Open Access Article
Open Access ArticleOperando X-ray absorption and EPR evidence for a single electron redox process in copper catalysis†
Qingquan
Lu‡
a,
Jian
Zhang‡
 a,
Pan
Peng
a,
Pan
Peng
 a,
Guanghui
Zhang
a,
Guanghui
Zhang
 ac,
Zhiliang
Huang
ac,
Hong
Yi
a,
Jeffrey T.
Miller
cd and
Aiwen
Lei
*abc
ac,
Zhiliang
Huang
ac,
Hong
Yi
a,
Jeffrey T.
Miller
cd and
Aiwen
Lei
*abc
aCollege of Chemistry and Molecular Sciences, The Institute for Advanced Studies (IAS), Wuhan University, Wuhan 430072, Hubei, P. R. China. E-mail: aiwenlei@whu.edu.cn
bNational Research Center for Carbohydrate Synthesis, Jiangxi Normal University, Nanchang 330022, Jiangxi, P. R. China
cChemical Sciences and Engineering Division, Argonne National Laboratory, 9700 S. Cass Ave, Argonne, IL 60439, USA
dDepartment of Chemical Engineering, Purdue University, W. Lafayette, IN 47907, USA
First published on 26th May 2015
Abstract
An unprecedented single electron redox process in copper catalysis is confirmed using operando X-ray absorption and EPR spectroscopies. The oxidation state of the copper species in the interaction between Cu(II) and a sulfinic acid at room temperature, and the accurate characterization of the formed Cu(I) are clearly shown using operando X-ray absorption and EPR evidence. Further investigation of anion effects on Cu(II) discloses that bromine ions can dramatically increase the rate of the redox process. Moreover, it is proven that the sulfinic acids are converted into sulfonyl radicals, which can be trapped by 2-arylacrylic acids and various valuable β-keto sulfones are synthesized with good to excellent yields under mild conditions.
Copper is an essential trace element for nearly all aerobic organisms. Multi-copper oxidases (MCOs), for example, are encoded in genomes and play a critical role in the physiology of essentially all aerobes, including metal metabolism and O2-reduction.1 Copper has also been extensively used as a catalyst for the synthesis of organic and inorganic compounds,2 polymers,3 biomaterials4etc. Although copper-assisted chemistry has many important applications and is widely researched, the majority of studies are devoted to methodology development, rather than a detailed mechanistic understanding of the catalyst structure, the oxidation states and the effect of ligands on rate and selectivity. The lack of fundamental understanding has limited further developments.
Catalysis is the key technology for organic synthesis, and insight into the nature of the catalytic species has become particularly important in chemical manufacture.5 Until now, unambiguous identification of the oxidation states and structures of the catalytic species under realistic conditions was a major obstacle for mechanistic studies.6 As a result, redox states of +1/+3, +1/+2 and 0/+2, have been proposed for Cu catalyzed reactions, often with limited experimental evidence.2 As is well known, the active metal undergoes oxidation and reduction half reactions in a catalytic cycle. Oxidation of low valent copper, for example, oxidation of copper(I) to copper(II), has been extensively investigated for more than one century, particularly the oxidation of copper(I) by molecular oxygen due to its importance in chemistry and biology.7 While Cu(I) oxidation has been well studied, the reductive half reaction and factors that influence the Cu(II) reducibility are less well understood and remain rudimentary,1,8 which has been the main barrier for the development of new catalytic processes. Herein, we communicate a direct observation of the reduction of Cu(II) by a sulfinic acid at room temperature. A single electron transfer from a sulfinic acid to Cu(II) and the formation of Cu(I) are demonstrated using X-ray absorption and electron paramagnetic resonance spectroscopies.
The reaction between benzenesulfinic acid and Cu(II) under an inert atmosphere was initially monitored by electron paramagnetic resonance (EPR). As shown in Fig. 1, upon addition of excess benzenesulfinic acid to a DMF solution of CuBr2, the EPR signal of Cu(II) disappears rapidly at room temperature, suggesting that diamagnetic copper(I) has formed.9 This interesting phenomenon spurred us to investigate what really happened in the process.
