Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mesoscopic superstructures of flexible porous coordination polymers synthesized via coordination replication†
Kenji
Sumida
a,
Nirmalya
Moitra
b,
Julien
Reboul
a,
Shotaro
Fukumoto
b,
Kazuki
Nakanishi
b,
Kazuyoshi
Kanamori
b,
Shuhei
Furukawa
*a and
Susumu
Kitagawa
*a
aInstitute for Integrated Cell-Material Sciences (WPI-iCeMS), Kyoto University, Yoshida, Sakyo-ku, Kyoto 606-8501, Japan. E-mail: shuhei.furukawa@icems.kyoto-u.ac.jp; kitagawa@icems.kyoto-u.ac.jp
bDepartment of Chemistry, Graduate School of Science, Kyoto University, Kitashirakawa, Sakyo-ku, Kyoto, 606-8502, Japan
First published on 29th June 2015
Abstract
The coordination replication technique is employed for the direct conversion of a macro- and mesoporous Cu(OH)2–polyacrylamide composite to three-dimensional superstructures consisting of the flexible porous coordination polymers, Cu2(bdc)2(MeOH)2 and Cu2(bdc)2(bpy) (bdc2− = 1,4-benzenedicarboxylate, bpy = 4,4′-bipyridine). Detailed characterization of the replicated systems reveals that the structuralization plays an important role in determining the adsorptive properties of the replicated systems, and that the immobilization of the crystals within a higher-order architecture also affects its structural and dynamic properties. The polyacrylamide polymer is also found to be crucial for maintaining the structuralization of the monolithic systems, and in providing the mechanical robustness required for manual handling. In all, the results discussed here demonstrate a significant expansion in the scope of the coordination replication strategy, and further confirms its utility as a highly versatile platform for the preparation of functional three-dimensional superstructures of porous coordination polymers.
Introduction
The design and synthesis of porous coordination polymers (PCPs) or metal–organic frameworks (MOFs) has experienced an intensive focus in recent years,1 due to their potential use in applications such as gas storage, molecular separations, and heterogeneous catalysis.2 These compounds are assembled from metal-containing nodes bridged by organic linkers, which form porous structures that are characterized by high surface areas, as well as tunable pore dimensions and pore surface chemistry. While the ability to conveniently construct new materials from the combination of a metal salt and organic ligand (in the so-called modular approach) has provided researchers with a tremendously large library of compounds, there is an urgent need for versatile synthetic strategies for the convenient fabrication of PCPs in a structuralized form.3 Here, synthetic routes have begun to emerge for the bottom-up preparation of zero- (e.g. hollow spheres), one- (rods), two- (films), and three-dimensional (monolithic) superstructures of PCPs.3a,b A feature common to the preparative methodologies of the systems reported so far is that they provide a precise control of the crystallization interface at which PCP formation occurs, resulting in the precipitation of the PCP with the desired structuralized architecture.An elegant technique that has recently emerged for the preparation of three-dimensional superstructures of PCPs is the so-called coordination replication strategy.4–7 In this method, a structuralized metal source (such as a metal oxide) is employed as a template, which undergoes conversion in a ligand solution into a three-dimensional PCP superstructure with retention of the original structure. While this technique has been successfully demonstrated with a small number of PCP systems so far,4 investigations of the incorporation of molecular-scale flexibility within structuralized systems with sophisticated dynamic properties are yet to emerge. While studies of this type are of high interest from a fundamental perspective due to the prospects of new phenomena emerging from the embedding of such dynamic building blocks in a structuralized form, the identification of suitable starting materials and PCP systems is challenging due to the difficulty in preparing metal-based compounds in well-defined structures, as well as the currently limited scope of structuring techniques.
