, experimental lattice spacing. | ||||
| 30 °C Colhex/h | 28.4 | 28.4 | (100) | a = 32.8 Å, c = 69.5 Å, 3.4 Å, (π–π stacking) |
| 26.3 | 26.4 | (101) | ||
| 22.1 | 22.2 | (102) | ||
| 17.9 | 17.8 | (103) | ||
| 16.4 | 16.4 | (110) | ||
| 14.2 | 14.3 | (200) | ||
| 13.9 | 13.9 | (005) | ||
| 13.1 | 13.1 | (202) | ||
| 12.5 | 12.4 | (105) | ||
| 12.1 | 12.1 | (203) | ||
| 11.0 | 11.0 | (204) | ||
| 60 °C Colhex/h | 28.4 | 28.4 | (100) | a = 32.8 Å, c = 69.5 Å, 3.4 Å, (π–π stacking) |
| 22.1 | 22.3 | (102) | ||
| 17.9 | 17.8 | (103) | ||
| 16.4 | 16.4 | (110) | ||
| 14.2 | 14.3 | (200) | ||
| 13.9 | 13.8 | (005) | ||
| 12.5 | 12.5 | (105) | ||
| 70 °C Colhex/o | 28.3 | 28.3 | (100) | a = 32.7 Å, 3.5 Å, (π–π stacking) |
| 16.4 | 16.4 | (110) | ||
| 14.2 | 14.2 | (200) | ||
| 100 °C Colhex/o | 27.7 | 27.7 | (100) | a = 31.9 Å, 3.5 Å, (π–π stacking) |
| 16.0 | 16.0 | (110) | ||
| 13.8 | 13.8 | (200) | ||
| 150 °C Colhex/o | 27.0 | 27.0 | (100) | a = 31.2 Å, 3.5 Å, (π–π stacking) |
| 15.6 | 15.6 | (110) | ||
| 13.3 | 13.4 | (200) | ||
| 160 °C Colhex/d | 26.7 | 26.7 | (100) | a = 30.9 Å |
| 15.5 | 15.4 | (110) | ||
| 13.4 | 13.5 | (200) | ||
According to our current work and previous research studies, complex factors such as mesogenic geometry,3–7 degree of branching,7b,h,8c,9a,19 peripheral chain length,12a,b,13,14e,20 and the size of the aromatic core6d,e,7h,14c,d may be involved in the intracolumnar molecular packing. These factors work cooperatively, and thereby it is difficult to say individually the effect of each factor. Nevertheless, some factors may be considered independently to elucidate the packing tendency in intracolumnar assemblies. Especially, for the helical columnar formation, the molecular shape including mesogenic and peripheral chain parts is crucial, although other molecular parameters like a stereogenic chiral group11 is also taken into consideration. In the intracolumnar helical structure, mesogens should stack on top of each other with a certain mutual rotation angle. At a given peripheral chain, symmetric star-shaped or propeller-like mesogens may be more advantageous than flat aromatic cores. In terms of helical spacing-filling, the former with a radially undulated molecular shape can pack tightly via an interdigitated stacking on top of each other, which is more likely to form a helical stacking than the latter with the planar shape.
Likewise, the density of peripheral chains in DLCs should be symmetric and unequally distributed in the design of the helical columnar assembly. The chain density distribution is affected by the degree of chain branching and chain length relative to the aromatic core. This is exemplified in molecules 1 and 2 with the identical aromatic core. The Colhex/h structure is not observed in 1 with ninefold octyl peripheries. Thus, the number of octyl peripheries significantly influences the helical space-filling in a column, and guides the stacking mode of the propeller-like aromatic mesogen.