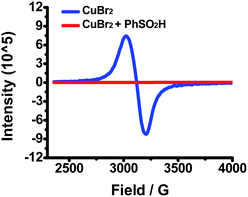 | ||
| Fig. 1 Blue line: CuBr2 (0.4 mmol) in DMF (4.0 mL) at r.t. under N2; red line: CuBr2 (0.4 mmol) and benzenesulfinic acid 2a (0.80 mmol) in DMF (4.0 mL) kept at r.t. for 5 min under N2. | ||
To identify the variation of the valence states of copper in the solution, this reaction was also studied using X-ray absorption near-edge structure (XANES) spectroscopy. Initially, the XANES spectrum of the DMF solution of CuBr2 gave a spectrum with a small pre-edge energy peak at 8977.3 eV, which is typical of Cu(II) compounds (Fig. 2, blue line).8c,10 However, when benzenesulfinic acid was added at room temperature, a new Cu species with an edge energy of 8981.3 eV was observed (Fig. 2, red line), confirming that Cu(II) was reduced to Cu(I) during the reaction.8c,10 These results provided direct evidence that the sulfinic acid reduces Cu(II) through a single electron redox process.
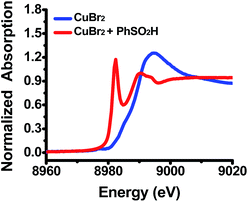 | ||
| Fig. 2 Blue line: CuBr2 (0.4 mmol) in DMF (4.0 mL) at r.t. under N2; red line: CuBr2 (0.4 mmol) and benzenesulfinic acid (0.80 mmol) in DMF (4.0 mL) kept at r.t. for 5 min under N2. | ||
Furthermore, in order to determine the number and types of ligands on Cu(I), an EXAFS spectrum of the Cu(I) species was next fitted. The k3-weighted R-space EXAFS spectrum shows that the CuI center was coordinated to two Br ligands with an average distance of 2.24 Å (Fig. 3). The Cu(I) species, therefore, was assigned as diBr anion complex [CuIBr2]−1.
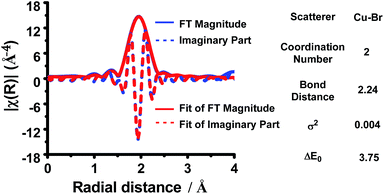 | ||
| Fig. 3 k 3-Weighted R-space EXAFS spectrum of the solution of the reaction of CuBr2 (0.4 mmol) and benzenesulfinic acid (0.80 mmol) in DMF (k-range: 2.93–13.67 Å−1; R-range: 1.40–2.50 Å). | ||
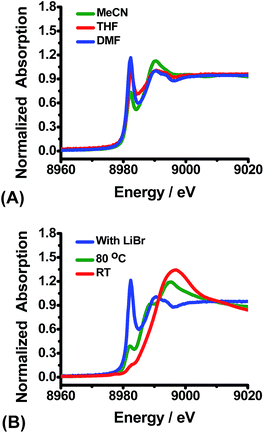
XANES spectra was also applied to explore the influence of different factors which affect the reduction of Cu(II). It was found that the choice of solvent has only a slight influence, and that the reduction of CuBr2 proceeds in polar solvents, such as DMF, or low-polar solvents including MeCN and THF at room temperature (Fig. 4A). While the solvent has little effect on the reducibility, the type of anion is very important. For example, Cu(OAc)2 shows little reduction at room temperature (Fig. 4B, red line), and reduces only slightly at 80 °C (Fig. 4B, green line). Addition of a stoichiometric amount of LiBr, however, leads to rapid reduction of Cu(OAc)2 with benzenesulfinic acid even at room temperature (Fig. 4B, blue line, edge energy is 8981.6 eV).8c,10
The influence of Br− ions on the reducibility of Cu(OAc)2 was also investigated using EPR spectroscopy. As shown in Fig. 5, Cu(OAc)2 exhibits a very weak EPR signal in DMF, which is consistent with being present as an EPR-silent paddlewheel dimer.9 However, once LiBr was added to the Cu(OAc)2 solution, a strong EPR signal was observed, which increased in intensity with increasing amounts of LiBr. The addition of LiBr appears to dissociate Cu(OAc)2 into mononuclear Cu(II) species.9,11 More importantly, upon addition of benzenesulfinic acid to the solution of Cu(OAc)2 and LiBr, the Cu(II) EPR signal disappeared immediately and as shown by the XANES spectrum in Fig. 4B, Cu(I) is formed concurrently. These results suggest that coordinative saturation of Cu(OAc)2 and the inability for substrate coordination may be the obstacle to Cu(II) reduction by benzenesulfinic acid.
