Open Access Article
Open Access ArticleVisualizing changes in mitochondrial Mg2+ during apoptosis with organelle-targeted triazole-based ratiometric fluorescent sensors†
G.
Zhang
,
J. J.
Gruskos
,
M. S.
Afzal
and
D.
Buccella
*
Department of Chemistry, New York University, New York 10003, USA. E-mail: dbuccella@nyu.edu
First published on 28th October 2015
Abstract
Magnesium is one of the most abundant metals in cells and is essential for a wide range of cellular processes. Magnesium imbalance has been linked to a variety of diseases, but the scarcity of sensors suitable for detection of Mg2+ with subcellular resolution has hampered the study of compartmentalization and mobilization of this ion in the context of physiological and pathological processes. We report herein a family of fluorescent probes for targeted detection of free Mg2+ in specific intracellular organelles, and its application in the study of programmed cell death. The new sensors feature a triazole unit that plays both structural and electronic roles by serving as an attachment group for targeting moieties, and modulating a possible internal charge transfer process for ratiometric ion sensing. A probe decorated with an alkylphosphonium group was employed for the detection of mitochondrial Mg2+ in live HeLa cells, providing the first direct observation of an increase in free Mg2+ levels in this organelle in the early stages of Staurosporine-induced apoptosis.
Introduction
Magnesium is essential for numerous cellular processes, playing a role in activation of enzymes, structural stabilization of nucleic acids and proteins, modulation of ion channels, and as a second messenger.1,2 In mammalian cells, Mg2+ is the most abundant divalent cation, with a total concentration typically maintained in the mid-millimolar range in most cell types.3,4 Abnormal levels of serum or cellular magnesium have been linked to various conditions including cardiovascular disease, diabetes, neurodegeneration, and cancer.5–9Despite the importance of Mg2+ homeostasis in human health, details of the mechanisms that regulate the concentration of this ion at the cellular and subcellular level have remained partially obscure, primarily due to the paucity of efficient tools for the measurement of Mg2+ with the required spatial and temporal resolutions.10 In particular, the ability to study intracellular ion distribution and mobilization between subcellular domains has been hampered by the scarcity of probes capable of reporting organelle-specific levels of Mg2+. In this regard, Oka and coworkers developed a rosamine-based Mg2+ turn-on indicator that spontaneously localizes to mitochondria.11 More recently, the same group reported a related turn-on biarsenical dye that can be anchored to tetracysteine-tagged proteins expressed in specific compartments, thus enabling the visualization of Mg2+ dynamics upon mitochondrial membrane depolarization.12 Genetically encoded protein-based FRET fluorescent sensors reported by Merkx and coworkers have been targeted to other intracellular compartments.13 A general platform suitable for organelle-targeted ratiometric detection of Mg2+ with small-molecule indicators, however, is still lacking.
The activation of apoptotic pathways bears close connection with cellular homeostasis of divalent cations, with Ca2+ playing a major role in regulation of the intrinsic (mitochondrial) pathway.14–16 The role of Mg2+, on the other hand, has not been clearly established. Changes in cytosolic Mg2+ concentration have been observed in glycodeoxycolate-induced apoptosis of hepatocytes,17 during proanthocyanidin/doxorubicin-induced apoptosis in K562/DOX cells,18 and in Fas ligand-induced apoptosis of B lymphocytes.19 In the latter example, an increase in cytosolic free Mg2+ was found to be independent of the extracellular concentration of the metal, which led to the hypothesis that mitochondria could be acting as an intracellular source. Until now, however, the dynamics of mitochondrial Mg2+ during apoptosis have not been observed directly in whole cells. In this report, we introduce a new family of fluorescent sensors for targeted ratiometric detection of Mg2+ in organelles of interest (Fig. 1), and present the first direct observation of the changes in free Mg2+ levels in mitochondria during early stages of Staurosporine-induced apoptosis in HeLa cells.