Like the stacking model proposed in Fig. 1b, the aromatic mesogen of 2 is assumed to interdigitate to some extent on top of each other along the columnar axis. This assumption can be clarified by investigating the number (N) of molecules in a columnar slice of the RT structure, Colhex/o and Colhex/h for 1 and 2, respectively. The N value can be calculated by the volume (Vcs) of a columnar slice/the molecular volume (Vmol). By dividing the molecular weight (M.W.) by the measured density (ρ) at RT, the Vmol values for 1 and 2 are obtained to be 3220.5 Å3 and 2518.9 Å3, respectively. On the basis of the X-ray data, the Vcs values can be estimated by the columnar cross-sectional area (S = a × d(100)) × the thickness (h) of a columnar slice. Although the Vmol of 2 is approximately 22% smaller than that of 1, their Vcs values are almost identical. Using the obtained Vcs and Vmol, the N values at RT are calculated to be 1.00 and 1.26 for 1 and 2, respectively (Table 3). Similar to conventional flat discogens, a single molecule of 1 constitutes the columnar slice. In contrast, the columnar slice of 2 is composed of more than one molecule. The non-unit N value indicates an interdigitated stacking of the aromatic mesogens. The dendritic wings of the molecular propeller primarily interdigitate in the columnar stacking. This stacking mode may enable the efficient filling of columnar space. In terms of space-filling, the interdigitation occurs more effectively in the columnar assembly of 2 with the sixfold octyl chains. Since its dendritic wings are less bulky than those of 1, more empty space will be generated if only a molecule covers the columnar slice.
| M.W. (g mol−1) | ρ (g cm−3) | V mol (Å3) | S (Å2) | h (Å) | V cs (Å3) | N (Vcs/Vmol) | |
|---|---|---|---|---|---|---|---|
| a ρ, density measured at RT; Vmol, molecular volume; S, columnar cross-sectional area; h, thickness of a columnar cross-section from WAXS data; Vcs, volume of a columnar slice; N, number of molecules in a columnar slice. | |||||||
| Colhex/o (1) | 1661.5 | 0.857 | 3220.5 | 916.4 | 3.5 | 3207.4 | 1.00 |
| Colhex/h (2) | 1276.8 | 0.842 | 2518.9 | 933.4 | 3.4 | 3173.6 | 1.26 |
It is worth noting that in our recent work, an analogous C3-symmetric molecule with slightly flexible benzylic units formed a lamellar structure rather than a columnar one by adopting a Y-shaped conformer.21 Through the conformational variation, the analogue LC could fill the space efficiently without vacancy. However, 2 with the fully conjugated aromatic mesogen cannot adopt such a Y-shaped conformer, but it primarily forms the C3-symmetric conformer due to its limited conformation. Consequently, interdigitation stacking is the way to meet the space-filling requirement by keeping the C3-symmetric conformer.
Upon heating above the Tg, the Colhex/h phase of 2 is transformed into a Colhex/o phase. In the GIXS data, the (005) reflection at the meridian and the diagonal reflections disappear, while the two-dimensional hexagonal reflections at the equator and the aromatic stacking reflection at the meridian still remain. This GIXS pattern is consistent with the bulk X-ray data (Fig. 6a). In addition, the off-meridional reflections at q = 1.61 Å−1 disappear in the Colhex/o phase, as shown in the azimuthal plot (Fig. 6b). This means that the torsional angle of the triazolyl group with respect to the central benzenyl group is not fixed, and the bond begins to rotate upon transitioning into the Colhex/o phase (Fig. 6c). In the Colhex/o phase, the degree of interdigitation reduces, and thereby the average shape of the aromatic mesogen may become a two-dimensional disk.
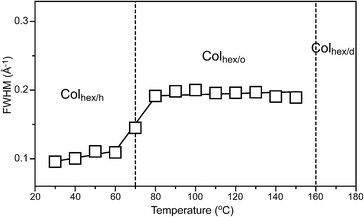
The conformational motions increase the mesogenic fluctuation along the columnar axis. This can be confirmed by examining the full width at half maximum (FWHM) of the peak near 1.80 Å−1. Like the way to determine the Colhex/o-to-Colhex/d transition temperature of 1, the peak corresponding to the aromatic stacking is able to be isolated by the Gaussian peak separation of the WAXS data. The peak isolation of 2 can be performed only up to 150 °C. In the Colhex/d phase, the peak cannot be extracted from the peak separation. In Fig. 7, the FWHM increases suddenly near 70 °C. Interestingly, this transitional temperature is consistent with the Tg in the DSC data. This result indicates that the long-range correlation between aromatic mesogens somewhat decreases by the conformational rotations of the aromatic mesogen.
