Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An enzyme-responsive metal-enhanced near-infrared fluorescence sensor based on functionalized gold nanoparticles†
Zhanghua
Zeng
a,
Shin
Mizukami
ac,
Katsumasa
Fujita
b and
Kazuya
Kikuchi
*ac
aDivision of Advanced Science and Biotechnology, Osaka University, Osaka, 565-0871, Japan. E-mail: kkikuchi@mls.eng.osaka-u.ac.jp
bDepartment of Applied Physics, Graduate School of Engineering, Osaka University, Osaka, 565-0871, Japan
cImmunology Frontier Research Centre, Osaka University, Osaka, 565-0871, Japan
First published on 19th June 2015
Abstract
Near-infrared (NIR) fluorescence imaging is promising due to the high penetration depths and minimal levels of autofluorescence in living systems. However, it suffers from low fluorescent quantum yield, and metal-enhanced fluorescence (MEF) is considered to be a promising technique to overcome this. Stimuli-responsive NIR fluorescence enhancement shows remarkable potential for applications in medical imaging and diagnosis. Herein, we successfully fabricated an enzyme-responsive near-infrared sensor based on MEF by functionalizing gold nanoparticles with NIR fluorophores and enzyme-responsive self-aggregation moieties. The NIR fluorescence of fluorophores on the gold nanoparticles was significantly enhanced due to increases both in the light scattering intensity and in the radiative decay rate (kr) of the NIR fluorophores, along with relatively small variation in the nonradiative decay rate. This novel strategy for NIR fluorescent sensors should be particularly promising for NIR fluorescence imaging of enzyme activities and early diagnosis based on rationally designed nanomaterials.
Introduction
Near-infrared (NIR) fluorescence imaging continues to attract attention because, in the physiologically relevant optical window (700–900 nm), the features of this technique include minimal levels of interfering absorption from biological samples, reduced scattering, enhanced tissue penetration depth, and minimal levels of autofluorescence.1–6 However, most NIR fluorophores exhibit fluorescence quantum yields (ΦF) much lower than those of visible-wavelength fluorophores. For instance, indocyanine green (ICG), one of the most commonly used dyes in medical diagnostics, has a low ΦF of 0.012 in aqueous media,7 whereas fluorescein, a typical green fluorescent dye, has a ΦF of 0.91.8 The low ΦF of NIR fluorophores significantly limits their applications in medical imaging. Moreover, the development of NIR fluorescent sensors based on fluorescence resonance energy transfer or photo-induced electron transfer is more problematic and less efficient than for visible-wavelength fluorophores. Therefore, the development of a general approach for NIR fluorescence enhancement has become highly important and urgent.Recently, many reports have shown that localized surface plasmon resonance (LSPR) of noble metals can enhance the fluorescence of proximal fluorophores when the architectures of metallic nanostructures and fluorophores are properly engineered.9–21 This phenomenon is named as metal-enhanced fluorescence (MEF).22 Plenty of surface-immobilized metallic nanostructures for MEF have been developed and the advantages of MEF include the increased photostability of fluorophores,23 and improved detection of protein24 and DNA/RNA.25 Although the mechanism of MEF is not fully understood, it is indicated that the strong local electric field of LSPR causes an increase in the radiative decay rate (kr) of fluorophores near the metallic nanostructure.12,26 On the other hand, it is well known that noble metal nanostructures such as gold nanoparticles (AuNPs) strongly quench the fluorescence of neighboring fluorophores. When the distance between the gold surface and the fluorophore is very short (<5 nm), or when the electromagnetic resonance coupling between the LSPR and the fluorophore emission is very small, fluorescence quenching is observed due to the predominance of the nonradiative decay rate (knr).27–31 In principle, the change from fluorescence enhancement to fluorescence quenching depends on the relative magnitudes of kr and knr of the fluorophore. Thus, the fluorescence enhancement caused by AuNPs is a function of both the proximity and the electromagnetic resonance coupling of the fluorophores and AuNPs. So far, most studies on MEF have focused on the fabrication of systems that allow for a more selective control of the distance between the fluorophore and the gold film surface, and of the electromagnetic resonance coupling.32–38 Nevertheless, stimuli-responsive enhancement of NIR fluorescence using noble metal LSPR, especially that based on the tuning of LSPR, has hardly been developed, despite the fact that it shows more potential for significant imaging applications in medicine.