Results and discussion
Sensor design and synthesis
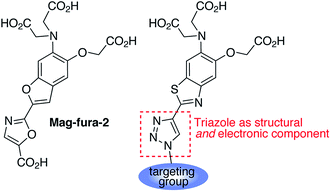
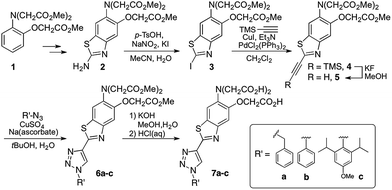
1,2,3-Triazoles assembled by copper catalyzed alkyne–azide cycloaddition (CuAAC)20 have been used extensively as structural linkages in fluorophore biocojugation, but only recently have their electronic features been exploited to influence the properties of fluorescent labels and sensors.21 We envisioned a sensor design incorporating a 1,2,3-triazole moiety as part of the fluorophore, replacing the oxazole group in furaptra22 and related ‘fura’ dyes.23 The triazole is thus intended to serve a dual purpose, namely, a structural role as an attachment group between fluorophore and an organelle-targeting moiety, and a possible electronic role as a modulator of an internal charge transfer (ICT) process for fluorescence-based ion sensing.We synthesized an alkynyl-functionalized benzothiazole, 5, to be employed as a precursor for rapid assembly of targeted ratiometric sensors via CuAAC (Scheme 1). This compound was obtained from 2-aminobenzothiazole 2, which was prepared by modification of a protocol reported by Metten and coworkers.24 The amino function was converted by diazotization and treatment with potassium iodide, followed by Sonogashira coupling with trimethylsilylacetylene and subsequent deprotection. The late stage click reaction with the resulting alkyne may be used to tune the chemical and biological properties of the final sensors with minimum synthetic effort, based on sensible choice of azide.
With the goal of evaluating the performance of our sensor design, model sensors 7a and 7b were prepared by reaction of alkyne 5 with benzyl- and phenylazide, respectively, followed by ester hydrolysis. Cycloaddition was performed on the ester-protected sensors in order to minimize residual copper binding to the metal-recognition unit, which may interfere with metal sensing in subsequent studies.
Spectroscopic properties of fluorescent sensors
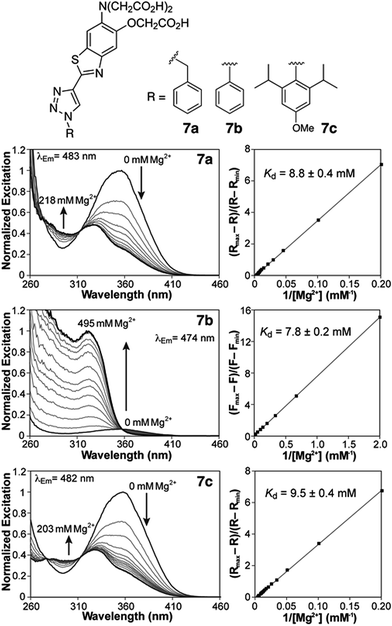
Photophysical characterization of sensors 7a,b was conducted in aqueous buffer mimicking physiological ionic strength (Table 1). The new triazole-based probes show large Stokes shifts in aqueous solution, and respond to Mg2+ with a significant blue shift in the fluorescence excitation and emission maxima (Table 1 and Fig. 2). These observations are consistent with a destabilizing effect of the cation on an excited state characterized by a large dipole moment. A similar response is observed with the related furaptra (Mag-fura-2) dye,22 suggesting a common ICT mechanism with the nitrogen of the metal-recognition unit acting as a donor.25 This notion is currently being investigated computationally.| Absorption λmax (nm), ε × 103 (M−1 cm−1) | Excitation λmax (nm) | Emission λmax (nm), Φb | R max/Rmin | K d,Mg2+ (mM) | K d,Ca2+ (μM) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Unbound | Mg2+-saturated | Unbound | Mg2+-saturated | Unbound | Mg2+-saturated | ||||
| a Measurements performed in 50 mM PIPES, 100 mM KCl, pH 7.0 at 25 °C. Molar absorptivity coefficients, fluorescence quantum yields and dissociation constants are averages of three determinations; numbers in parenthesis represent the uncertainty on the last significant figure. N.D. = not determined. b Quinine sulfate in 0.5 M H2SO4 (Φ347 = 0.546)28,29 was employed as a fluorescence standard. | |||||||||
| 7a | 354, 18.7(1) | 328, 17.9(7) | 354 | 328 | 493, 0.42(1) | 483, 0.235(8) | 2.7 | 8.8(4) | 64(3) |
| 7b | 356, 20.7(7) | 323, 17.4(2) | 356 | 323 | 495, 0.0053(3) | 474, 0.080(4) | N.D. | 7.8(2) | 58.9(8) |
| 7c | 356, 21.2(6) | 330, 16(1) | 356 | 330 | 495, 0.42(1) | 482, 0.25(2) | 2.5 | 9.5(4) | 71(4) |
| Mag-mito | 356, N.D. | 330, N.D. | 356 | 330 | 495, N.D. | 482, N.D. | 2.7 | 6.7(3) | 53.5(9) |
Both benzyl and phenyl derivatives 7a and 7b exhibit similar absorption and fluorescence emission wavelengths in their metal-free and -bound forms (Table 1), but the phenyl derivative exhibits a lower quantum yield than the benzyl derivative, well below 10%. We postulated that rotation around the triazole-phenyl bond may provide an efficient non-radiative decay pathway for 7b, via distortion of the excited state26 and/or access to a non-emissive twisted intramolecular charge transfer state.27 Attempts to test this hypothesis through measurements in solvents of increasing viscosity proved inconclusive. However, incorporation of sterically demanding isopropyl substituents on the ortho position of the phenyl ring (see derivative 7c, Scheme 1), which increase the barrier of rotation and disrupt a possible coplanar arrangement of phenyl and triazole rings, resulted in the recovery of the fluorescence quantum yields to values comparable to those of benzyl derivative 7a.
Compounds 7a and 7c are useful for ratiometric detection of Mg2+ (Fig. 2 and S1–S3, ESI†), with apparent dissociation constants in the low millimolar range at 25 °C (Kd,Mg2+ = 8.8 ± 0.4 and 9.5 ± 0.4 mM for 7a and 7c, respectively). On the other hand, the difference in brightness for the metal-free and -bound forms of phenyl derivative 7b makes it more suitable for a turn-on application (∼13-fold turn-on, Kd,Mg2+ = 7.8 ± 0.2 mM). It is important to note that these indicators detect free Mg2+, and do not respond to bound forms of the ion such as MgATP. In this regard, the fluorescence response of a solution of compound 7c treated with increasing amounts of Mg2+ in the presence of 18.4 mM ATP (Fig. S5†) can be modelled by considering a single binding event for the complexation of Mg2+ by the sensor. The fluorescence ratio expressed as a function of [Mg2+]free, calculated from the amount of total magnesium and dissociation of MgATP (Kd = 50 μM (ref. 30)), matches the isotherms obtained in the absence of the ATP (Fig. S5B†).
The optical properties of derivatives 7a–c were also tested in the presence of high concentrations of other biologically relevant divalent metal ions, including Ca2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, and Zn2+ (Fig. S6–S8†). The metal selectivity of the new probes is comparable to that of other related o-aminophenol-N,N,O-triacetic acid (APTRA)-based metal ion indicators.23,31 In addition to Mg2+, the compounds respond to mid-micromolar concentrations of Ca2+ (Table 1), thus could be employed as low-affinity Ca2+ indicators for the study of systems with particularly high concentrations of this ion. For compounds 7a and 7c, the changes in spectral properties upon Ca2+ coordination are similar to those observed in the presence of Mg2+ (Fig. S9 and S11†). For 7b, on the other hand, binding of Ca2+ leads to a blue shift in excitation with no significant increase in the emission efficiency, i.e. no turn-on response is obtained (Fig. S10†). The compounds also respond to the micromolar concentrations of Zn2+ tested.32 With few exceptions, however, the typical sub-nanomolar intracellular concentrations of this ion should not interfere with Mg2+ detection.33 Finally, the sensors are insensitive to variations in pH in the 5.5 to 8.0 range (Fig. S13†).