Upon further heating to the Colhex/d phase, aromatic mesogens become distributed more randomly within a column. In the WAXS data, no peak is observed near 1.80 Å−1 (Fig. 4b). The long-range correlation of the aromatic cores disappears because the conformational motions become even greater in the Colhex/d phase. It is worth noting that the sequential phase transitions from the Colhex/h to Colhex/o to Colhex/d phase are second-order transitions as identified by the DSC data. Therefore, by considering that the change in the conformational motions does not require a considerable energy, the conformational argument for the sequential intercolumnar transitions is reasonable.
Thermally-induced intercolumnar phase transitions are led by the variation in the degree of mesogenic stacking order which is influenced by peripheral chain motions. At an intercolumnar transition, mesogenic ordering should be altered. According to previous publications, molecular examples showing only the fragmentary intercolumnar transitions (e.g., Colhex/o-to-Colhex/d, Colhex/h-to-Colhex/o) mostly consist of fused aromatic cores such as hexa-peri-hexabenzocoronenes,9c phthalocyanine,11c triphenylene,12d,13a and porphyrazine.13b In comparison to molecule 2 showing the multiple intercolumnar transitions in this study, these fused aromatic mesogens are considered to interact more strongly with each other due to their large, planar and rigid mesogenic structures, assuming that an identical peripheral chain group is attached. Strong intermesogenic interactions may cause the discogen stacking order to be less sensitive to the variation of flexible chain motions. Accordingly, DLCs with the large fused aromatic mesogens may show only a single intercolumnar transformation if any. In contrast, the C3-symmetric mesogen of 2 is composed of rotatable aromatic segments. Therefore, the mesogenic stacking order may be altered readily in response to the conformational motions of peripheral chains.
Conclusions
In this study, we prepared two C3-symmetric molecules 1 and 2 with ninefold and sixfold octyl peripheries, respectively, using click chemistry. The structural analysis proved that 1 underwent a second-order phase transition from the Colhex/o to the Colhex/d LC phases as the temperature increased. On the other hand, 2 with less bulky dendritic wings displayed a helical hexagonal columnar phase (Colhex/h) at RT as well as the subsequent Colhex/o to Colhex/d phases upon heating (Fig. 8). In particular, the unique sequential intercolumnar phase transformation of 2 can be elucidated by the stepwise conformational variation of the C3-symmetric mesogen and the optimized longitudinal space-filling. Therefore, an interdigitated packing of the propeller-like mesogens can be proposed as the plausible model for the helical formation. The C3-symmetric molecular system provides a versatile approach to fine-tune the aromatic packing structure within the 1-D aromatic column, and thereby the assembling concept will be useful in the design of 1-D electro-active materials with morphology-dependent tuneable mobility.Acknowledgements
The present research was conducted by the research fund of Dankook University in 2014. We acknowledge the Pohang Accelerator Laboratory (Beamline 9A), Korea.Notes and references
- (a) J. Nelson, Science, 2001, 293, 1059–1060 CrossRef CAS PubMed; (b) C. W. Tang, Appl. Phys. Lett., 1986, 48, 183–185 CrossRef CAS PubMed; (c) L. Schmidt-Mende, A. Fechtenkötter, K. Müllen, E. Moons, R. H. Friend and J. D. MacKenzie, Science, 2001, 293, 1119–1122 CrossRef CAS PubMed; (d) J. P. Schmidtke, R. H. Friend, M. Kastler and K. Müllen, J. Chem. Phys., 2006, 124, 174704 CrossRef PubMed; (e) H. E. Katz, A. J. Lovinger, J. Johnson, C. Kloc, T. Siegrist, W. Li, Y.-Y. Lin and A. Dodabalapur, Nature, 2000, 404, 478–481 CrossRef CAS PubMed; (f) W. Pisula, A. Menon, M. Stepputat, I. Lieberwirth, U. Kolb, A. Tracz, H. Sirringhaus, T. Pakula and K. Müllen, Adv. Mater., 2005, 17, 684–689 CrossRef CAS; (g) M. Katsuhara, I. Aoyagi, H. Nakajima, T. Mori, T. Kambayashi, M. Ofuji, Y. Takanishi, K. Ishikawa, H. Takezoe and H. Hosono, Synth. Met., 2005, 149, 219–223 CrossRef CAS PubMed; (h) A. Kraft, ChemPhysChem, 2001, 2, 163–165 CrossRef CAS.