Thus, we planned to develop MEF-based NIR fluorescent sensors by adequately tuning the distance between the metal surface and fluorophores in response to analytes. Recently, we reported that β-galactosidase (β-gal) can shift the LSPR absorption spectrum of AuNPs functionalized with specific β-gal substrate ligands from the visible to the NIR region.39 This result prompted us to exploit enzyme-triggered LSPR changes to obtain MEF. Here we report a β-gal-responsive metal-enhanced NIR fluorescence sensor based on functionalized AuNPs.
Results and discussion
The mechanism of the enzyme-responsive NIR fluorescence enhancement is illustrated in Scheme 1. The prepared nanoparticles, NGal-NIR-AuNPs and CHO-NIR-AuNPs, were anchored with β-gal-substrate ligands (Lip-NGal) and with aldehyde ligands (Lip-CHO), respectively. NIR-fluorophore ligands (Lip-Cy5.5m) and polyethylene glycol ligands (Lip-PEG400) as co-ligands for stabilization in solution were attached to both types of AuNPs. The synthetic routes to the ligands Lip-NGal, Lip-CHO, and Lip-Cy5.5m are summarized in Schemes S1–S3†. Details of the synthetic procedures, the characterization of the intermediates and ligands, the synthesis of gold nanoparticles, and the fabrication of functionalized gold nanoparticles (NGal-NIR-AuNPs and CHO-NIR-AuNPs) are also provided in the ESI.†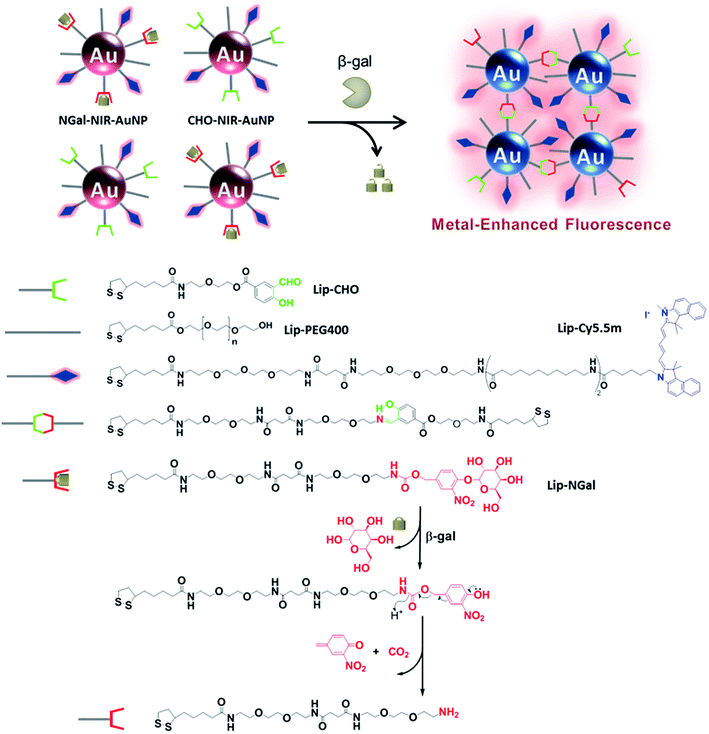 | ||
| Scheme 1 Schematic illustration of the proposed mechanism of the enzyme-responsive fluorescence enhancement by NGal-NIR-AuNPs/CHO-NIR-AuNPs. | ||
The ratio of the attached ligands on the nanoparticle surface was determined by the dye molar extinction coefficient and HPLC analysis (Scheme S4†). As a result, the ratio of Lip-NGal (or Lip-CHO), Lip-Cy5.5m, and Lip-PEG400 was 1/0.12/1.26 and 1/0.10/1.19 for the NGal-NIR-AuNPs and CHO-NIR-AuNPs, respectively. The results show slight differences from those of the modification-reaction solutions (Lip-NGal (or Lip-CHO)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lip-Cy5.5m
Lip-Cy5.5m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lip-PEG400 = 1/0.2/1), presumably due to the nature of the ligand pendants (hydrophilic sugar versus hydrophobic dye), lengths of the linkers, and the overall sizes of the ligands.40–42 The fluorescence of the surface-attached Cy5.5m was expected to be quenched by AuNPs due to the negligibly small overlap between the LSPR absorption (∼520 nm) and the emission (∼707 nm) spectra (Fig. S1†). After the enzymatic substrate hydrolysis of NGal-NIR-AuNPs, a self-immolative elimination reaction via the formation of a quinone methide occurs and the subsequent decarboxylation rapidly produces a primary amine that reacts with an aldehyde on a neighboring CHO-NIR-AuNP to form a Schiff base. As a result, the inter-nanoparticle distance decreases and the LSPR shifts towards the NIR region, as Mirkin et al. reported.43 Such an increase in the spectral overlap between LSPR and emission enhances the electromagnetic resonance coupling, and the fluorescence is enhanced.