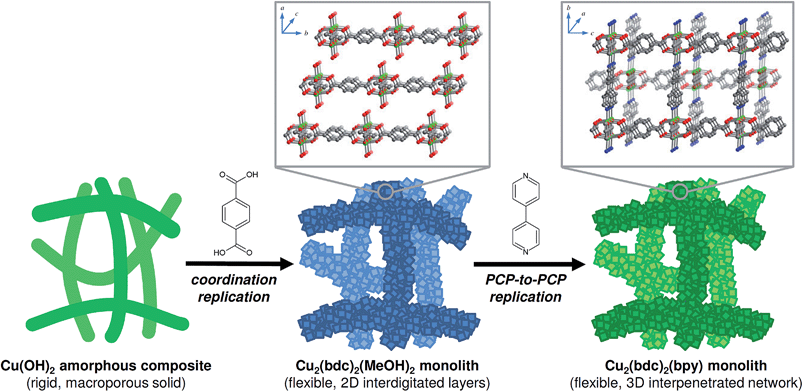
In this work, we address these challenges via the structuring of flexible copper-based PCPs, namely Cu2(bdc)2(MeOH)2, which has a two-dimensional interdigitated structure, and Cu2(bdc)2(bpy), which comprises a three-dimensional interpenetrated structure, into three-dimensional monolithic superstructures (bdc2− = 1,4-benzenedicarboxylate, bpy = 4,4′-bipyridine).8 A macro- and mesoporous Cu(OH)2–polyacrylamide (PAAm) monolithic material was chosen as a precursor for the coordination replication strategy, which was firstly successfully converted into a Cu2(bdc)2(MeOH)2 monolith (“daughter” phase), followed by a PCP-to-PCP replication to fabricate a Cu2(bdc)2(bpy) monolith (“granddaughter” phase) via the pillar ligand (bpy) insertion process (see Fig. 1). Importantly, unique adsorptive and dynamic properties are observed following immobilization of the PCPs within the three-dimensional superstructures, and the potential origins of these effects are discussed in the context of both the composition and the structures of the monoliths.
Experimental section
General considerations
Unless otherwise noted, all reagents were obtained from commercial vendors and used as received. While all syntheses were carried out in the air, the desolvated forms of each of the compounds were handled and stored in a nitrogen-filled glove box. Solvothermal syntheses were carried out in a DKN302 constant temperature oven (Yamato Scientific Co., Ltd) using glass vials sealed with Teflon-lined lids. Nitrogen and methanol adsorption measurements were carried out on a BELSORP-max adsorption analyser (BEL Japan, Inc.) equipped with a constant temperature bath. Powder X-ray diffraction patterns were collected using a Smartlab X-ray Diffractometer (Rigaku Corp.) equipped with a Cu Kα source.Field-emission scanning electron microscopy (FE-SEM)
Scanning electron microscopy (SEM) images were collected using a JEOL JSM-7001F4 electron microscope. Powder and monolith samples were evacuated to remove any residual solvent molecules, and attached to a 13.5 mm substrate using double-sided carbon tape. The samples were then coated with osmium nanoparticles to a thickness of 5 nm, and transferred to the SEM instrument. The images were collected using an emission voltage between 10 and 15 kV.Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), water (1.10 mL), ethanol (0.30 mL), glycerol (2.40 mL), and propylene oxide (1.47 mL, 21.0 mmol). The as-synthesized form of the monolith was stored in 2-propanol, and was rinsed with methanol prior to the coordination replication procedure. Note that after washing, some Cl− ions still remain in the composition (ca. 4 wt%),9 but we refer to the starting structure as “Cu(OH)2–polyacrylamide monolith” for simplicity.
000), water (1.10 mL), ethanol (0.30 mL), glycerol (2.40 mL), and propylene oxide (1.47 mL, 21.0 mmol). The as-synthesized form of the monolith was stored in 2-propanol, and was rinsed with methanol prior to the coordination replication procedure. Note that after washing, some Cl− ions still remain in the composition (ca. 4 wt%),9 but we refer to the starting structure as “Cu(OH)2–polyacrylamide monolith” for simplicity.
Results and discussion
Synthesis and characterization
The coordination replication technique is an attractive method for the structuralization of PCP materials, since a potentially wide variety of metal-based precursors can be shaped into a desired form via conventional fabrication techniques, such as sol–gel processing. Here, the main requirement for precursor materials is a slow dissolution rate relative to the crystallization rate of the target PCP crystals, such that crystal growth is spatially constrained at the interface between the solid precursor and the ligand solution.4 This represents one of the main challenges in expanding the scope of coordination replication synthesis, and precursors that offer the correct balance between solubility and reactivity under the reaction conditions for the PCP formation process have remained limited so far.Among the solid sources containing Cu2+ considered for the formation of the Cu2(bdc)2(MeOH)2 and Cu2(bdc)2(bpy) frameworks, Cu(OH)2 was chosen for further study owing to its low solubility in polar organic solvents and high reactivity toward acids.10 Consequently, synthetic conditions for the synthesis of the two compounds were developed using a commercially-available bulk crystalline powder of Cu(OH)2. The screening of various parameters, including the reaction solvent, the metal-to-ligand ratio, and the reaction time revealed that the addition of a stoichiometric quantity of Cu(OH)2 to a refluxing solution of H2bdc in methanol afforded Cu2(bdc)2(MeOH)2 after a reaction time of 3 days. Next, a suspension of Cu2(bdc)2(MeOH)2 was treated with an excess of bpy in methanol, resulting in the installation of bpy pillars between every second square grid layer to produce the interpenetrated Cu2(bdc)2(bpy) framework (see Fig. S3†). SEM observation confirmed a plate-like crystal morphology (Fig. S4†), and N2 adsorption measurements (Fig. S5†) at 77 K gave a BET surface area11 of 1030 m2 g−1 (Langmuir surface area: 1300 m2 g−1) which is somewhat higher than the corresponding value of 700 m2 g−1 measured previously for a sample prepared from a conventional method that uses CuSO4 as the Cu2+ source.8a
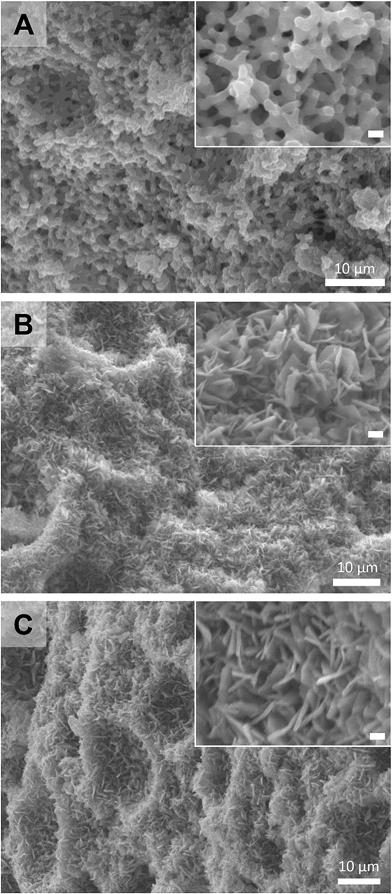
Following the successful demonstration of the synthesis of Cu2(bdc)2(MeOH)2 and Cu2(bdc)2(bpy) from crystalline Cu(OH)2 powders, a structuralized form of Cu(OH)2 was required for coordination replication studies. Recently, a method for the preparation of an amorphous macro- and mesoporous Cu(OH)2–polyacrylamide composite material via sol–gel processing accompanied by phase separation, and its conversion to the prototypical and rigid PCP, Cu3(btc)2, was reported.9,12 The amorphous nature of the Cu(OH)2 within this parent phase is expected to have a similar (or enhanced) reactivity compared to crystalline Cu(OH)2, and was identified as a suitable candidate for further study. In this case, the synthetic procedure of the Cu(OH)2–polyacrylamide parent phase was adapted to prepare a monolithic solid featuring continuous macropores with a diameter of ca. 1 μm (see Fig. 2, left, and Fig. 3A). Analysis of the porosity of the parent monolith used in this work via N2 adsorption isotherms afforded a type-IV profile typical of a mesoporous solid (Fig. S6†). The determination of a pore size distribution based on this data revealed a maximum density at ca. 5 nm for the mesopores. Note that the large pores present within this monolith are expected to facilitate the diffusion of the organic linkers throughout the solid, which is required for full conversion during the coordination replication procedure.
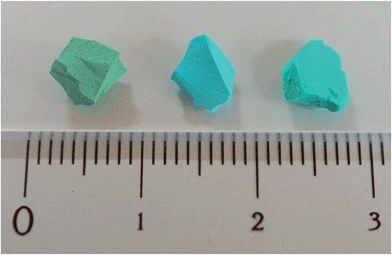
The Cu(OH)2–polyacrylamide monolith was suspended and heated within a solution of H2bdc in methanol for an extended period of 7 days (with daily exchange of the mother liquor), which resulted in a color change of the solid from green to sky-blue. Importantly, the external dimensions of the monolith and its mechanical integrity were retained despite the long period of treatment (see Fig. 2, center).13 Observation of the surface of the monolith following replication by SEM revealed the growth of square plate-like crystals approximately 1 μm in width from the walls of the co-continuous structure (see Fig. 3B). SEM observation following slicing of a monolith sample to expose the cross-section (depth direction) of the structure showed crystals of the same morphology had uniformly formed throughout the material (see Fig. S7†), but the macropores were almost completely eliminated. This is because the conversion from Cu(OH)2 to Cu2(bdc)2(MeOH)2 results in a volume increase of approximately 10 times (based on Cu2+ density in the bulk, crystalline forms of both compounds). The complete conversion of the Cu(OH)2 of the parent phase was further confirmed by thermogravimetric analysis (TGA), which did not exhibit a weight loss at the decomposition temperature of Cu(OH)2 of ca. 80 °C (see Fig. S8†). The TGA data could also be used to estimate a polyacrylamide content of 15.0 wt%, which is close to the composition employed during the preparation of the Cu(OH)2–polyacrylamide monolith of ca. 20.0 wt%. Note that, in the preparation of the Cu2(bdc)2(MeOH)2 monolith, the reaction conditions developed for the preparation of bulk powders of the same compound from crystalline Cu(OH)2 was successfully used for monolith conversion. This agrees with our experience using the coordination replication method for the synthesis of Al-based PCP architectures from Al2O3 phases,4 which has demonstrated that amorphous or less-dense variants of an inorganic compound tend to dissolve faster or have higher reactivities since they have lower lattice energies. This results in the right balance between precursor dissolution and PCP crystallization, which is required for preservation of the structuring of the parent phase.
Next, the Cu2(bdc)2(MeOH)2 monolith was immersed in a methanol solution of bpy to induce pillaring of the square grid layers of the two-dimensional framework to afford the three dimensional Cu2(bdc)2(bpy) compound. After several hours, the color of the monolith changed from sky-blue to blue-green (see Fig. 2, right). SEM data revealed the retention of the structuralization of the monolith following replication accompanied with a slight increase in the thickness of the crystals, which is consistent with the insertion of the bpy pillars between the dinuclear copper paddlewheels of every second square grid layer (Fig. 3C). Estimation of the composition of the monolith via TGA data revealed a polyacrylamide content of 2.0 wt% (Fig. S16†), the loss of which, as discussed in the following section, has important consequences with respect to the properties of the monoliths.
Structural features and structural flexibility of the replicated frameworks
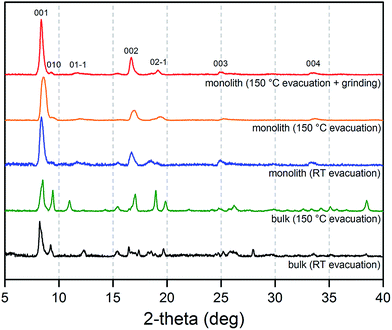
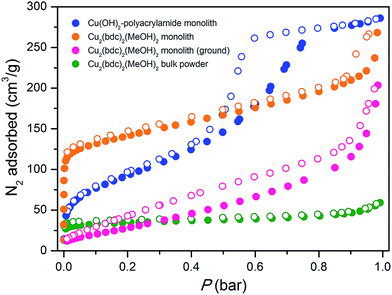
The origins of this unusual feature of the powder X-ray diffraction data were further probed by N2 adsorption analysis at 77 K after activation of the monolith at 150 °C.16Fig. 5 displays data collected for the parent Cu(OH)2–polyacrylamide monolith, the Cu2(bdc)2(MeOH)2 monolith and a bulk Cu2(bdc)2(MeOH)2 powder sample. Remarkably, while the bulk material showed a negligible N2 uptake owing to the inability of N2 to open and access the interlayer spacing, the structuralized variant exhibited significant uptake at low pressures, reminiscent of a type-I isotherm observed for a microporous solid. Indeed, a BET analysis of the sorption data (see Fig. S9†) afforded a surface area of 520 m2 g−1,17 which is significantly greater than can be accounted for by the sorption properties of the parent Cu(OH)2–polyacrylamide phase and bulk Cu2(bdc)2(MeOH)2. This suggests that the structural influence of the interactions between the Cu2(bdc)2(MeOH)2 crystals and the polyacrylamide chains at the molecular scale in turn impart considerably different sorption properties to the PCP phase compared to its bulk counterpart.
The powder diffraction and adsorption data observed here can be reconciled by considering the role of the polyacrylamide polymer in the replicated system. The polyacrylamide content of the Cu2(bdc)2(MeOH)2 monolith of approximately 15.0 wt% is a component required for the integrity of the three-dimensional structuralization. Here, it is expected that the anchoring of the crystals to the polymer occurs by way of Cu2+–amide interactions, which inherently requires the polymer to become partially incorporated between the layers of the framework (i.e. by coordination to the dinuclear paddlewheels). This is expected to disrupt the periodicity of the PCP in the crystallographic a-direction of the framework (while leaving the crystallinity of the bc plane unaffected), and the creation of uneven spacings between the square grid layers, some of which are sufficiently large for N2 to be incorporated at low temperatures. This phenomenon is unique to Cu2(bdc)2(MeOH)2 in a structuralized state, since such points of anchoring do not exist in the bulk form. Further, it demonstrates the importance of molecular scale interactions between the PCP crystals and the support in determining the adsorptive and dynamic behavior of the system as a whole.
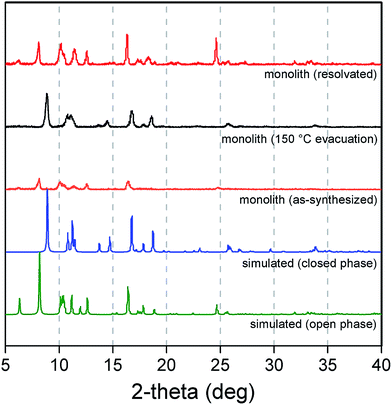
The impact of structuralization in the Cu2(bdc)2(MeOH)2 system was further investigated by destroying the architecture by mechanical grinding of the monolith into a fine powder. Although the crystallinity of the sample was preserved following this process (see Fig. 4), N2 adsorption data at 77 K revealed the complete loss of microporosity once in a ground powder form (see Fig. S10†). This can be ascribed to the pulverization of the crystals as observed by SEM (Fig. S11†), which leads to most of the crystalline fragments no longer being bound by the polyacrylamide polymer. Indeed, while the microporous region of the N2 isotherm no longer shows a significant uptake, the profile exhibits a monotonic increase up to 190 cm3 g−1 at a pressure of 1 bar, consistent with surface adsorption of N2 to the polyacrylamide polymer surface. In addition, preparation of Cu(bdc)2(MeOH)2 from a uniformly ground sample of the Cu(OH)2–polyacrylamide parent phase (prepared under the same reaction conditions as bulk Cu(bdc)2(MeOH)2) yielded a sample of the same composition as the Cu(bdc)2(MeOH)2 monolith. However, unlike the monolith form, the material displays little microporosity despite the presence of polyacrylamide in the overall composition (see Fig. S12 and S13†). This further supports the observation that the immobilization of the Cu(bdc)2(MeOH)2 crystals within the three-dimensional architecture provides the additional microporosity observed here.
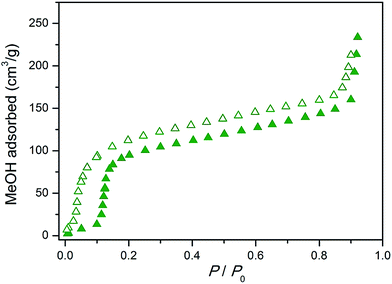 | ||
| Fig. 7 Methanol adsorption isotherm collected at 298 K for the Cu2(bdc)2(bpy) monolith. Closed and open symbols represent adsorption and desorption, respectively. | ||
Next, the effect of structuralization of the Cu2(bdc)2(bpy) compound in a monolith form was probed by comparing its methanol adsorption isotherm after mechanical grinding of the framework. Surprisingly, in contrast to the Cu2(bdc)2(MeOH)2 monolith, little change was observed after the destruction of the structuralization with regard to both the gate-opening pressure and the quantity of methanol adsorbed (Fig. S15†). Furthermore, comparison with a bulk powder of Cu2(bdc)2(bpy) also revealed an almost identical adsorption profile, revealing that both the structural flexibility and the adsorption properties of the monolith are a good match to those of a bulk sample of the same compound. This is a somewhat surprising result given that, based on the unusual properties observed for the Cu2(bdc)2(MeOH)2 monolithic system, the immobilization of the Cu2(bdc)2(bpy) crystals in a monolith form might be expected to influence the adsorptive and dynamic properties of the system.
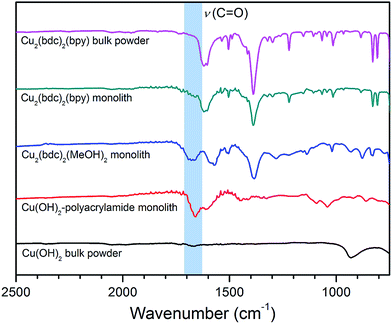
In order to elucidate the origin of this result, IR and TGA data were collected to evaluate the composition of the Cu2(bdc)2(bpy) replicate. As is clear from the IR data presented in Fig. 8, the spectrum observed for the activated form of the Cu2(bdc)2(bpy) monolith shows a close match with that of a bulk sample of the same framework. However, in comparison with the parent and Cu2(bdc)2(MeOH)2 monolith, the C–N stretch at approximately 1660 cm−1 originating from the amide moiety of the polyacrylamide polymer is greatly diminished, suggesting that the polymer component is excluded from the structure during the insertion of the bpy pillars. This was further confirmed by the TGA data shown in Fig. S16,† which allowed the polyacrylamide content to be calculated as 2.0 wt%, compared with 20.0 wt% and 15.0 wt% in the parent Cu(OH)2–polyacrylamide and Cu2(bdc)2(MeOH)2 monoliths, respectively. The loss of polyacrylamide from the structure is also consistent with a decrease in the mechanical robustness of the Cu2(bdc)2(bpy) monolith, emphasizing its key role in providing the effect of structuralization of the monolithic structure following replication.
The origin of the loss of polyacrylamide from the structure was probed via a number of control experiments. Immersion of the parent Cu(OH)2–polyacrylamide and Cu2(bdc)2(MeOH)2 monoliths in methanol resulted in no change to the composition or the structuralization, which provided clear evidence of the stability of the monoliths (and its associated polymer content) under these conditions. Furthermore, immersion of the parent Cu(OH)2–polyacrylamide compound in a methanol solution of bpy resulted in no loss in the polyacrylamide component from the structure as evaluated by TGA data (Fig. S20†). Thus, the polyacrylamide is only lost when the Cu2(bdc)2(MeOH)2 undergoes pillaring by the bpy molecules during the second PCP-to-PCP replication step. While an exact mechanism for the loss of polyacrylamide is not yet available, a plausible sequence of events is as follows. In the conversion of the Cu(OH)2–polyacrylamide monolith to the Cu2(bdc)2(MeOH)2 replicate, the polyacrylamide directly binds to the Cu2(bdc)2(MeOH)2 framework via amide sidechains as described above. This leads to the polymer chains, which are originally buried beneath a colloidal network of Cu(OH)2 particles, to become exposed after replication. This is due in part to the plate-shaped crystals of Cu2(bdc)2(MeOH)2 that are not expected to uniformly protect the polymer chains from access at the molecular scale. Then, upon exposure of the monolith to a solution containing bpy, the amide moieties are displaced from the Cu2+ centers, leaving the chains unbound and susceptible to dissolution out of the monolith. This dissolution process may additionally be assisted by a partial hydrolysis of the polymer chains, which is known to occur in the presence of basic species. Note that analysis of the reactant solution by IR and 1H NMR did not reveal the presence of free acrylamide monomers, suggesting a complex decomposition pathway for the PAAm component into a variety of products. As such, after the removal of the polyacrylamide component from the monolith, the limited intergrowth between the Cu2(bdc)2(bpy) crystals leads to the structural and sorption properties of the monolith largely reflecting those of a bulk powder, despite the retention of the monolithic structure.
Conclusions and future outlook
The foregoing results have detailed the synthesis and properties of three-dimensional superstructures consisting of the flexible Cu2(bdc)2(MeOH)2 and Cu2(bdc)2(bpy) frameworks via coordination replication from a structuralized macro- and mesoporous Cu(OH)2–polyacrylamide composite parent phase. The synthesis of these monolithic systems expands on the scope of the coordination replication technique to include flexible PCPs, but perhaps more importantly, provides monolithic systems that exhibit properties that differ from bulk powders as a result of structuralization. In the case of the Cu2(bdc)2(MeOH)2 system, the anchoring of the two-dimensional framework by the polyacrylamide polymer leads to their immobilization within the superstructure, but also results in an amorphization of the interlayer direction of the framework structure. This provides the framework with an ability to adsorb N2, which is not observed in the absence of structuralization. For the Cu2(bdc)2(bpy) system, the framework flexibility is preserved after immobilization, leading to a flexible monolith system. In this case, the sorption and dynamic properties largely reflect the characteristics of the bulk form owing to the dissolution of the polymer phase during the PCP-to-PCP replication step. This emphasizes the importance of the polymer phase in maintaining the connectivity between crystals and in providing the system with the effects of structuralization.The results presented here further demonstrate the versatility of the coordination replication technique, and it is envisaged that a greater library of structuralized PCPs will emerge in the near future for specific applications in areas such as molecular separations and heterogeneous catalysis. In addition, the new properties observed for the structuralized forms of the compounds suggest that new, rich phenomena could emerge as a result of detailed studies of this type. However, as revealed here, there is an urgent need for preparative routes to new parent materials that are optimized for coordination replication, and care is also needed in the selection of the target PCP system. Specifically, a high degree of crystal intergrowth is desired in order to achieve cooperative effects stemming from material structuralization. While the polyacrylamide polymer serves as an adhesive between the crystals in this case, greater intergrowth between the PCP crystals themselves would preclude the need for the use of a composite system. For example, optimization of both the crystal size (i.e. smaller crystals) and morphology (i.e. block-shaped crystals) of the PCP phase is expected to facilitate a greater preservation of the original structure of the parent material with a greater degree of intergrowth. Such optimizations of the crystal parameters have already appeared in the case of bulk crystals via the coordination modulation technique,18 and studies using this strategy for the fabrication of three-dimensionally structuralized systems composed of other functional PCP systems are already underway.