- (a) I. Paraschiv, K. de Lange, M. Giesbers, B. van Langen, F. C. Grozema, R. D. Abellon, L. D. A. Siebbeles, E. J. R. Sudhölter, H. Zuilhof and A. T. M. Marcelis, J. Mater. Chem., 2008, 18, 5475–5481 RSC; (b) S. Sergeyev, W. Pisula and Y. H. Geerts, Chem. Soc. Rev., 2007, 36, 1902–1929 RSC; (c) R. J. Bushby and O. R. Lozman, Curr. Opin. Colloid Interface Sci., 2002, 7, 343–354 CrossRef CAS; (d) S. Kumar, Chem. Soc. Rev., 2006, 35, 83–109 RSC.
- (a) K. Isoda, T. Yasuda and T. Kato, Chem.–Asian J., 2009, 4, 1619–1625 CrossRef CAS PubMed; (b) Y. Matsuo, A. Muramatsu, Y. Kamikawa, T. kato and E. Nakamura, J. Am. Chem. Soc., 2006, 128, 9586–9587 CrossRef CAS PubMed; (c) T. Hatano and T. Kato, Chem. Commun., 2006, 1277–1279 RSC; (d) K. Sato, Y. Itoh and T. Aida, J. Am. Chem. Soc., 2011, 133, 13767–13769 CrossRef CAS PubMed; (e) B. Xu and T. M. Swager, J. Am. Chem. Soc., 1993, 115, 1159–1160 CrossRef CAS.
- (a) X. H. Cheng, S. Diele and C. Tschierske, Angew. Chem., Int. Ed., 2000, 39, 592–595 CrossRef CAS; (b) A. Pegenau, T. Hegmann, C. Tschierske and S. Diele, Chem.–Eur. J., 1999, 5, 1643–1660 CrossRef CAS; (c) A. Pegenau, P. Göring and C. Tschierske, Chem. Commun., 1996, 2563–2564 RSC.
- (a) S. T. Trzaska, H.-F. Hsu and T. M. Swager, J. Am. Chem. Soc., 1999, 121, 4518–4519 CrossRef CAS; (b) H. Zheng and T. M. Swager, J. Am. Chem. Soc., 1994, 116, 761–762 CrossRef CAS.
- (a) M. Lehmann, R. I. Gearba, M. H. J. Koch and D. A. Ivanov, Chem. Mater., 2004, 16, 374–376 CrossRef CAS; (b) M. Lehmann and M. Jahr, Chem. Mater., 2008, 20, 5453–5456 CrossRef CAS; (c) M. Lehmann, M. Jahr and J. Gutmann, J. Mater. Chem., 2008, 18, 2995–3003 RSC; (d) M. Lehmann, M. Jahr, F. C. Grozema, R. D. Abellon, L. D. A. Siebbeles and M. Müller, Adv. Mater., 2008, 20, 4414–4418 CrossRef CAS; (e) M. Lehmann, M. Jahr, B. Donnion, R. Graf, S. Gemming and I. Popov, Chem.–Eur. J., 2008, 14, 3562–3576 CrossRef PubMed.
- (a) M. Peterca, M. R. Imam, C.-H. Ahn, V. S. K. Balagurusamy, D. A. Wilson, B. M. Rosen and V. Percec, J. Am. Chem. Soc., 2011, 133, 2311–2328 CrossRef CAS PubMed; (b) J. Barberá, L. Puig, P. Romero, J. L. Serrano and T. Sierra, J. Am. Chem. Soc., 2006, 128, 4487–4492 CrossRef PubMed; (c) Q. Xiao, T. Sakurai, T. Fukino, K. Akaike, Y. Honsho, A. Saeki, S. Seki, K. Kato, M. Takata and T. Aida, J. Am. Chem. Soc., 2013, 134, 18268–18271 CrossRef PubMed; (d) T. Metzroth, A. Hoffmann, R. Mrtín-Rapún, M. M. J. Smulders, K. Pieterse, A. R. A. Palmans, J. A. J. M. Vekemans, E. W. Meijer, H. W. Spiess and J. Gauss, Chem. Sci., 2011, 2, 69–76 RSC; (e) J. J. van Gorp, J. A. J. M. Vekemans and E. W. Meijer, J. Am. Chem. Soc., 2002, 124, 14759–14769 CrossRef CAS PubMed; (f) M. L. Bushey, T.-Q. Nguyen and C. Nuckolls, J. Am. Chem. Soc., 2003, 125, 8264–8269 CrossRef CAS PubMed; (g) M. L. Bushey, A. Hwang, P. W. Stephens and C. Nuckolls, J. Am. Chem. Soc., 2001, 123, 8157–8158 CrossRef CAS; (h) E. Beltrán, J. L. Serrano, T. Sierra and R. Giménez, J. Mater. Chem., 2012, 22, 7797–7805 RSC.