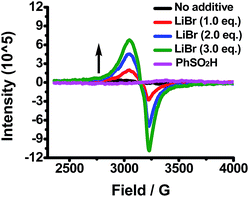 | ||
| Fig. 5 LiBr (0.4 mmol) × 3 and PhSO2H (0.80 mmol) were successively added to the DMF (4.0 mL) solution of Cu(OAc)2 (0.4 mmol) at r.t. under N2. | ||
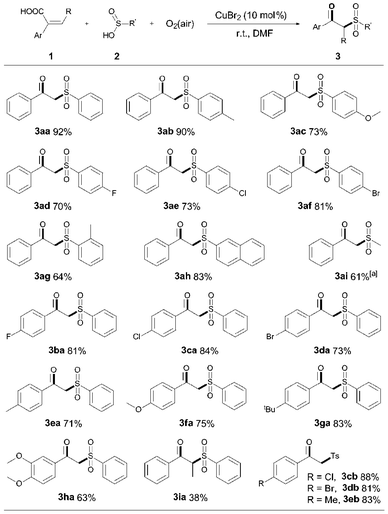
Based on the above results, it appears likely that the sulfinic acid serves as the one electron donor for reduction of Cu(II) to Cu(I) with formation of oxidized sulfonyl radicals. If true, it is reasonable to expect that a catalytic redox process could be used for the synthesis of fine chemicals with suitable radical acceptors. When 2-arylacrylic acid was reacted with CuBr2/sulfinic acid, the product β-keto sulfones 3 could be isolated in good to excellent yields at room temperature under air (Scheme 1).
As shown in Scheme 1, a variety of aryl sulfinic acids, bearing either electron-donating groups (R = OMe, Me) or electron-withdrawing groups (R = F, Cl, Br) on the aryl ring, reacted smoothly with 2-phenylarcylic acid, affording the corresponding β-keto sulfones 3ab–3ah in good to excellent yields. Alkyl sulfinic acids, as exemplified by methyl sulfinic acid, also efficiently reacted with 1a and the product 3ai was obtained in 61% yield. Besides, the arylacrylic acids, with either electron-rich or electron-poor substituents on the aromatic ring, were all compatible with this transformation, leading to the corresponding decarboxylative products in good yields (3ba–3ha). Non-terminal arylacrylic acid derivatives, such as α-ethylidene benzeneacetic acid, exhibited relatively low reactivity and the product 3ia was afforded in moderate yield, presumably due to the steric hindrance from the pyramidal structure of the sulfonyl radical.12 Arylacrylic acids containing chloro, bromo and methyl groups also reacted effectively with p-toluenesulfinic acid, offering the expected products 3cb–3eb in high yields (81–88%). Furthermore, 18O labeling experiments indicated that the carbonyl oxygen in the β-keto sulfones comes from dioxygen (for details, see ESI†). To the best of our knowledge, this is the first example of the combination of S–H bond alkylation, C–C σ bond cleavage, and aerobic oxygenation in a single step.13 These results not only serve as indirect evidence to support the formation of the sulfonyl radical in situ, but also provide a useful insight into the mechanism of Cu(II) reduction and further synthetic applications.
Conclusions
In conclusion, X-ray absorption and electron paramagnetic resonance spectroscopies provide direct evidence for the single electron redox process between sulfinic acids and Cu(II), forming Cu(I) and a sulfonyl radical at room temperature. Addition of bromide ions increases the rate of Cu(II) reduction. Based on these observations, catalytic oxysulfonylation of arylacrylic acids under simple and mild conditions was developed for the first time. Ongoing research including further mechanistic details and expanding the substrate scope is currently underway.Acknowledgements
This work was supported by the 973 Program (2012CB725302), the National Natural Science Foundation of China (21390400, 21025206, 21272180, and 21302148), the Research Fund for the Doctoral Program of Higher Education of China (20120141130002) and the Ministry of Science and Technology of China (2012YQ120060). The Program of Introducing Talents of Discipline to Universities of China (111 Program) is also appreciated. Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract no. DE-AC02-06CH11357. MRCAT operations are supported by the Department of Energy and the MRCAT member institutions.Notes and references
- Selected reviews, see:
(a) D. J. Kosman, JBIC, J. Biol. Inorg. Chem., 2010, 15, 15 CrossRef CAS PubMed
; (b) K. Nakamura and N. Go, Cell. Mol. Life Sci., 2005, 62, 2050 CrossRef CAS
; (c) E. I. Solomon, P. Chen, M. Metz, S.-K. Lee and A. E. Palmer, Angew. Chem., Int. Ed., 2001, 40, 4570 CrossRef CAS
; (d) G. Vashchenko and R. MacGillivray, Nutrients, 2013, 5, 2289 CrossRef CAS PubMed
.