Targeted, organelle-specific sensing of free Mg2+
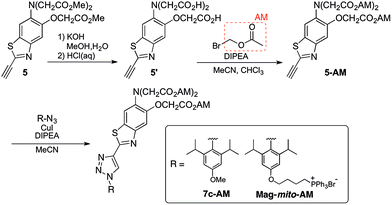
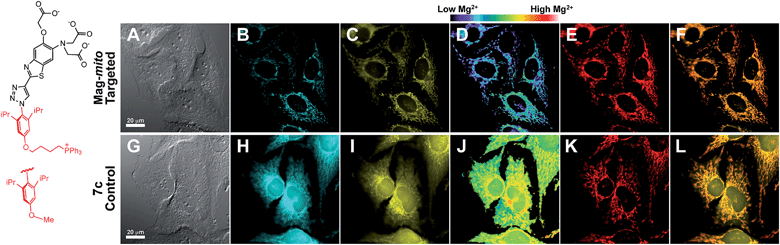
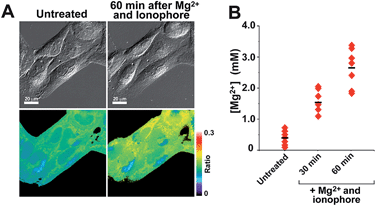
With insight gained from the model compounds characterized in vitro, we focused on the design of a mitochondria-targeted sensor. Mitochondria are regarded as intracellular reservoirs of Mg2+, and are invoked often as central players in the regulation of Mg2+ homeostasis due to their ability to take up and extrude this metal ion in a respiration-dependent manner.11,34,35 The potential caused by the proton gradient across the mitochondrial membrane can be exploited to direct the accumulation of small-molecules to this organelle. With this feature in mind, a derivative functionalized with a lipophilic cationic alkylphosphonium group36 (Mag-mito, Scheme 2) was prepared. This targeted sensor shows similar photophysical properties and metal response as those displayed by the analogue compound 7c, devoid of the targeting moiety (Table 1 and Fig. S4 and S12†).Mag-mito was tested for the excitation ratiometric imaging of mitochondrial Mg2+ in live HeLa cells by widefield fluorescence microscopy, using filter sets available for Mag-fura-2 and the Ca2+-sensitive analog Fura-2 (Fig. 3). To facilitate cell loading of the compound, the metal-binding carboxylate groups were masked as acetoxymethyl (AM) esters, which are readily cleaved by intracellular esterases after probe uptake.37 Cells were incubated with 1 μM of the sensor for 30 min at room temperature, rinsed, and then allowed to incubate for another 30 min for full de-esterification of the internalized probe. Successful targeting of the desired organelle was evidenced by a Pearson correlation coefficient of 0.83 in the co-localization analysis with MitoTracker green FM (Molecular Probes, Fig. 3F).38 This analysis was conducted over the three-dimensional volume of the cell, reconstructed from a z-stacked series of images (Fig. S14†). To the best of our knowledge, this is the first example of targeted ratiometric detection of mitochondrial Mg2+ with a fluorescent probe.39 For comparison, the non-targeted analog 7c, devoid of the alkylphosphonium group, was tested under the same conditions. This sensor showed relatively unselective staining of various compartments (Fig. 3H–J), with a correlation coefficient of 0.55 for the co-localization analysis with the reference mitochondrial stain. The ability of the indicators to respond to changes in intracellular Mg2+ concentrations was confirmed by collecting two sets of images of cells stained with non-targeted compound 7c, before and after treatment with non-fluorescent ionophore 4-bromo-A-23187 (Molecular Probes) and 20 mM of MgCl2 for 60 min. An increase in the average fluorescence ratio per cell (∼20%, Fig. 4 and S15†) was observed in response to the increase in intracellular free Mg2+ concentration mediated by the ionophore. Furthermore, the fluorescence excitation spectrum of 7c-loaded HeLa cells treated with ionophore and 50 mM EDTA for 30 min was acquired on a plate reader, showing a red-shift consistent with decreasing concentrations of intracellular Mg2+ (Fig. S16†).