Acknowledgements
K. S. is grateful to JSPS Postdoctoral Fellowship Program for Foreign Researchers. This work was supported by a Grant-in-Aid for Scientific Research (no. 25708010 and 15H03785 (S. F.)) from MEXT, Japan. iCeMS is supported by World Premier International Research Initiative (WPI), MEXT, Japan. The authors thank CeMI for assistance with electron microscopy, and J. A. Mason for helpful discussions.Notes and references
- (a) O. M. Yaghi, M. O'Keeffe, N. W. Ockwing, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS PubMed; (b) S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS PubMed; (c) G. Férey, Chem. Soc. Rev., 2008, 37, 191 RSC.
- For recent reviews covering the emerging applications of metal–organic frameworks, refer to the special themed issues in Chem. Rev., and more recently Chem. Soc. Rev.: (a) H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673 CrossRef CAS PubMed; (b) H.-C. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415 RSC , and references therein.
- (a) A. Carné-Sánchez, I. Imaz, K. C. Stylianou and D. Maspoch, Chem.–Eur. J., 2014, 20, 5192 CrossRef PubMed; (b) S. Furukawa, J. Reboul, S. Diring, K. Sumida and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5700 RSC; (c) Q.-L. Zhu and Q. Zhu, Chem. Soc. Rev., 2014, 43, 5468 RSC.
- J. Reboul, S. Furukawa, N. Horike, M. Tsotsalas, K. Hirai, H. Uehara, M. Kondo, N. Louvain, O. Sakata and S. Kitagawa, Nat. Mater., 2012, 11, 717 CrossRef CAS PubMed.
- (a) K. Khaletskaya, J. Reboul, M. Meilikhov, M. Nakahama, S. Diring, M. Tsujimoto, S. Isoda, F. Kim, K. Kamei, R. A. Fischer, S. Kitagawa and S. Furukawa, J. Am. Chem. Soc., 2013, 135, 10998 CrossRef CAS PubMed; (b) M. Nakahama, J. Reboul, K. Kamei, S. Kitagawa and S. Furukawa, Chem. Lett., 2014, 43, 1052 CrossRef CAS; (c) J. Reboul, K. Yoshida, S. Furukawa and S. Kitagawa, CrystEngComm, 2015, 17, 323 RSC.
- K. Okada, R. Ricco, Y. Tokudome, M. J. Styles, A. J. Hill, M. Takahashi and P. Falcaro, Adv. Funct. Mater., 2013, 14, 1969 Search PubMed.
- I. Stassen, N. Campagnol, J. Fransaer, P. Vereecken, D. De Vos and R. Ameloot, CrystEngComm, 2013, 15, 9308 RSC.
- (a) K. Seki, Phys. Chem. Chem. Phys., 2002, 4, 1968 RSC; (b) Y. Sakata, S. Furukawa, M. Kondo, K. Hirai, N. Horike, Y. Takashima, H. Uehara, N. Louvain, M. Meilikhov, T. Tsuruoka, S. Isoda, W. Kosaka, O. Sakata and S. Kitagawa, Science, 2013, 339, 193 CrossRef CAS PubMed.
- S. Fukumoto, K. Nakanishi and K. Kanamori, New J. Chem., 2015 10.1039/C5NJ00479A.