- (a) D. Miyajima, F. Araoka, H. Takezoe, J. Kim, K. Kato, M. Takata and T. Aida, Science, 2012, 336, 209–213 CrossRef CAS PubMed; (b) T. Yasuda, T. Shimizu, F. Liu, G. Ungar and T. Kato, J. Am. Chem. Soc., 2011, 133, 13437–13444 CrossRef CAS PubMed; (c) J. Kim, S. Cho and B.-K. Cho, Chem.–Eur. J., 2014, 20, 12734–12739 CrossRef CAS PubMed.
- (a) J. Wu, M. D. Watson and K. Müllen, Angew. Chem., Int. Ed., 2003, 42, 5329–5333 CrossRef CAS PubMed; (b) W. Pisula, Ž. Tomović, M. D. Watson, K. Müllen, J. Kussmann, C. Ochsenfeld, T. Metzroth and J. Gauss, J. Phys. Chem. B, 2007, 111, 7481–7487 CrossRef CAS PubMed; (c) J. Wu, M. D. Watson, L. Zhang, Z. Wang and K. Müllen, J. Am. Chem. Soc., 2004, 126, 177–186 CrossRef CAS PubMed.
- (a) F. Vera, J. L. Serrano and T. Sierra, Chem. Soc. Rev., 2009, 38, 781–796 RSC; (b) E. Beltrán, E. Cavero, J. Barberá, J. L. Serrano, A. Elduque and R. Giménez, Chem.–Eur. J., 2009, 15, 9017–9023 CrossRef PubMed.
- (a) Ž. Tomović, J. van Dongen, S. J. George, H. Xu, W. Pisula, P. Leclére, M. M. J. Smulders, S. D. Feyter, E. W. Meijer and A. P. H. J. Schenning, J. Am. Chem. Soc., 2007, 129, 16190–16196 CrossRef PubMed; (b) S. Ishihara, Y. Furuki and S. Takeoka, Polym. Adv. Technol., 2008, 19, 1097–1104 CrossRef CAS; (c) C. F. van Nostrum, A. W. Bosman, G. H. Gelinck, P. G. Schouten, J. M. Warman, A. P. M. Kentgens, M. A. C. Devillers, A. Meijerink, S. J. Picken, U. Sohling, A.-J. Schouten and R. J. M. Nolte, Chem.–Eur. J., 1995, 1, 171–182 CrossRef CAS.
- (a) V. Percec, H.-J. Sun, P. Leowanawat, M. Peterca, R. Graf, H. W. Spiess, X. Zeng, G. Ungar and P. A. Heiney, J. Am. Chem. Soc., 2013, 135, 4129–4148 CrossRef CAS PubMed; (b) V. Percec, S. D. Hudson, M. Peterca, P. Leowanawat, E. Aqad, R. Graf, H. W. Spiess, X. Zeng, G. Ungar and P. A. Heiney, J. Am. Chem. Soc., 2011, 133, 18479–18494 CrossRef CAS PubMed; (c) V. Percec, M. Peterca, T. Tadjiev, X. Zeng, G. Ungar, P. Leowanawat, E. Aqad, M. R. Imam, B. M. Rosen, U. Akbey, R. Graf, S. Sekharan, D. Sebastiani, H. W. Spiess, P. A. Heiney and S. D. Hudson, J. Am. Chem. Soc., 2011, 133, 12197–12219 CrossRef CAS PubMed; (d) E. Fontes and P. A. Heiney, Phys. Rev. Lett., 1988, 61, 1202–1205 CrossRef CAS; (e) V. Percec, E. Aqad, M. Peterca, J. G. Rudick, L. Lemon, J. C. Ronda, B. B. De, P. A. Heiney and E. W. Meijer, J. Am. Chem. Soc., 2006, 128, 16365–16372 CrossRef CAS PubMed; (f) D. Adam, P. Schuhmacher, J. Simmerer, L. Häussling, K. Siemensmeyer, K. H. Etzbach, H. Ringsdorf and D. Haarer, Nature, 1994, 371, 141–143 CrossRef CAS.