Mitochondrial changes in free Mg2+ during apoptosis
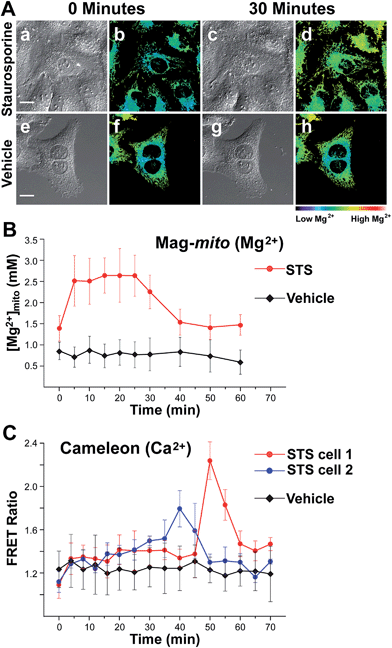
With a probe capable of detecting free Mg2+ in mitochondria, we investigated the changes in ion levels in these organelles during apoptosis induced by Staurosporine (STS) in HeLa cells. Live cells pre-loaded with Mag-mito were treated with 1 μM of the alkaloid on the fluorescence microscope stage, and monitored over the course of 120 min (Fig. 5). MitoTracker green was employed to confirm the localization of the Mg2+ probe and a caspase indicator was used to verify apoptosis, whereas ethidium homodimer-1 was used to rule out possible cell lysis from necrosis. Changes in the fluorescence ratio of the sensor revealed a roughly threefold increase in concentration of free Mg2+, which plateaued at 2.6 mM within 10 min and decreased slowly after ∼25 min as the process continued (Fig. 5B). Signal of the sensor and MitoTracker started to appear diffuse after approximately 40 min of observation, likely due to dye leakage upon depolarization of the mitochondrial membrane that makes the estimation of ion concentration less reliable at later points. Morphological changes associated with apoptosis such as mitochondrial fragmentation and cell blebbing were also observed. The caspase indicator became activated after ∼90 min, revealing the downstream events of the apoptosis cascade (Fig. S17†). For comparison, no significant changes were observed in cells treated with vehicle over the same period of time, showing a basal mitochondrial level of 0.8 mM free Mg2+ that remained constant throughout the experiment.Given the weak Ca2+ binding ability of APTRA-based sensors, we sought to rule out possible Ca2+-induced signal in our experiment by comparing the fluorescence response of Mag-mito with that obtained with a genetically encoded Ca2+-specific indicator. We conducted a similar experiment with HeLa cells transiently expressing cameleon 4mtD3cpv, which has been optimized for the detection of Ca2+ in mitochondria.40 The protein-based FRET indicator revealed Ca2+ elevations in mitochondria clusters starting after 30–40 min of treatment with the drug (Fig. 5C). The clear differences in the onset and duration of the Ca2+ signal in comparison with the response obtained by Mag-mito are consistent with the detection of Mg2+, and not Ca2+, by the small molecule probe. Another control experiment was conducted by adding tris-(2-pyridylmethyl)amine (TPA), a rapid picomolar Zn2+ chelator,41 15 min after induction of apoptosis. The fluorescence ratio did not show a decrease within the typical response time of the chelator, ruling out the interference of Zn2+ in our measurement (Fig. S18†). To the best of our knowledge, these results represent the first direct observation of changes in mitochondrial free Mg2+ during programmed cell death. The source of this pool of free Mg2+ is unknown at this time, but it could be attributed to its release from bound forms abundant in the mitochondrion (e.g. MgATP), or to an extra-mitochondrial origin. Significantly, studies conducted with isolated mitochondria by Martinou and coworkers have shown that Mg2+ may potentiate the release of cytochrome c from these organelles,42 thus hinting to the possible relevance of an early increase in free Mg2+ in the apoptotic cascade.
Conclusions
The ability to study metal compartmentalization and mobilization in cells in the context of physiological and pathological processes depends on the availability of fluorescence indicators that enable rapid detection of the ions with subcellular resolution. We have designed a new family of triazole-based fluorescent probes for targeted ratiometric detection of Mg2+ in intracellular organelles by fluorescence microscopy. The sensors are rapidly assembled by copper catalyzed alkyne–azide cycloaddition between an alkynyl benzothiazole, functionalized with an APTRA Mg2+ recognition unit, and an azide-functionalized organelle-targeting group of choice. The resulting triazole moiety plays both structural and electronic roles in the new sensors, by serving as an attachment group to organelle-targeting moieties and participating in a possible ICT process useful for ion sensing. With appropriate changes to the metal-binding functionality, the sensor design presented herein may be adapted for the targeted detection of other cations of biological relevance.We developed a sensor functionalized with a lipophilic cationic alkylphosphonium group, i.e.Mag-mito, which displays selective localization in mitochondria thus enabling the targeted ratiometric imaging of free Mg2+ within these organelles in live cells. A time-course fluorescence imaging study conducted on HeLa cells treated with Staurosporine provided the first direct observation of an increase in free Mg2+ levels in mitochondria during early stages of apoptosis. The onset of this change appears to precede Ca2+ entry into the organelle. Future studies will be aimed at identifying the origin and destination of this mitochondrial pool of free Mg2+ and its influence in the downstream events in the apoptotic cascade.