Lip-PEG400 = 1/0.2/1), presumably due to the nature of the ligand pendants (hydrophilic sugar versus hydrophobic dye), lengths of the linkers, and the overall sizes of the ligands.40–42 The fluorescence of the surface-attached Cy5.5m was expected to be quenched by AuNPs due to the negligibly small overlap between the LSPR absorption (∼520 nm) and the emission (∼707 nm) spectra (Fig. S1†). After the enzymatic substrate hydrolysis of NGal-NIR-AuNPs, a self-immolative elimination reaction via the formation of a quinone methide occurs and the subsequent decarboxylation rapidly produces a primary amine that reacts with an aldehyde on a neighboring CHO-NIR-AuNP to form a Schiff base. As a result, the inter-nanoparticle distance decreases and the LSPR shifts towards the NIR region, as Mirkin et al. reported.43 Such an increase in the spectral overlap between LSPR and emission enhances the electromagnetic resonance coupling, and the fluorescence is enhanced.
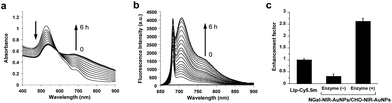
To verify the β-gal-induced LSPR absorption spectral changes of the mixture of the two different gold nanoparticles (NGal-NIR-AuNPs/CHO-NIR-AuNPs), the evolution of the visible-NIR absorbance spectrum of NGal-NIR-AuNPs/CHO-NIR-AuNPs was measured over time (Fig. 1a). The spectra show a typical LSPR peak at 525 nm, which is 5 nm red-shifted with respect to that of the citrate-capped gold nanoparticles due to the ligand-exchange effect,44 and they have a shoulder around 680 nm that arises from the absorbance of Cy5.5m in phosphate buffered saline (PBS) (Fig. S2†). As expected, the addition of β-gal causes a decrease in the intensity of the absorbance at 525 nm over time, along with a gradual increase in absorption near 700 nm due to the aggregation of gold nanoparticles. In comparison, the absorption spectrum of NGal-NIR-AuNPs does not change in the presence of β-gal (Fig. S3†). These results indicate that the formation of the Schiff base induces a shift of the LSPR absorption toward the NIR region.
The fluorescence of NGal-NIR-AuNPs/CHO-NIR-AuNPs in PBS at 37 °C was partially quenched relative to that of Lip-Cy5.5m, because the fluorophores are deposited on the Au surface via a moderately long linker (>9 nm). Upon the addition of β-gal, the fluorescence was enhanced in a time-dependent manner by a factor of 5.2 at 1 h, 6.7 at 2 h, and 7.8 at 6 h of incubation, respectively (Fig. 1b and S4†). These results indicate that the response time was relatively fast initially, and afterwards became slower. This could be satisfactory for in-demand imaging techniques, such as in vitro enzyme detection and early diagnosis. However, we realize the improvement of reaction kinetics is very important for versatile imaging applications as well as improvement of the enhancement factor. Relative to the fluorescence of free ligand Lip-Cy5.5m (at the same concentration) in PBS, the fluorescence was enhanced by a factor of 2.6 (Fig. 1c and Scheme S4†).
The excitation spectra were similarly enhanced (Fig. S5†). A prolonged incubation time did not afford significant further spectral changes. The fluorescence enhancement was also examined in a biological solution containing BSA (0.1%, w/w) and glycine (0.25%, w/w). Under these conditions, the enhancement factor slightly decreased to 4.6-fold at 1 h and 6.2-fold at 6 h, keeping around 90% and 81% enhancement, respectively, compared to that in PBS (Fig. S4†). The small amount of disruption indicates its practicability in biological media, although complete bioorthogonality is more promising.