- (a) B.-K. Cho and S.-H. Kim, Soft Matter, 2014, 10, 553–559 RSC; (b) K. Ohta, S. Azumane, W. Kawahara, N. Kobayashi and I. Yamamoto, J. Mater. Chem., 1999, 9, 2313–2320 RSC.
- (a) V. Percec, M. Goldde, M. Peterca, A. Rapp, I. Schnell, H. W. Spiess, T. K. Bera, Y. Miura, V. S. K. Balagurusamy, E. Aqad and P. A. Heiney, Chem.–Eur. J., 2006, 12, 6298–6314 CrossRef CAS PubMed; (b) V. Percec, E. Aqad, M. Peterca, M. R. Imam, M. Glodde, T. K. Bera, Y. Miura, V. S. K. Balagurusamy, P. C. Ewbank, F. Würthner and P. A. Heiney, Chem.–Eur. J., 2007, 12, 3330–3345 CrossRef PubMed; (c) V. Percec, M. R. Imam, M. Peterca, D. A. Wilson and P. A. Heiney, J. Am. Chem. Soc., 2009, 131, 1294–1304 CrossRef CAS PubMed; (d) V. Percec, M. R. Imam, M. Peterca, D. A. Wilson, R. Graf, H. W. Spiess, V. S. K. Balagurusamy and P. A. Heiney, J. Am. Chem. Soc., 2009, 131, 7662–7677 CrossRef CAS PubMed; (e) H. C. Holst, T. Pakula and H. Meier, Tetrahedron, 2004, 60, 6765–6775 CrossRef CAS PubMed.
- S. Choi and B.-K. Cho, Soft Matter, 2013, 9, 4241–4248 RSC.
- (a) M.-H. Ryu, J.-W. Choi and B.-K. Cho, J. Mater. Chem., 2010, 20, 1806–1810 RSC; (b) K. Han and B.-K. Cho, Soft Matter, 2014, 10, 7588–7594 RSC.
- (a) M. Yoshio, T. Mukai, H. ohno and T. Kato, J. Am. Chem. Soc., 2004, 126, 994–995 CrossRef CAS PubMed; (b) V. Percec, C. M. Mitchell, W.-D. Cho, S. Uchida, M. Glodde, G. Ungar, X. Zeng, Y. Liu, V. S. K. Balagurusamy and P. A. Heiney, J. Am. Chem. Soc., 2004, 126, 6078–6094 CrossRef CAS PubMed.
- M. Yoshio, T. Kagata, K. Hoshino, T. Mukai, H. Ohno and T. Kato, J. Am. Chem. Soc., 2006, 128, 5570–5577 CrossRef CAS PubMed.
- (a) E. Beltrán, J. L. Serrano, T. Sierra and R. Giménez, Org. Lett., 2010, 12, 1404–1407 CrossRef PubMed; (b) J. Barberá, L. Puig, J. L. Serrano and T. Sierra, Chem. Mater., 2004, 16, 3308–3317 CrossRef.
- (a) K. Katoh, S. Vehara, S. Kohmoto and K. Kishikawa, Chem. Lett., 2006, 35, 322–323 CrossRef; (b) J. Barberá, J. Jiménez, A. Laguna, L. Orion, S. Pérez and J. L. Serrano, Chem. Mater., 2006, 18, 5437–5445 CrossRef; (c) M. Lehmann, G. Kestemont, R. G. Aspe, C. Buess-Herman, M. H. J. Koch, M. G. Debije, J. Piris, M. P. de Haas, J. M. Warman, M. D. Watson, V. Lemaur, J. Cornil, Y. H. Greets, R. Gearba and D. A. Ivanov, Chem.–Eur. J., 2005, 11, 3349–3362 CrossRef CAS PubMed.
- S. Park, M.-H. Ryu, T. J. Shin and B.-K. Cho, Soft Matter, 2014, 10, 5804–5809 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, synthesis schemes, 1H and 13C-NMR data, GPC data, optical textures, GIXS data of 1, and Gaussian peak separation of the WAXS data of 1. See DOI: 10.1039/c4sm02004a |
| This journal is © The Royal Society of Chemistry 2015 |