Acknowledgements
This research was supported by start-up funds granted to D. B. by New York University. The Bruker Avance-400 NMR spectrometer was acquired through the support of the National Science Foundation under Award Number CHE-01162222. pcDNA-4mtD3cpv was a gift from Amy Palmer & Roger Tsien (Addgene plasmid # 36324).References
- J. A. Cowan, in The Biological Chemistry of Magnesium, ed. J. A. Cowan, VCH Publishers, New York, 1995, pp. 1–23 Search PubMed.
- F.-Y. Li, B. Chaigne-Delalande, C. Kanellopoulou, J. C. Davis, H. F. Matthews, D. C. Douek, J. I. Cohen, G. Uzel, H. C. Su and M. J. Lenardo, Nature, 2011, 475, 471–476 CrossRef CAS PubMed.
- R. D. Grubbs, Biometals, 2002, 15, 251–259 CrossRef CAS PubMed.
- A. M. P. Romani, Arch. Biochem. Biophys., 2011, 512, 1–23 CrossRef CAS PubMed.
- N.-E. L. Saris, E. Mervaala, H. Karppanen, J. A. Khawaja and A. Lewenstam, Clin. Chim. Acta, 2000, 294, 1–26 CrossRef CAS.
- J. A. M. Maier, Mol. Aspects Med., 2003, 24, 137–146 CrossRef CAS PubMed.
- M. Barbagallo and L. J. Dominguez, Arch. Biochem. Biophys., 2007, 458, 40–47 CrossRef CAS PubMed.
- T. Hashimoto, K. Nishi, J. Nagasao, S. Tsuji and K. Oyanagi, Brain Res., 2008, 1197, 143–151 CrossRef CAS PubMed.
- A. M. P. Romani, in Interrelations between Essential Metal Ions and Human Diseases, ed. A. Sigel, H. Sigel and R. K. O. Sigel, SpringerNetherlands, 2013, vol. 13, ch. 3, pp. 49–79 Search PubMed.
- For reviews of recent advances in magnesium detection with fluorescent indicators, see: (a) V. Trapani, M. Schweigel-Roentgen, A. Cittadini and F. I. Wolf, Methods Enzymol., 2012, 505, 421–444 CAS; (b) V. Trapani, G. Farruggia, C. Marraccini, S. Iotti, A. Cittadini and F. I. Wolf, Analyst, 2010, 135, 1855–1866 RSC.
- Y. Shindo, T. Fujii, H. Komatsu, D. Citterio, K. Hotta, K. Suzuki and K. Oka, PLoS One, 2011, 6, e23684 CAS.
- T. Fujii, Y. Shindo, K. Hotta, D. Citterio, S. Nishiyama, K. Suzuki and K. Oka, J. Am. Chem. Soc., 2014, 136, 2374–2381 CrossRef CAS PubMed.
- L. H. Lindenburg, J. L. Vinkenborg, J. Oortwijn, S. J. A. Aper and M. Merkx, PLoS One, 2013, 8, e82009 Search PubMed.
- S. Orrenius, B. Zhivotovsky and P. Nicotera, Nat. Rev. Mol. Cell Biol., 2003, 4, 552–565 CrossRef CAS PubMed.
- R. Rizzuto, P. Pinton, D. Ferrari, M. Chami, G. Szabadkai, P. J. Magalhães, F. D. Virgilio and T. Pozzan, Oncogene, 2003, 22, 8619–8627 CrossRef CAS PubMed.
- P. Pinton, C. Giorgi, R. Siviero, E. Zecchini and R. Rizzuto, Oncogene, 2008, 27, 6407–6418 CrossRef CAS PubMed.
- T. Patel, S. F. Bronk and G. J. Gores, J. Clin. Invest., 1994, 94, 2183–2192 CrossRef CAS PubMed.
- X.-Y. Zhang, W.-G. Li, Y.-J. Wu, D.-C. Bai and N.-F. Liu, Can. J. Physiol. Pharmacol., 2005, 83, 309–318 CrossRef CAS PubMed.
- M. M. Chien, K. E. Zahradka, M. K. Newell and J. H. Freed, J. Biol. Chem., 1999, 274, 7059–7066 CrossRef CAS PubMed.
- M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952–3015 CrossRef CAS PubMed.
- For examples of triazole-containing molecules in sensing, see: M. Watkinson, in Click Triazoles, ed. J. Košmrlj, SpringerBerlin Heidelberg, 2012, pp. 109–136 Search PubMed.
- B. Raju, E. Murphy, L. A. Levy, R. D. Hall and R. E. London, Am. J. Physiol.: Cell Physiol., 1989, 256, C540–C548 CAS.
- R. P. Haugland, Handbook of Fluorescent Probes and Research Products, Molecular Probes Inc., Eugene, Oregon, 9th edn, 2002 Search PubMed.
- B. Metten, M. Smet, N. Boens and W. Dehaen, Synthesis, 2005, 2005, 1838–1844 CrossRef.
- B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3–40 CrossRef CAS.
- H. L. Kee, C. Kirmaier, L. Yu, P. Thamyongkit, W. J. Youngblood, M. E. Calder, L. Ramos, B. C. Noll, D. F. Bocian, W. R. Scheidt, R. R. Birge, J. S. Lindsey and D. Holten, J. Phys. Chem. B, 2005, 109, 20433–20443 CrossRef CAS PubMed.
- Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899–4032 CrossRef PubMed.
- W. H. Melhuish, J. Phys. Chem., 1961, 65, 229–235 CrossRef CAS.
- A. M. Brouwer, Pure Appl. Chem., 2011, 83, 2213–2228 CrossRef CAS.
- R. K. Gupta, P. Gupta, W. D. Yushok and Z. B. Rose, Biochem. Biophys. Res. Commun., 1983, 117, 210–216 CrossRef CAS PubMed.
- M. S. Afzal, J.-P. Pitteloud and D. Buccella, Chem. Commun., 2014, 50, 11358–11361 RSC.
- The related Mag-fura-2 sensor responds to Zn2+ with an apparent dissociation constant of 20 nM. See T. J. B. Simons, J. Biochem. Biophys. Methods, 1993, 27, 25–37 CrossRef CAS PubMed.
- R. A. Colvin, W. R. Holmes, C. P. Fontaine and W. Maret, Metallomics, 2010, 2, 306–317 RSC.
- D. W. Jung and G. P. Brierley, J. Bioenerg. Biomembr., 1994, 26, 527–535 CrossRef CAS PubMed.
- T. Kubota, Y. Shindo, K. Tokuno, H. Komatsu, H. Ogawa, S. Kudo, Y. Kitamura, K. Suzuki and K. Oka, Biochim. Biophys. Acta, Mol. Cell Res., 2005, 1744, 19–28 CrossRef CAS PubMed.
- B. C. Dickinson, D. Srikun and C. J. Chang, Curr. Opin. Chem. Biol., 2010, 14, 50–56 CrossRef CAS PubMed.
- R. Y. Tsien, Nature, 1981, 290, 527–528 CrossRef CAS PubMed.
- S. Bolte and F. P. Cordelières, J. Microsc., 2006, 224, 213–232 CrossRef CAS PubMed.
- The only other instances of selective detection of magnesium in mitochondria in live cells have been reported by the group of Oka and coworkers with KMG-301 and KMG-104-AsH, both intensity-based turn-on probes. See ref. 11 and 12.
- A. E. Palmer and R. Y. Tsien, Nat. Protoc., 2006, 1, 1057–1065 CrossRef CAS PubMed.
- Z. Huang, X.-a. Zhang, M. Bosch, S. J. Smith and S. J. Lippard, Metallomics, 2013, 5, 648–655 RSC.
- R. Eskes, B. Antonsson, A. Osen-Sand, S. Montessuit, C. Richter, R. Sadoul, G. Mazzei, A. Nichols and J.-C. Martinou, J. Cell Biol., 1998, 143, 217–224 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, metal selectivity plots, determination of apparent dissociation constants, fluorescence microscopy co-localization analysis, and supporting figures. See DOI: 10.1039/c5sc02442k |
| This journal is © The Royal Society of Chemistry 2015 |