Although various ratios of Lip-NGal (or Lip-CHO) and Lip-Cy5.5m (from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at the concentration ratio in the modification reaction) were employed to modulate the properties of the nanoparticles, variation of these ratios proved to have no significant effect on the enhancement factor (Fig. S6†). The fluorescence of NGal-NIR-AuNPs is hardly affected by the presence of β-gal (Fig. S7†). This strongly indicates that the enzyme-responsive fluorescence enhancement of NGal-NIR-AuNPs/CHO-NIR-AuNPs is induced by an increase in the spectral overlap between the LSPR of gold nanoparticles and the emission of the fluorophores, which causes the electromagnetic resonance coupling.
1 at the concentration ratio in the modification reaction) were employed to modulate the properties of the nanoparticles, variation of these ratios proved to have no significant effect on the enhancement factor (Fig. S6†). The fluorescence of NGal-NIR-AuNPs is hardly affected by the presence of β-gal (Fig. S7†). This strongly indicates that the enzyme-responsive fluorescence enhancement of NGal-NIR-AuNPs/CHO-NIR-AuNPs is induced by an increase in the spectral overlap between the LSPR of gold nanoparticles and the emission of the fluorophores, which causes the electromagnetic resonance coupling.
It was difficult to directly monitor the enzymatic hydrolysis and Schiff base formation on the AuNP surface. Alternatively, the enzymatic reaction of Lip-NGal was analyzed. The absorption spectra (Fig. S8†) and HPLC chart (Fig. S9†) show the reaction proceeded in a conversion yield of almost 100% within 10 min. The subsequent Schiff base formation with Lip-CHO proceeded relatively slowly, and the reaction yield was almost 50–60% after 4 h (Fig. S10†). These results suggest that the slow kinetics of the β-gal-triggered spectral changes of NIR-AuNPs (Fig. 1a and b) is mainly due to the slow rate of the Schiff base formation.
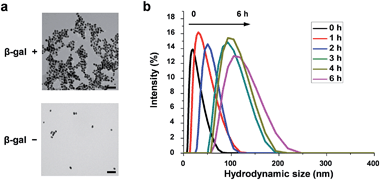
The morphological variation was confirmed by transmission electron microscopy (TEM) (Fig. 2a). Without β-gal, NGal-NIR-AuNPs/CHO-NIR-AuNPs were quite well dispersed. Upon addition of β-gal, the nanoparticles aggregated. The hydrodynamic size of nanoparticles measured by dynamic light scattering (DLS) showed a gradual increase with β-gal from 25 nm to 137 nm (Fig. 2b). Time-dependent zeta-potential (ζ) measurements showed a drastic increase from −3.4 to +6.2 mV in 20 min and a subsequent slow decrease to +3.5 mV over time (Fig. S11†). All data indicate that enzymatic production of primary amines on the nanoparticle surface occurs in the initial stage (∼20 min) and the Schiff-base formation accompanied with the consumption of the amines progresses more slowly, in agreement with HPLC analysis (Fig. S9 and S10†).
Next, to validate the mechanism of enzyme-responsive NIR fluorescence enhancement, we synthesized hollow AuNPs (HGNs) with an LSPR peak at 700 nm (matching well with the emission of Cy5.5m),45 and modified them with Lip-Cy5.5m (Fig. S12†). The fluorescence of these fluorophore-modified Lip-Cy5.5m@HGNs was remarkably enhanced by a factor of 4.2 relative to that of Lip-Cy5.5m at the same concentration (Fig. S13†), indicating that the sufficient spectral overlap between LSPR and NIR emission efficiently induces the fluorescence enhancement.
Then, the essential mechanism of responsive MEF was investigated. The increase in scattering efficiency enhances the amount of light absorbed by the proximal fluorophores and contributes to the fluorescence enhancement.12,14 To evaluate the light scattering effect, fluorescent dye-free NGal-AuNPs/CHO-AuNPs were prepared on the assumption that they show the same light scattering intensity changes as the dye-containing NGal-NIR-AuNPs/CHO-NIR-AuNPs. In the presence of β-gal, the scattering intensity of NGal-AuNPs/CHO-AuNPs drastically increased in the NIR region (Fig. 3).
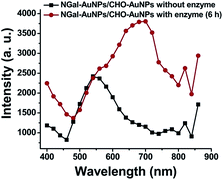 | ||
| Fig. 3 Light scattering spectral shift of NGal-AuNPs/CHO-AuNPs (1.0 nM) in the absence and presence of enzyme (6 h) in PBS (pH 7.4) at 37 °C. | ||
The effect of AuNPs aggregation on the fluorescence radiation process was also investigated. Fluorescence lifetime measurements revealed the average fluorescence lifetimes (τave) of Lip-Cy5.5m, and NGal-NIR-AuNPs/CHO-NIR-AuNPs in the absence and presence of β-gal were 835 ps, 274 ps and 202 ps, respectively (Table 1 and S2, and Fig. S14†). The ΦF of Lip-Cy5.5m and NGal-NIR-AuNPs/CHO-NIR-AuNPs without and with enzyme were 0.18, 0.057, and 0.36, respectively (Table 1). From the τave and ΦF, the kinetic parameters of the radiation process were calculated (Table 1). The nonradiative kinetic parameter knr of NGal-NIR-AuNPs/CHO-NIR-AuNPs without enzyme is 3.3 × 109 s−1, which is a factor of 3.3 higher than that of Lip-Cy5.5m (1.0 × 109 s−1). Concomitantly, the radiative kinetic parameter kr is 2.1 × 108 s−1, which is almost unchanged from that of Lip-Cy5.5m (2.2 × 108 s−1). These results indicate that the AuNPs significantly quench the fluorescence of the surface-modified fluorophores and that the small enhancement of kr is probably due to negligible coupling between LSPR and emission. Interestingly, kr with enzyme is 1.8 × 109 s−1, which is an enhancement by a factor of 8.2 and 8.6 relative to Lip-Cy5.5m and NGal-NIR-AuNPs/CHO-NIR-AuNPs without enzyme, respectively. On the other hand, knr is 3.2 × 109 s−1, which is slightly lower than that without enzyme and about 3 times larger than that of Lip-Cy5.5m. Overall, NGal-NIR-AuNPs/CHO-NIR-AuNPs show an enhancement of the ΦF by a factor of approximately 6 in response to β-gal, and it is mostly due to a significant increase in kr.
| Samples | Φ F | k r (s−1) | k nr (s−1) |
|---|---|---|---|
| Lip-Cy5.5m | 0.18 | 2.2 × 108 | 1.0 × 109 |
| NGal-NIR-AuNPs/CHO-NIR-AuNPs without enzyme | 0.057 | 2.1 × 108 | 3.3 × 109 |
| NGal-NIR-AuNPs/CHO-NIR-AuNPs with enzyme (6 h) | 0.36 | 1.8 × 109 | 3.2 × 109 |
| Lip-Cy5.5m@HGNs | 0.63 | 5.3 × 109 | 3.1 × 109 |
Given that knr depends on the distance and orientation between the fluorophore and AuNPs,46 the slight change in knr suggests that the enzyme-triggered aggregation induces a very small disruption of the architecture of the nanostructure, such as the distance between the fluorophore and the AuNPs surface. The kr of Lip-Cy5.5m on HGNs drastically increases by a factor of 25, and knr is similar to those of NGal-NIR-AuNPs/CHO-NIR-AuNPs with and without enzyme (Table 1). These results indicate that the enzyme-triggered enhancement of NIR fluorescence results from an aggregation-induced LSPR shift that increases the electromagnetic resonance coupling, which in turn increases kr, but has little effect on knr (Fig. 4).
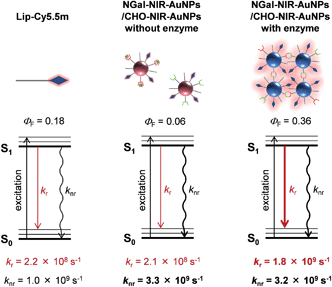 | ||
| Fig. 4 Plausible mechanism of the β-gal-responsive metal-enhanced NIR fluorescence enhancement of NIR-AuNPs by the consideration of the photophysical parameters. | ||
Conclusions
In summary, we developed a novel enzyme-triggered NIR fluorescence enhancement nanosystem, which was obtained by the deliberate design and construction of functionalized AuNPs. The NIR fluorescence on the AuNPs was enhanced by a factor of 7.8 by β-gal. The increase induced by β-gal in electromagnetic resonance coupling between the AuNPs and the fluorophores gave rise to an increase in kr and light scattering intensity, both of which substantially contribute to the fluorescence enhancement. This novel strategy for the design of NIR fluorescent sensors will be widely applicable for the development of various enzyme-detection systems by the introduction of specific substrates on AuNPs. The new NIR fluorescence enhancement principle described here will be useful in the in-demand imaging techniques, such as in vivo enzyme detection and early diagnostic imaging. Although some challenges remain for the in vivo applications, they will be overcome by the integration with some advanced technologies such as stimuli-responsive polymers or active targeting technologies.Acknowledgements
This work was financially supported by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan (Grant nos 24685028, 25620133, 24651259, 25220207, 24115513 and 24108724, 15H03120, and 15H00818), by the MEXT Photonics Advanced Research Center (PARC) program in Osaka University, by the Japanese Society for the Promotion of Science (JSPS) through its Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program), by CREST from JST, and by Asahi Glass Foundation. TEM measurements were taken at the Research Center for Ultra-High Voltage Electron Microscopy, Osaka University.References
- V. Ntziachristos, J. Ripoll and R. Weissleder, Opt. Lett., 2002, 27, 1652 CrossRef.
- R. Weissleder, Nat. Biotechnol., 2001, 19, 316–317 CrossRef CAS PubMed.
- J. V. Rangioni, Curr. Opin. Chem. Biol., 2003, 7, 626–634 CrossRef PubMed.
- B. Ballou, L. A. Ernst and A. S. Waggoner, Curr. Med. Chem., 2005, 12, 795–805 CrossRef CAS.
- K. Licha, Top. Curr. Chem., 2002, 222, 1–29 CAS.
- J. Rao, A. Dragulescu-Andrasi and H. Yao, Curr. Opin. Biotechnol., 2007, 18, 17–25 CrossRef CAS PubMed.
- R. C. Benson and H. A. Kues, Phys. Med. Biol., 1978, 23, 159–163 CAS.
- D. Magde, R. Wong and P. G. Seybold, Photochem. Photobiol., 2002, 75, 327–334 CrossRef CAS.
- R. R. Chance, A. Prock and R. Silbey, J. Chem. Phys., 1974, 60, 2744–2748 CrossRef CAS PubMed.
- K. H. Drexhage, J. Lumin., 1970, 1, 693–701 CrossRef.
- W. L. Barnes, J. Mod. Opt., 1998, 45, 661–699 CrossRef CAS PubMed.
- J. R. Lakowicz, Anal. Biochem., 2005, 337, 171–194 CrossRef CAS PubMed.
- P. Anger, P. Bharadwaj and L. Novotny, Phys. Rev. Lett., 2006, 96(113002), 1–4 Search PubMed.
- F. Tam, G. P. Goodrich, B. R. Johnson and N. J. Halas, Nano Lett., 2007, 7, 496–501 CrossRef CAS PubMed.
- R. Bardhan, N. K. Grady and N. J. Halas, Small, 2008, 4, 1716–1722 CrossRef CAS PubMed.
- G. Hong, S. M. Tabakman, K. Welsher, H. Wang, X. Wang and H. Dai, J. Am. Chem. Soc., 2010, 132, 15920–15923 CrossRef CAS PubMed.
- R. Wilson and D. V. Nicolau, Angew. Chem., Int. Ed., 2011, 50, 2151–2215 CrossRef CAS PubMed.
- H. Peng, C. M. Strohsahl, K. E. Leach, T. D. Krauss and B. L. Miller, ACS Nano, 2009, 3, 2265–2273 CrossRef CAS PubMed.
- Y. Zhang, A. Dragan and C. D. Geddes, J. Phys. Chem. C, 2009, 113, 12095–12100 CAS.
- V. G. Kravets, G. Zoriniants, C. P. Burrows, F. Schedin, A. K. Geim, W. L. Barnes and A. N. Grigorenko, Nano Lett., 2010, 10, 874–879 CrossRef CAS PubMed.
- W. Deng and E. M. Goldys, Langmuir, 2012, 28, 10152–10163 CrossRef CAS PubMed.
- Metal-Enhanced Fluorescence, ed. C. D. Geddes, John Wiley and Sons, New Jersey, June 2010, ISBN: 978-0-470-22838-8 Search PubMed.
