Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Isomerisation of nido-[C2B10H12]2− dianions: unprecedented rearrangements and new structural motifs in carborane cluster chemistry†
David
McKay‡
 *,
Stuart A.
Macgregor
and
Alan J.
Welch
*,
Stuart A.
Macgregor
and
Alan J.
Welch
Institute of Chemical Sciences, School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh, EH14 4AS, UK. E-mail: dm228@st-andrews.ac.uk; Fax: +44 (0)1314513180; Tel: +44 (0)1314518031
First published on 24th March 2015
Abstract
Dianionic nido-[C2B10]2− species are key intermediates in the polyhedral expansion from 12- to 13-vertex carboranes and metallacarboranes, and the isomer adopted by these nido intermediates dictates the isomeric form of the 13-vertex product. Upon reduction and metallation of para-carborane up to five MC2B10 metallacarboranes can be produced (Angew. Chem., Int. Ed., 2007, 46, 6706), the structures of which imply the intermediacy of 1,7-, 3,7-, 4,7-, 7,9- and 7,10-isomers of the nido-[C2B10]2− species. In this paper we use density functional theory (DFT) calculations to characterise the reduction of closo-C2B10H12 carboranes and the subsequent isomerisations of the nido-[C2B10H12]2− dianions. Upon reduction para-carborane initially opens to [1,7-nido-C2B10H12]2− (abbreviated to 1,7) and [4,7-nido-C2B10H12]2− (4,7) and isomerisation pathways connecting 1,7 to 7,9, 4,7 to 7,10 and 1,7 to 3,7 have been characterised. For ortho- and meta-carborane the experimental reduction produces 7,9 in both cases and computed pathways for both processes are also defined; with ortho-carborane rearrangement occurs via7,8, whereas with meta-carborane 7,9 is formed directly. The 7,9 isomer is the global minimum nido-structure. The characterisation of these isomerisation processes uncovers intermediates that adopt new structural motifs that we term basket and inverted nido. Basket intermediates feature a two-vertex basket handle bridging the remaining 10 vertices; inverted nido intermediates are related to known nido species, in that they have 5- and 6-membered belts, but where the latter, rather than the former, is capped, leaving a 5-membered open face. These new intermediates exhibit similar stability to the nido species, which is attributed to their relation to the 13-vertex docosahedron through the removal of 5-connected vertices. Isomerisation pathways starting from nido geometries are most often initiated by destabilisation of the cluster through a DSD process causing the 3-connected C7 vertex to move into a 4-connected site and a neighbouring B vertex to become 3-connected. The ensuing rearrangement of the cluster involves processes such as the pivoting of a 4-vertex diamond about its long diagonal, the pivoting of two 3-vertex triangles about a shared vertex and DSD processes. These processes are all ultimately driven by the preference for carbon to occupy low-connected vertices on the open 6-membered face of the resulting nido species.
Introduction
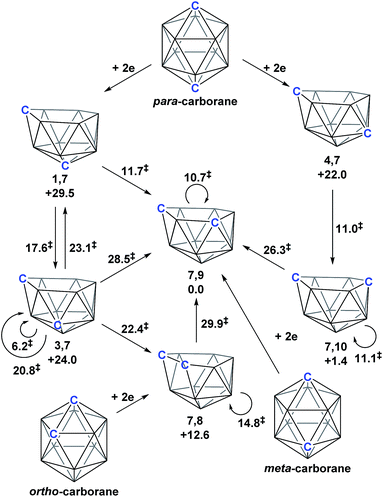
As predicted by Wade's Rules,1 the addition of a skeletal electron pair (SEP) to a closo polyhedron (with [n + 1] SEPs, where n is the number of vertices) results in the formation of the corresponding nido cluster ([n + 2] SEPs). A key synthetic route that relies upon this is the polyhedral expansion method, whereby 2-electron reduction of a closo precursor (normally a 12-vertex carborane) results in the formation of a nido fragment, which can then be capitated with a {BR} or {M} fragment. Application of this method led to both the first 13-vertex metallacarborane2 and the first 13-vertex carborane.3 In 2007 we showed that the polyhedral expansion of a single carborane precursor, [1,12-Ph2-1,12-closo-C2B10H10], with {M} = {Ru(p-cymene)} led to the formation of five isomeric supraicosahedral metallacarboranes of the form RuC2B10 (Fig. 1).4 This implies the presence of five isomeric nido fragments following reduction, i.e.1,7-, 3,7-, 4,7-, 7,9- and [7,10-Ph2-7,10-nido-C2B10H10]2−. A concurrent computational study on [1,12-closo-C2B10H12] (para-carborane) suggested the first nido-fragments formed upon reduction were [1,7-nido-C2B10H12]2− (termed 1,7 in the following) and [4,7-nido-C2B10H12]2− (4,7). At the time, the further isomerisation of these species to the remaining nido species was not considered, however computing these directly provided relative energies, H (enthalpies, 0 K) of +29.5, +24.0, +22.0 and +1.4 kcal mol−1 for 1,7, 3,7, 4,7 and 7,10 respectively, all relative to 7,9 at 0.0 kcal mol−1. In contrast, related experimental studies adopting ortho- or meta-carborane precursors show that 7,9 is the only nido species formed.2,5–7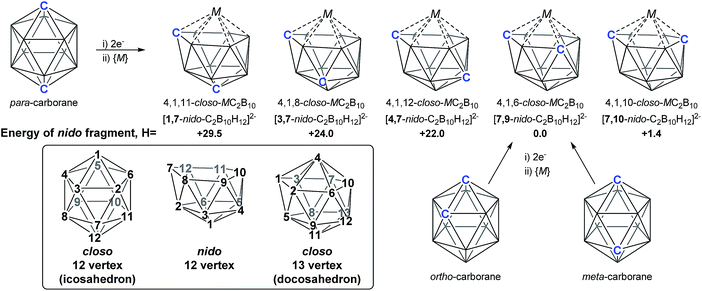 | ||
| Fig. 1 Results of polyhedral expansion of 12-vertex carboranes to 13-vertex metallacarboranes. For para-carborane {M} = {Ru(p-cymene)}, {C} = {CPh} relating to ref. 4; for ortho-carborane, e.g. {M} = {Ru(p-cymene)} or {CoCp}, {C} = e.g. {CH}.2,5 Computed energies of [nido-C2B10H12]2− species given relative to 7,9 in kcal mol−1. See inset for formal numbering. | ||
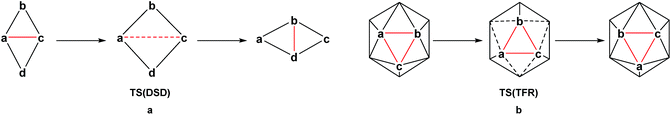
The thermal rearrangement of 12-vertex closo (hetero)boranes has been the subject of continued investigation. Starting from ortho-carborane, the conversion to meta-carborane at 450 °C and then to para-carborane at 700 °C has been known for over 50 years and indeed was the route to their first syntheses.8–10 The processes involved in their rearrangement have been studied for many years, both theoretically11–16 and experimentally through labelling studies.17–19 In 1966, Lipscomb13 introduced the diamond-square-diamond (DSD) mechanism (Scheme 1a), while in the same year, Zakharkin and Kalinin16 suggested the triangular face rotation (TFR) mechanism, which can also be described as three concerted DSD processes (Scheme 1b). The DSD process in particular has since been recognised as key to carborane rearrangement. Wales15 adopted an eigenvector following method to map the potential energy surface of C2B10H12 at the Hartree–Fock (HF) level. Multi-step DSD-derived processes were found to dominate, however two TFR-type pathways were also located. High symmetry processes involving multiple simultaneous DSDs (e.g. the hextuple DSD process leading to a cuboctahedral geometry suggested by Lipscomb13) were discounted due to their unfeasibly high activation energies. Later studies also demonstrate an energetic preference for low symmetry processes.11,12,14 Wales also ruled out the closo-nido-closo rearrangement pathway, which requires opening of the cage to a high energy pseudo-nido intermediate.
More recently, Brown and McKee11 showed, through density functional theory (DFT) calculations, that a single step TFR process was favoured in the ortho- to meta-carborane isomerisation, while a two-step DSD pathway was preferred for isomerisation from meta- to para-carborane. Brown and McKee had discounted a two-step pathway from ortho- to meta-carborane, due to a high initial barrier between ortho-carborane and the intermediate involved. We later showed that the equivalent intermediate was formed upon the oxidation of 7,9 and characterised a lower energy process to form ortho-carborane, in agreement with experiment.12 Most recently, Sugden and co-workers14 investigated both of these isomerisation pathways through ab initio molecular dynamics (DFT-MD) adopting the PBE functional.
In stark contrast, few computational studies have involved reduced 12-vertex carboranes, despite the isomeric form of the reduced species ultimately dictating the isomer of the supraicosahedral product. McKee et al.20 computed 7,9 directly, through HF calculations, for comparison with plausible protonated [nido-C2B10H13]− structures. Later, Hermansson and co-workers21 showed in a study of the electron affinities of carboranes (also at the HF level) that sequential addition of two electrons to meta-carborane resulted in 7,9, while geometries produced from reduction of ortho- and para-carborane showed only minor distortions and did not resemble nido fragments. More recently, 12-vertex nido carboranes and (bis)carboranes have featured in our investigations of the aforementioned oxidation of 7,9 to ortho-carborane,12 the room-temperature C–C activation of an arene at a 13-vertex metallacarborane,22 co-production of isomeric 13-vertex cobaltacarboranes from polyhedral expansion of a tethered carborane precursor23 and in the rational design of derivatives of 7,9 stabilised towards aerial oxidation.24
Herein we report a computational study of the isomerisation processes that follow from the initial 2e reduction of para-carborane and formation of 1,7 and 4,7, revealing pathways interconnecting all five nido fragments inferred experimentally (see Fig. 1). The reduction of ortho-carborane is shown to initially produce 7,8, before rearranging to the experimental product, 7,9. meta-Carborane reduction proceeds to 7,9 directly, where the barrierless rearrangement process is rationalised by relation to the nido isomerisation pathways. In the completion of this work, we uncover and rationalise new dianionic 12-vertex carborane structures which we refer to as basket and inverted nido intermediates and characterise new, unexpectedly complex processes interconnecting nido species, ultimately linking all intermediates to the global minimum, 7,9.
Results
1. Formation of 1,7 and 4,7
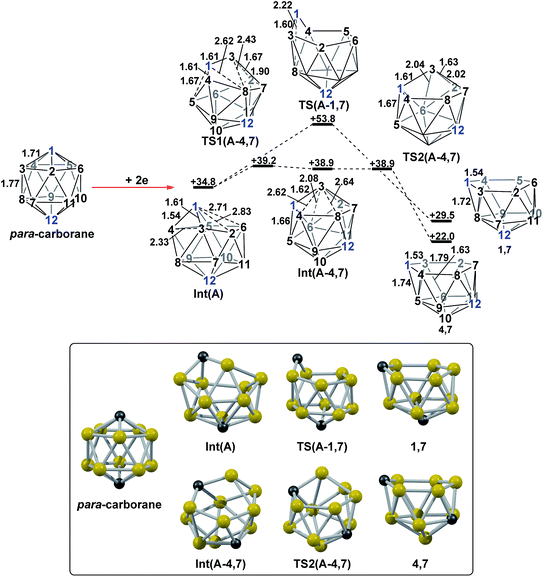
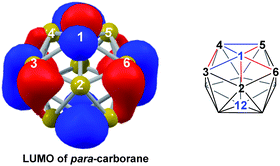
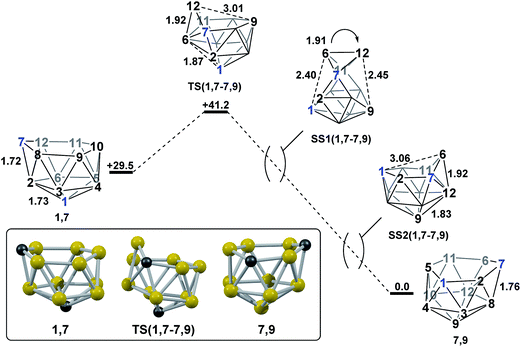
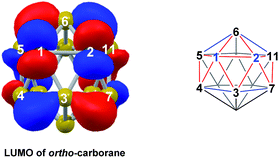
DFT calculations were performed at the BP86/6-31G** level using Gaussian 03 and we report zero-point corrected electronic energies, H, for all computed species relative to 7,9 (see Computational details). In order to model the reduction of para-carborane, first the neutral geometry was optimised, then two electrons were added and the system was re-optimised. This resulted in an initial reduced minimum, Int(A) (H = +34.8 kcal mol−1; Fig. 2). Int(A) features 4-membered (B3–B8–B4–C1) and 5-membered (B3–C1–B5–B6–B2) open faces and is related to the nido geometry, 1,7, by a DSD process in the C1–B3–B8–B4 face. This was characterised by decreasing the C1⋯B8 distance, giving TS(A-1,7) (H = +53.8 kcal mol−1). Through this process, C1, which is 3-connected in Int(A), becomes 2-connected in the transition state and returns to 3-connected in 1,7, giving a barrier of 19.0 kcal mol−1. Alternatively, movement of B3 across the 5-membered face towards B6 in Int(A) gives Int(A-4,7), through a lower barrier of 4.4 kcal mol−1. Int(A-4,7) is topologically equivalent to Int(A) and features 4-membered C1–B3–B2–B6 and 5-membered C1–B3–B7–B8–B4 faces. We refer to such a topology as a basket intermediate (discussed in detail later) where here C1 and B3 vertices bridge the remaining 10 vertices in a way that resembles a basket handle. From Int(A-4,7) decreasing the B3⋯B6 distance causes the 4-membered face to close viaTS2(A-4,7) (H = +38.9 kcal mol−1) in what is effectively a barrierless process. Thereafter, a DSD process occurs in the C1–B3–B6–B5 diamond, breaking the C1–B6 connection and forming the B3–B5 connection, allowing C1 to become 3-connected and furnishing the nido geometry, 4,7. These energy profiles suggest that processes decreasing the number of connections to carbon vertices and increasing the number of connections to boron vertices are favoured. The lower barrier to formation of 4,7 than of 1,7 also suggests that processes involving movement of boron are easier than those involving movement of carbon. This is supported by electronegativity arguments; the radial orbitals of the carbon vertices, being more contracted than those of boron, do not allow stabilisation of higher-connected sites or longer connections. The movement of vertices from the initial para-carborane geometry to give Int(A) can be rationalised by visualisation of the LUMO of para-carborane (Fig. 3). This features a π-antibonding interaction along the C1–B6 connection and a further antibonding interaction between B3 and B4. Therefore the 2e occupation of this orbital is consistent with the breaking of these interactions to give a 4-membered C1–B3–B8–B4 and a 5-membered C1–B3–B2–B6–B5 face in Int(A).2. Onward isomerisations of 1,7 and 4,7: general strategies
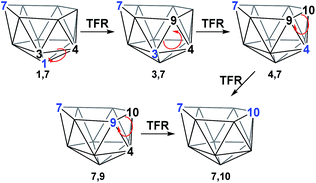
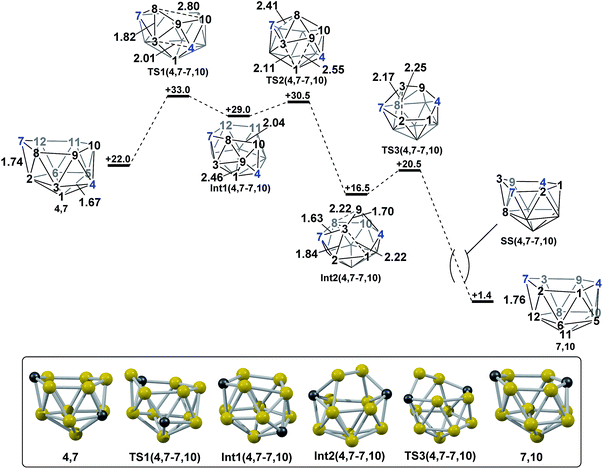
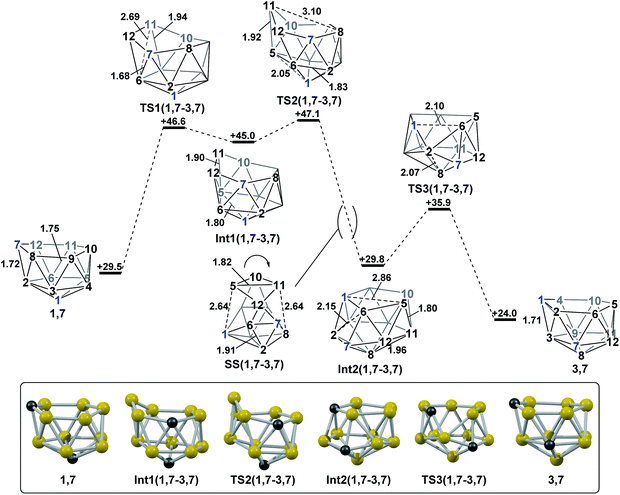
The geometries of 1,7 and 4,7 were considered as starting points towards formation of the remaining nido species, 3,7, 7,9 and 7,10. Initially, we considered the possibility of TFR processes linking nido isomers (Scheme 2); in 1,7, rotation of the C1–B3–B4 triangle could be envisaged to interconvert 1,7 and 3,7 and rotation of the same triangle again, or C3–B9–B4, would exchange 3,7 and 4,7. Likewise, in 4,7, rotation of the C4–B9–B10 triangle could give 7,9 and 7,10. However, attempts to characterise such processes through potential energy surface searching (linear transits) were unsuccessful from either 1,7 or 4,7. Several atoms had to be fixed in position in order to prevent non-targetted rearrangement of the cluster. This suggested that the TFR process, though relevant to closo carborane isomerisation, was higher in energy than other processes available to the more flexible nido species. It was noted that the lowest energy vibrational mode of all nido species computed involves rotation of the 6-membered face above the 5-membered 2–3–4–5–6 belt, where the largest displacement is seen in the 7-position. Taking the lead from the mode-following approach of Wales,15 we used the transition state (TS) optimisation option in Gaussian 03,25 which follows the lowest energy vibrational mode to a saddle-point on the PES, thus allowing low energy transition states to be sought a priori, direct from selected minima. The present C2B10H12 clusters (which lack polyatomic exopolyhedral substituents) lend themselves to mode-following since the lowest energy vibrational mode always involves displacement of cluster vertices and so is productive towards cluster rearrangement. Mode-following was therefore always attempted in the first instance for any transition state search (see Computational Details). From 1,7 and 4,7 this revealed surprising and contrasting isomerisation processes, which see rearrangement of 1,7 to 7,9 in a single step and 4,7 to 7,10 in a multi-step process.3. Reductions of ortho- and meta-carborane
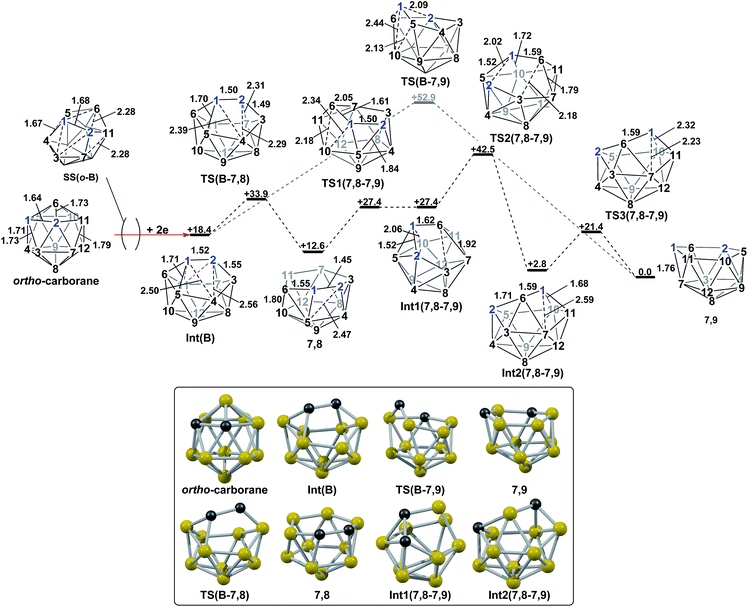
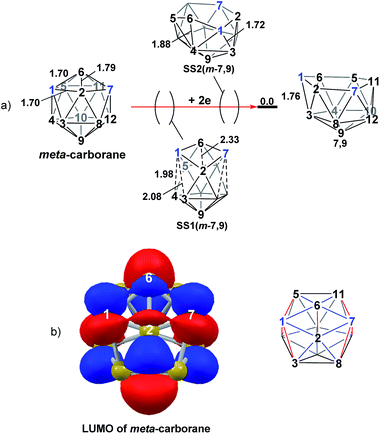
The experimental reductions of ortho- and meta-carborane each lead to 7,9.2,5–7 Hermansson et al.21 showed in a study of carborane electron affinities that, at the HF level of theory, sequential addition of two electrons to meta-carborane produced a 7,9nido geometry, whereas ortho-carborane was only slightly distorted. Through the present DFT calculations, we have now characterised the rearrangement processes undergone by both of these species following 2e reduction, ultimately giving 7,9, which can be rationalised by relating them to the processes seen above. By analogy to the computational treatment of the reduction of para-carborane, 2e were added to the optimised geometry of ortho-carborane and the structure re-optimised as a dianion. This gave Int(B) (H = +18.4 kcal mol−1 above 7,9; Fig. 8). Int(B) is another example of a basket intermediate, where here the geometry is C2 symmetric and the C1 and C2 vertices form the basket handle. The C1–C2 connection in Int(B) is shortened to 1.52 Å w.r.t. 1.64 Å in ortho-carborane, indicative of single bond character. From Int(B), a basket collapse process was characterised through TS(B-7,9) (H = +52.9 kcal mol−1; see Fig. 8, grey pathway) and involves DSD processes in the C1–B5–B10–B6 diamond (with C1⋯B10 and B5⋯B6 distances of 2.44 Å and 2.58 Å respectively) and breaking of the C1–C2 connection to give 7,9 in a single step. However, this process exhibits a high overall barrier of 34.5 kcal mol−1, consistent with it being dominated by the breaking of a C–C connection with single bond character. An alternative basket collapse process was characterised through mode-following (Fig. 8, black pathway). This pathway initially maintains the C1–C2 connection, giving 7,8 through a low barrier of 15.5 kcal mol−1 in which the C1–C2 connection is shortened still further to 1.45 Å. Mode-following from 7,8 led to a degenerate rearrangement involving a DSD process in the C1–C2–B4–B5 diamond (as numbered in Fig. 8), forming a C2–B5 connection through a barrier of 14.8 kcal mol−1. Linear transits were therefore conducted to discover a pathway leading to 7,9. A low energy transition state, TS1(7,8-7,9) (H = +27.4 kcal mol−1) was located, and provided a basket intermediate, Int1(7,8-7,9). From here, C1–C2 bond breaking proceeds through TS2(7,8-7,9) at over 10 kcal mol−1 lower than TS(B-7,9) (H = +42.5 kcal mol−1), giving a barrier of 29.9 kcal mol−1 from 7,8. This process leads to Int2(7,8-7,9) at H = +2.8 kcal mol−1, which is identical to Int(7,10-7,9). Therefore the basket collapse process described above to give 7,9 is repeated in this pathway; here forming the C1–B7 connection through a DSD process in the C1–B11–B7–B6 diamond of the Int2(7,8-7,9)basket. The nido-7,8 isomer is implicated experimentally in the synthesis if 4,1,2-MC2B10 species from ortho-carborane, where exopolyhedral hydrocarbyl or silyl tethers connecting the C-vertices ensure the C positions remain adjacent.3,26,27 With the removable silyl tether metallation with M = {CoCp} leads to the concurrent formation of the expected 4,1,2-MC2B10 species, but also the 4,1,6-MC2B10 isomer, indicating that isomerisation of the nido-7,8 fragment to the 7,9 form is possible.23 The initial strengthening of the C1–C2 connection in Int(B)cf. ortho-carborane contrasts with the 2e addition to 1,2-Ph2-1,2-closo-C2B10H10 (towards [7,9-Ph2-7,9-nido-C2B10H10]2−), where the C1–C2 connection breaks due to a σ-C–C antibonding component in the LUMO orbital of the neutral species.24 In the LUMO of ortho-carborane (Fig. 9a) a π-antibonding interaction is seen between the C vertices, suggesting the connection would indeed be lengthened on occupation of the orbital. Further antibonding interactions are seen between C2 and the B3–B7 edge and those equivalent by C2v symmetry (C1–{B3–B4}, C1–{B5–B6} and C2–{B6–B11}) and along the B4–B5 (and B7–B11) connections. Upon visualising the optimisation an initial lengthening of connections with antibonding interactions was noted (see ESI† for Movie). A snapshot of this (Fig. 8, SS(o-B)) shows the C1–C2 connection initially lengthens from 1.64 Å in ortho-carborane to ca. 1.7 Å. In addition, the distances from the C vertices to B3 and B6 and the B4–B5 and B7–B11 connections are also lengthened at SS(o-B). As the optimisation continues the C2v symmetry is reduced to C2 by reformation of the C2–B3 (1.55 Å), C1–B5 (1.71 Å) and C1–C2 (1.52 Å) connections in Int(B).Addition of 2e to meta-carborane led directly to the location of 7,9 (Fig. 10a) (see ESI† for movie). During the optimisation the structure initially distorts with retention of C2v symmetry, consistent with the population of the LUMO of meta-carborane (Fig. 10b and see snap-shot geometry SS1(m-7,9) in Fig. 10a). Subsequently, B6–C7 lengthens and the symmetry is lost. At SS2(m-7,9), a B6–B4 connection is formed and the connections from C7 to B8, B11 and B12 have reformed. SS2(m-7,9) is equivalent to SS2(1,7-7,9) (Fig. 4) and indeed undergoes a related DSD process, here in the B3–B4–B6–C1 diamond to give 7,9.
Discussion
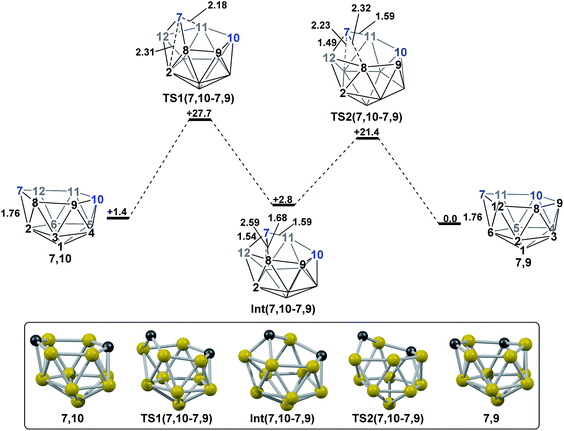
Polyhedral expansion of closo-C2B10 carboranes with metal fragments produces a range of MC2B10 species which imply the intermediacy of 1,7-, 3,7-, 4,7-, 7,9- and 7,10-isomers of the nido-[C2B10]2− species. Here we have used DFT calculations to characterise the isomerisation pathways that link these various nido isomers. Our study has revealed several unusual new intermediates and their unforeseen rearrangement pathways which are categorised and rationalised below.Following the addition of two electrons to the optimised geometry of para-carborane, 1,7 and 4,7 are formed as the initial nido species (Scheme 3). Thereafter, 1,7 connects to 7,9, through a single transition state, with a barrier of 11.7 kcal mol−1. In contrast, the isomerisation of 4,7 proceeds through a facile multi-step process to form 7,10, but with a similar overall barrier of 11.0 kcal mol−1. The remaining nido species, 3,7, is formed in an alternative 3-step process from 1,7 with a barrier of 17.6 kcal mol−1. In order to connect all nido species to the global minimum, 7,9, additional pathways were sought from 7,10, 3,7 and 4,7. From 7,10, a two-step process was characterised with a barrier of 26.3 kcal mol−1. From 3,7, while a single step process was characterised for isomerisation to 7,9 with a barrier of 28.5 kcal mol−1 (see ESI Fig. S2†) this is higher than the reverse process from 3,7 to 1,7 (above; ΔH‡ = 23.1 kcal mol−1) and therefore 3,7 likely isomerises to 7,9via 1,7. Similarly, a direct pathway from 4,7 to 7,9 was not found and so formation of 7,9 from 4,7 is thought to proceed through 7,10 (ΔH‡ = 11.0 kcal mol−1). An additional nido species, 7,8, was found to be formed following 2e addition to ortho-carborane and isomerises to 7,9 through a barrier of 29.9 kcal mol−1. 2e reduction of meta-carborane leads directly to 7,9. Degenerate pathways, where the start and end points of a rearrangement are the same nido species, were characterised for 7,9 (ΔH‡ = 10.7 kcal mol−1), 7,10 (ΔH‡ = 11.1 kcal mol−1), two examples for 3,7 (ΔH‡ = 6.2 kcal mol−1 and 20.8 kcal mol−1) and 7,8 (ΔH‡ = 14.8 kcal mol−1).
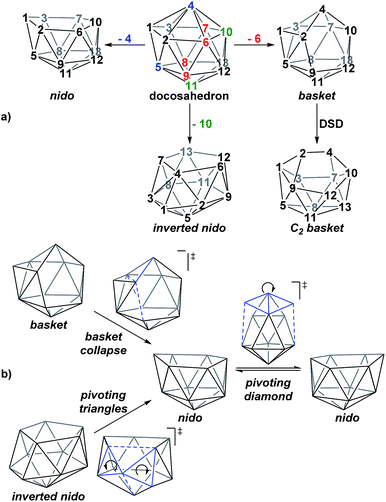
A family of basket intermediates, which are often energetically comparable to conventional nido fragments, were located along several of the characterised pathways. In a basket intermediate, two vertices form a basket handle bridging the remaining 10 vertices, with examples located in this study including Int(A), Int(A-4,7), Int(7,10-7,9), Int1(7,8-7,9) and Int2(7,8-7,9) (all with C1 symmetry) and Int2(4,7-7,10) and Int(B) (with C2 symmetry with the CH vertices in the bridgehead positions and basket handle positions respectively). As shown in Scheme 4a, starting from the docosahedron, a C1-basket may be produced through removal of one of the 5-connected vertices 6–9 (red), with lengthening of the 1–4 distance to produce the requisite 4- and 5-membered faces. The C2-basket intermediates are related to the relevant C1-basket by a DSD in the 1–2–5–9 diamond and lengthening of the 3–4 distance. Two additional key intermediates, Int1(4,7-7,10) and Int2(1,7-3,7), we refer to as inverted nido geometries, due to the 6-membered belt of vertices, rather than the 5-membered belt, being capped by a single vertex. The inverted nido motif is derived from the docosahedron by removal of a 5-connected vertex (10 or 11, green). A classical nido geometry is produced through removal of a 6-connected vertex (4 or 5, blue).28 In order to test the validity of this empirical observation, the idealised [B13H13]2− docosahedron was computed, the appropriate vertices removed and the structure re-optimised as [B12H12]4− fragments. The nido and inverted nido were located as minima (H = 0.0 and +25.8 respectively; see ESI†). The basket geometry was found to collapse to a nido structure, suggesting the C vertices are required to stabilise the distorted geometry, however, the C2basket was located as a transition state that exchanges equivalent nido structures (H = +18.6 kcal mol−1; see ESI†).
The structural types discussed above tend to access specific rearrangement processes. From nido species, the initial step in the isomerisation is most often to move the 3-connected C7 vertex into a 4-connected position. This then triggers movement of the neighbouring vertices resulting in net rotation of the 6-membered belt of vertices above the 5-membered belt. Such processes are also responsible for the degenerate exchanges characterised in 7,8, 7,9 and 7,10. Three additional processes are found (Scheme 4b): a common DSD process by which basket intermediates undergo basket collapse to give nido species (Scheme 4b, upper left); the pivoting of a 4-vertex diamond about its long diagonal to directly exchange nido geometries (Scheme 4b, right; seen in the isomerisations from 1,7 to 7,9, from 1,7 to 3,7 and in the higher energy degenerate process at 3,7 (see ESI, Fig. S1†)) and the pivoting of two triangles about a shared vertex exchanging inverted nido and nido geometries (Scheme 4b lower left; seen in the isomerisations of 4,7 to 7,10 and 1,7 to 3,7).
Conclusions
The rearrangements of dianionic 12-vertex nido-carboranes, [C2B10H12]2−, have been characterised through DFT calculations. para-Carborane rearranges to 1,7 and 4,7 as the first nido species, where experimentally, 1,7, 3,7, 4,7, 7,9 and 7,10 are produced. Isomerisation processes have been characterised connecting 1,7 to 7,9, 4,7 to 7,10, 1,7 to 3,7 and 7,10 to 7,9, thus rationalising the experimental formation of these species and showing how they are interconnected. Reduction of ortho-carborane gives 7,8 as the first nido species which subsequently isomerises to the expected 7,9, while meta-carborane rearranges directly to 7,9. The initial movements of vertices away from closo geometries, following the addition of two electrons, is related to the LUMO of the neutral species.In the characterisation of these isomerisation processes, where possible through a priori mode-following calculations, a series of common intermediate topologies as well as the unexpectedly complex processes by which they interconnect have been uncovered and rationalised. Basket intermediates (e.g.Int(A), Int(A-4,7), Int2(4,7-7,10), Int(7,10-7,9) and Int(B)) are characterised by a two-vertex basket handle bridging the remaining 10 vertices; inverted nido intermediates (Int1(4,7-7,10) and Int2(1,7-3,7)) exhibit a 5-membered belt and a 6-membered belt capped by the remaining vertex. The geometries of these new intermediates, like nido species themselves, are related to the 13-vertex docosahedron by removal of a single vertex. The pathways through which carborane dianions isomerise, driven by the thermodynamic preference for low-connected C vertices, are most often initiated by movement of the 3-connected C7 vertex, common to all nido species, into a 4-connected position through a DSD step, forcing a B vertex into a destabilised 3-connected site and leading to rearrangement of the cluster. Isomerisation continues through processes such as the pivoting of a 4-vertex diamond about its long diagonal. This can directly lead to a nido geometry or produce a basket or inverted nido intermediate. Basket intermediates can undergo a basket collapse process, characterised by DSD steps, giving rise to a nido geometry, while inverted nido intermediates convert to nido geometries through the pivoting of two 3-vertex triangles about a shared vertex.
Computational details
Calculations were performed using Gaussian 03, Revision D.01 employing the BP86 functional29,30 and 6-31G** basis sets31 for B, C and H atoms. Zero-point corrected energies, H, are reported in kcal mol−1 relative to 7,9. Analytical frequency calculations were used to confirm geometries as minima (all positive eigenvalues) or transition states (one negative eigenvalue). Transition states were further characterised through IRC calculations.32,33 Mode-following calculations used the ‘OPT = TS’ option in Gaussian along with the GDIIS algorithm, where convergence constraints were set to ‘verytight’ in order to force the optimisation to move away from a formally minimum energy starting geometry, itself optimised with default convergence constraints. Synchronous Transit-Guided Quasi-Newton (STQN) calculations were run with the ‘QST2’ option (two intermediate geometries given as input) and the structure generated used as input in a transition state optimisation.Acknowledgements
We thank the EPSRC for support (DMcK funded by project EP/E02971X/1).Notes and references
- K. Wade, Adv. Inorg. Chem. Radiochem., 1976, 18, 1 CrossRef CAS
.
- G. B. Dunks, M. M. McKown and M. F. Hawthorne, J. Am. Chem. Soc., 1971, 93, 2541 CrossRef
.
- A. Burke, D. Ellis, B. T. Giles, B. E. Hodson, S. A. Macgregor, G. M. Rosair and A. J. Welch, Angew. Chem., Int. Ed., 2003, 42, 225 CrossRef CAS PubMed
.
- S. Zlatogorsky, M. J. Edie, D. Ellis, S. Erhardt, M. E. Lopez, S. A. Macgregor, G. M. Rosair and A. J. Welch, Angew. Chem., Int. Ed., 2007, 46, 6706 CrossRef CAS PubMed
.
- M. R. Churchill and B. G. DeBoer, J. Chem. Soc., Chem. Commun., 1972, 1326 RSC
.
- G. B. Dunks, R. J. Wiersema and M. F. Hawthorne, J. Am. Chem. Soc., 1973, 95, 3174 CrossRef CAS
.
- T. D. Getman, C. B. Knobler and M. F. Hawthorne, Inorg. Chem., 1990, 29, 158 CrossRef CAS
.
- D. Grafstein and J. Dvorak, Inorg. Chem., 1963, 2, 1128 CrossRef CAS
.
- S. Papetti and T. L. Heying, J. Am. Chem. Soc., 1964, 86, 2295 CrossRef CAS
.
- V. I. Stanko, V. A. Brattsev and Y. V. Gol'tyapin, Zh. Obshch. Khim., 1969, 39, 2686 CAS
.
- C. A. Brown and M. L. McKee, J. Mol. Model., 2006, 12, 653 CrossRef CAS
.
- B. W. Hutton, F. MacIntosh, D. Ellis, F. Herisse, S. A. Macgregor, D. McKay, V. Petrie-Armstrong, G. M. Rosair, D. S. Perekalin, H. Tricas and A. J. Welch, Chem. Commun., 2008, 5345 RSC
.
- W. N. Lipscomb, Science, 1966, 153, 373 CAS
.
- I. J. Sugden, D. F. Plant and R. G. Bell, Chem. Commun., 2013, 49, 975 RSC
.
- D. J. Wales, J. Am. Chem. Soc., 1993, 115, 1557 CrossRef CAS
.
- L. I. Zakharkin and V. N. Kalinin, Dokl. Akad. Nauk SSSR, 1966, 169, 590 CAS
.
- S. Robertson, D. Ellis, G. M. Rosair and A. J. Welch, Appl. Organomet. Chem., 2003, 17, 518 CrossRef CAS
.
- S. Robertson, D. Ellis, G. M. Rosair and A. J. Welch, J. Organomet. Chem., 2003, 680, 286 CrossRef CAS
.
- S. Robertson, R. M. Garrioch, D. Ellis, T. D. McGrath, B. E. Hodson, G. M. Rosair and A. J. Welch, Inorg. Chim. Acta, 2005, 358, 1485 CrossRef CAS PubMed
.
- M. L. McKee, M. Buehl and P. v. R. Schleyer, Inorg. Chem., 1993, 32, 1712 CrossRef CAS
.
- K. Hermansson, M. Wójcik and S. Sjöberg, Inorg. Chem., 1999, 38, 6039 CrossRef CAS PubMed
.
- D. Ellis, D. McKay, S. A. Macgregor, G. M. Rosair and A. J. Welch, Angew. Chem., Int. Ed., 2010, 49, 4943 CrossRef CAS PubMed
.
- A. McAnaw, M. Elena Lopez, G. Scott, D. Ellis, D. McKay, G. M. Rosair and A. J. Welch, Dalton Trans., 2012, 10957 RSC
.
- H. Tricas, M. Colon, D. Ellis, S. A. Macgregor, D. McKay, G. M. Rosair, A. J. Welch, I. V. Glukhov, F. Rossi, F. Laschi and P. Zanello, Dalton Trans., 2011, 40, 4200 RSC
.
-
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03, Revision D.01, Gaussian Inc., Wallingford, CT, USA, 2004 Search PubMed
.
- R. McIntosh, D. Ellis, J. Gil-Lostes, K. J. Dalby, G. M. Rosair and A. J. Welch, Dalton Trans., 2005, 1842 RSC
.
- J. Zhang, L. Deng, H.-S. Chan and Z. Xie, J. Am. Chem. Soc., 2007, 129, 18 CrossRef CAS PubMed
.
- M. A. Fox and K. Wade, Pure Appl. Chem., 2003, 75, 1315 CrossRef CAS
.
- A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098 CrossRef CAS
.
- J. P. Perdew, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 8822 CrossRef
.
- P. C. Hariharan and J. A. Pople, Theor. Chim. Acta, 1973, 28, 213 CrossRef CAS
.
- C. Gonzalez and H. B. Schlegel, J. Phys. Chem., 1990, 94, 5523 CrossRef CAS
.
- C. Gonzalez and H. B. Schlegel, J. Chem. Phys., 1989, 90, 2154 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Movies of the 1,7 to 7,9 isomerisation (17-79_isom.avi), ortho-carborane reduction (ortho-carborane_RED.avi) and meta-carborane reduction (meta-carborane_RED.avi); alternative reaction profiles; computed Cartesian coordinates and energies for all structures. See DOI: 10.1039/c5sc00726g |
| ‡ Present address: School of Chemistry, St Andrews University, North Haugh, St Andrews, Fife, KY16 9ST, UK. |
| This journal is © The Royal Society of Chemistry 2015 |