- For examples, see: (a) E. A. Nytko, J. S. Helton, P. Müller and D. G. Nocera, J. Am. Chem. Soc., 2008, 130, 2922 CrossRef CAS PubMed; (b) G. Majano and J. Pérez-Ramírez, Adv. Mater., 2013, 25, 1052 CrossRef CAS PubMed; (c) Y. Mao, L. Shi, H. Huang, W. Cao, J. Li, L. Sun, X. Jin and X. Peng, Chem. Commun., 2013, 49, 5666 RSC.
- K. S. Walton and R. Q. Snurr, J. Am. Chem. Soc., 2007, 129, 8552 CrossRef CAS PubMed.
- N. Moitra, S. Fukumoto, J. Reboul, K. Sumida, Y. Zhu, K. Nakanishi, S. Furukawa, S. Kitagawa and K. Kanamori, Chem. Commun., 2015, 51, 3511 RSC.
- Attempts to prepare monoliths of a sufficient size for mechanical strength measurements of the Cu2(bdc)2(MeOH)2 monolith were not successful in this case. While large monoliths (cylindrical tablets with a diameter of 1 cm and a height of 0.5 cm) of the Cu3(btc)2 framework were readily prepared within 30 min from the same starting precursor, 12 the conversion was found to be significantly slower in the case of Cu2(bdc)2(MeOH)2. The use of starting monoliths of a sufficient size resulted in samples with unreacted cores even after 14 days, likely due to preferential crystal growth at the exterior of the monolith resulting in macropore blockage, preventing diffusion of the organic linker throughout the solid. The significantly different behavior of the two systems highlights potential differences in both the molecular scale replication mechanism and the nature of the crystal growth, which are areas worthy of systematic investigation in order to optimize precursor design for specific PCP systems.
- Such effects are often observed in oriented samples or those with highly anisotropic crystal shapes, although this is not expected for the replicated phase studied here due to the random distribution of spatial orientations of the crystals within the monolith.
- Y. Hishiyama and M. Nakamura, Carbon, 1995, 33, 1399 CrossRef CAS.
- Note that this slightly lower activation temperature than for bulk powder samples allows the polyacrylamide component to be stably maintained within the framework, while allowing full removal of the methanol within the pores and bound to the Cu2+ ions of the dinuclear paddlewheel units.
- The macroporosity is largely eliminated and the mesoporosity significantly diminished upon replication, which is due to the Cu2(bdc)2(MeOH)2 crystals occupying a (up to 10 times) greater volume compared to the original Cu(OH)2 component based on the density of Cu2+ ions in the respective crystal structures. The plate-like morphology of the framework crystals may also provide a less contoured surface providing fewer cavities in the mesopore length scale.
- (a) T. Tsuruoka, S. Furukawa, Y. Takashima, K. Yoshida, S. Isoda and S. Kitagawa, Angew. Chem., Int. Ed., 2009, 48, 4739 CrossRef CAS PubMed; (b) S. Diring, S. Furukawa, Y. Takashima, T. Tsuruoka and S. Kitagawa, Chem. Mater., 2010, 22, 4531 CrossRef CAS; (c) A. Umemura, S. Diring, S. Furukawa, H. Uehara, T. Tsuruoka and S. Kitagawa, J. Am. Chem. Soc., 2011, 133, 15506 CrossRef CAS PubMed; (d) T. Uemura, Y. Hoshino, S. Kitagawa, K. Yoshida and S. Isoda, Chem. Mater., 2006, 18, 992 CrossRef CAS; (e) S. Hermes, T. Witte, T. Hikov, D. Zacher, S. Bahnmüller, G. Langstein, K. Huber and R. A. Fischer, J. Am. Chem. Soc., 2007, 129, 5324 CrossRef CAS PubMed; (f) A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem.–Eur. J., 2011, 17, 6643 CrossRef CAS PubMed; (g) F. Vermoortele, B. Bueken, G. Le Bars, B. Van de Voorde, M. Vandichel, K. Houthoofd, A. Vimont, M. Daturi, M. Waroquier, V. Van Speybroeck, C. Kirschhock and D. E. De Vos, J. Am. Chem. Soc., 2013, 135, 11465 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental procedures, powder X-ray diffraction data, thermogravimetric analysis data, adsorption isotherms, scanning electron microscopy data, and additional discussion. See DOI: 10.1039/c5sc02034d |
| This journal is © The Royal Society of Chemistry 2015 |