- A. I. Dragan, E. S. Bishop, J. R. Casas-Finet, R. J. Strouse, M. A. Schenerman and C. D. Geddes, Anal. Biochem., 2007, 396, 8–12 CrossRef PubMed.
- M. J. R. Previte, K. Aslan and C. D. Geddes, Anal. Chem., 2007, 79, 7042–7052 CrossRef CAS PubMed.
- K. Aslan, S. N. Malyn, G. Bector and C. D. Geddes, Analyst, 2007, 132, 1122–1129 RSC.
- A. Dragan and C. D. Geddes, J. Fluoresc., 2014, 24, 1715–1722 CrossRef CAS PubMed.
- S. Kuhn, U. Hakanson, L. Rogobete and V. Sandoghdar, Phys. Rev. Lett., 2006, 97(017402), 1–4 Search PubMed.
- E. Dulkeith, M. Ringler, T. A. Klar, J. Feldmann, A. M. Javier and W. J. Parak, Nano Lett., 2005, 5, 585–589 CrossRef CAS PubMed.
- P. Bharadwaj and L. Novotny, Opt. Express, 2007, 15, 14266–14274 CrossRef CAS.
- K. Aslan, I. Gryczynski, J. Malicka, E. Matveeva, J. R. Lakowicz and C. D. Geddes, Curr. Opin. Biotechnol., 2005, 16, 55–62 CrossRef CAS PubMed.
- R. Bardhan, N. K. Grady, J. R. Cole, A. Joshi and N. J. Halas, ACS Nano, 2009, 3, 744–752 CrossRef CAS PubMed.
- D. Darvill, A. Centeno and F. Xie, Phys. Chem. Chem. Phys., 2013, 15, 15709–15726 RSC.
- F. Xie, A. Centeno, M. R. Ryan, D. Riley, J. Riley and N. M. Alford, J. Mater. Chem. B, 2013, 1, 536–543 RSC.
- J. Zhang and J. R. Lakowicz, Opt. Express, 2007, 15, 2598–2606 CrossRef CAS.
- K. Aslan, M. Wu, J. R. Lakowicz and C. D. Geddes, J. Am. Chem. Soc., 2007, 129, 1524–1525 CrossRef CAS PubMed.
- R. Bardhan, W. Chen, C. Perez-Torres, M. Bartels, R. M. Huschka, L. L. Zhao, E. Morosan, R. G. Pautler, A. Joshi and N. J. Halas, Adv. Funct. Mater., 2009, 19, 3901–3909 CrossRef CAS PubMed.
- Y. Fu, J. Zhang and J. R. Lakowicz, J. Am. Chem. Soc., 2010, 132, 5540–5541 CrossRef CAS PubMed.
- M. Bauch, K. Toma, M. Toma, Q. Zhang and J. Dostalek, Plasmonics, 2014, 9, 781–799 CrossRef CAS.
- Z. Zeng, S. Mizukami and K. Kikuchi, Anal. Chem., 2012, 84, 9089–9095 CAS.
- X. Xia, M. Yang, Y. Wang, Y. Zheng, Q. Li, J. Chen and Y. Xia, ACS Nano, 2012, 6, 512–522 CrossRef CAS PubMed.
- S. D. Techane, J. Gamble and D. G. Castner, J. Phys. Chem. C, 2011, 115, 9432–9441 CAS.
- H. Hinterwirth, S. Kappel, T. Waitz, T. Prohaska, W. Lindner and M. Lämmerhofer, ACS Nano, 2013, 7, 1129–1136 CrossRef CAS PubMed.
- (a) C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607–609 CrossRef CAS PubMed; (b) R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078–1081 CrossRef CAS.
- K. Saha, S. S. Agasti, C. Kim, X. Li and V. M. Rotello, Chem. Rev., 2012, 112, 2739–2779 CrossRef CAS PubMed.
- Y. G. Sun and Y. N. Xia, Anal. Chem., 2002, 74, 5297–5305 CrossRef CAS.
- S. Vukovic, S. B. Corni and B. Mennucci, J. Phys. Chem. C, 2009, 113, 121–133 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed information on the synthetic routes to various ligands, characterization data, additional figures, schemes, tables and general experiment procedures are provided. See DOI: 10.1039/c5sc01850a |
| This journal is © The Royal Society of Chemistry 2015 |