- Selected reviews, see:
(a) A. Alexakis, J. E. Bäckvall, N. Krause, O. Pàmies and M. Diéguez, Chem. Rev., 2008, 108, 2796 CrossRef CAS PubMed
; (b) S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234 CrossRef CAS PubMed
; (c) I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337 CrossRef CAS PubMed
; (d) S. R. Chemler and P. H. Fuller, Chem. Soc. Rev., 2007, 36, 1153 RSC
; (e) D. Ma and Q. Cai, Acc. Chem. Res., 2008, 41, 1450 CrossRef CAS PubMed
; (f) L. M. Stanley and M. P. Sibi, Chem. Rev., 2008, 108, 2887 CrossRef CAS PubMed
; (g) A. E. Wendlandt, A. M. Suess and S. S. Stahl, Angew. Chem., Int. Ed., 2011, 50, 11062 CrossRef CAS PubMed
; (h) K.-i. Yamada and K. Tomioka, Chem. Rev., 2008, 108, 2874 CrossRef CAS PubMed
; (i) C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3464 RSC
.
-
(a) T. E. Patten and K. Matyjaszewski, Acc. Chem. Res., 1999, 32, 895 CrossRef CAS
; (b) T. Pintauer and K. Matyjaszewski, Chem. Soc. Rev., 2008, 37, 1087 RSC
.
- G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef CAS PubMed
.
-
(a) C. Bolm, J. Legros, J. Le Paih and L. Zani, Chem. Rev., 2004, 104, 6217 CrossRef CAS PubMed
; (b) S. Enthaler, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2008, 47, 3317 CrossRef CAS PubMed
; (c) A. Jutand, Chem. Rev., 2008, 108, 2300 CrossRef CAS PubMed
; (d) C. Sambiagio, S. P. Marsden, A. J. Blacker and P. C. McGowan, Chem. Soc. Rev., 2014, 43, 3525 RSC
.
-
(a) P. J. Ellis, I. J. S. Fairlamb, S. F. J. Hackett, K. Wilson and A. F. Lee, Angew. Chem., Int. Ed., 2010, 49, 1820 CrossRef CAS PubMed
; (b) A. R. Kapdi, A. C. Whitwood, D. C. Williamson, J. M. Lynam, M. J. Burns, T. J. Williams, A. J. Reay, J. Holmes and I. J. S. Fairlamb, J. Am. Chem. Soc., 2013, 135, 8388 CrossRef CAS PubMed
; (c) J. P. Reeds, M. P. Healy and I. J. S. Fairlamb, Catal. Sci. Technol., 2014, 4, 3524 RSC
.
-
(a) E. E. Chufán, S. C. Puiu and K. D. Karlin, Acc. Chem. Res., 2007, 40, 563 CrossRef PubMed
; (b) S. Itoh and S. Fukuzumi, Acc. Chem. Res., 2007, 40, 592 CrossRef CAS PubMed
; (c) M. Rolff, J. Schottenheim, H. Decker and F. Tuczek, Chem. Soc. Rev., 2011, 40, 4077 RSC
; (d) M. Suzuki, Acc. Chem. Res., 2007, 40, 609 CrossRef CAS PubMed
.
-
(a) G. Franc and A. Jutand, Dalton Trans., 2010, 7873 RSC
; (b) G. Lefèvre, G. Franc, A. Tlili, C. Adamo, M. Taillefer, I. Ciofini and A. Jutand, Organometallics, 2012, 31, 7694 CrossRef
; (c) G. Zhang, H. Yi, G. Zhang, Y. Deng, R. Bai, H. Zhang, J. T. Miller, A. J. Kropf, E. E. Bunel and A. Lei, J. Am. Chem. Soc., 2014, 136, 924 CrossRef CAS PubMed
.
- A. E. King, B. L. Ryland, T. C. Brunold and S. S. Stahl, Organometallics, 2012, 31, 7948 CrossRef CAS PubMed
.
- Y. Deng, G. Zhang, X. Qi, C. Liu, J. T. Miller, A. J. Kropf, E. E. Bunel, Y. Lan and A. Lei, Chem. Commun., 2015, 51, 318 RSC
.
- H. Grasdalen and I. Svare, Acta Chem. Scand., 1971, 25, 1089 CrossRef CAS PubMed
.
- C. Chatgilialoglu, B. C. Gilbert and R. O. C. Norman, J. Chem. Soc., Perkin Trans. 2, 1979, 770 RSC
.
- A tentative pathway for the oxysulfonylation of 2-arylacrylic acids is proposed in Scheme S1†.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc00807g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |