Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Very bright mechanoluminescence and remarkable mechanochromism using a tetraphenylethene derivative with aggregation-induced emission†
Bingjia
Xu‡
ab,
Jiajun
He‡
a,
Yingxiao
Mu
a,
Qiangzhong
Zhu
b,
Sikai
Wu
a,
Yifan
Wang
c,
Yi
Zhang
*a,
Chongjun
Jin
b,
Changcheng
Lo
c,
Zhenguo
Chi
*a,
Alan
Lien
d,
Siwei
Liu
a and
Jiarui
Xu
*a
aPCFM Lab, GD HPPC Lab, Guangdong Engineering Technology Research Center for High-performance Organic and Polymer Photoelectric Functional Films, State Key Laboratory of Opto-electronic Material and Technologies, School of Chemistry and Chemical Engineering, Sun Yet-sen University, Guangzhou 510275, China. E-mail: ceszy@mail.sysu.edu.cn; chizhg@mail.sysu.edu.cn; xjr@mail.sysu.edu.cn; Fax: +86 20 84112222; Tel: +86 20 84112712
bState Key Laboratory of Optoelectronic Material and Technologies, School of Physics and Engineering, Sun Yat-sen University, Guangzhou 510275, China
cShenzhen China Star Optoelectronics Technology Co., Ltd, Guangdong, China
dTCL Corporate Research, Guangdong, China
First published on 18th March 2015
Abstract
Organic materials exhibiting mechanoluminescence (ML) are promising for usage in displays, lighting and sensing. However, the mechanism for ML generation remains unclear, and the light-emitting performance of organic ML materials in the solid state has been severely limited by an aggregation-caused quenching (ACQ) effect. Herein, we present two strongly photoluminescent polymorphs (i.e., Cg and Cb) with distinctly different ML activities based on a tetraphenylethene derivative P4TA. As an aggregation-induced emission (AIE) emitter, P4TA perfectly surmounted the ACQ, making the resultant block-like crystals in the Cg phase exhibit brilliant green ML under daylight at room temperature. The ML-inactive prism-like crystals Cb can also have their ML turned on by transitioning toward Cg with the aid of dichloromethane vapor. Moreover, the Cg polymorph shows ML and mechanochromism simultaneously and respectively without and with UV irradiation under a force stimulus, thus suggesting a feasible design direction for the development of efficient and multifunctional ML materials.
Introduction
The mechanoluminescence (ML) phenomenon was first found by Francis Bacon in 1605.1 Heretofore, research on exploiting advanced ML materials has not yet been a major focus.2 Materials with brilliant ML are actually of great importance from both fundamental and practical viewpoints because they are promising for usage in displays, as well as light sources and sensors.2,3 However, a comprehensive understanding of the crystal properties required for ML activity and the corresponding mechanisms is less well demonstrated.4 This lack in understanding leads to feasible design principles for these emitters, particularly those with satisfactory ML brightness, being rarely found.5 As reported previously, the performance of organic ML compounds can be related to both their molecular and molecular-assembly structures.6 Therefore, controlling the molecular arrangements in the solid state and achieving a molecular-level understanding of the relationship between the molecular conformations and packing characteristics and the resulting optical properties are the essential issues in obtaining efficient ML materials.Notably, non-covalent intermolecular interactions, such as π–π stacking and hydrogen bonding, are important in constructing the supramolecular systems.7 These interactions are able to influence the final packing structure strongly, thereby making polymorphism with different ML activities more probable.6a,8 Nevertheless, in most cases, typical π–π stacking interactions often lead to aggregation-caused quenching, which poses significant difficulties for development of high-performance ML materials.4b,9 By contrast, a diametrically opposed effect was recently found to be operative in a class of chromophores with twisted conformations (e.g., tetraphenylethene derivatives), which exhibit enhanced emission in the solid state with respect to the fluid solution.10 The discovery of this abnormal phenomenon, known as aggregation-induced emission (AIE), has sparked a rapid expansion in the field of photoluminescent sensors and electroluminescent devices.11 AIE also provides new possibilities for designing highly mechanoluminescent materials.
This study presents a new polymorphic system that can be facilely and controllably constructed using a tetraphenylethene derivative [i.e., 5-(4-(1,2,2-triphenylvinyl)phenyl)thiophene-2-carbaldehyde (P4TA)] as the building block (Fig. 1). The two crystalline polymorphs of P4TA show strong blue- and green-colored photoluminescence (PL). The blue-light crystals are significantly ML inactive, whereas the green-light ones are highly mechanoluminescent because of their distinctly different molecular packing mode and unique AIE character. The existence of polymorphs from the same molecule with exactly opposite properties provides a unique prototype to investigate the crystalline structures required for ML activity and the effect of AIE properties on ML enhancement. The relationship between the ML and the mechanofluorochromism of P4TA is also presented.
Results and discussion
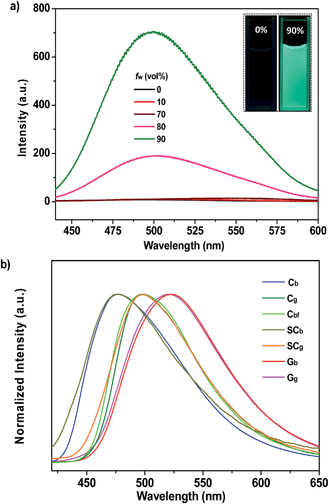
P4TA was straightforwardly prepared through a palladium-catalyzed coupling reaction by introducing 2-thiophenaldehyde to the tetraphenylethene moiety (Scheme S1†). The purified material was then characterized using nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography. The satisfactory data obtained fully confirmed its expected molecular structure (ESI†).The UV-visible absorption spectrum of P4TA was measured in a dichloromethane (DCM) solution. Two absorption bands centered at 317 and 366 nm were observed. These two bands were associated with the π–π* transition and intramolecular charge transfer, respectively (Fig. S1†). The large Stokes shift (68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 027 cm−1) between the absorption and fluorescence spectra (λem,max = 513 nm) of P4TA in DCM was indicative of the structural difference between the ground and excited states. The resulting P4TA compound would probably also be AIE-active on considering that tetraphenylethene is the most frequently used AIE unit. To confirm this probability, a tetrahydrofuran (THF) solution of P4TA was titrated with water and the change in the fluorescence emission was monitored. P4TA exhibited an extremely weak emission in the THF solution, where it was well dissolved (Fig. 2a). Hence, almost no PL signal was recorded. However, when 90% (v/v) water was present, a strong green emission that peaked at 499 nm was observed and the corresponding intensity (∼705 a. u.) dramatically increased by up to ∼100 times compared to that at the 0% water fraction (∼7 a. u.). Adding water to the THF solution of P4TA significantly induced the formation of nanoparticles because P4TA molecules contain highly hydrophobic aromatic rings. In other words, the emission enhancement was caused by molecule aggregation which suggests that P4TA is AIE-active.12
027 cm−1) between the absorption and fluorescence spectra (λem,max = 513 nm) of P4TA in DCM was indicative of the structural difference between the ground and excited states. The resulting P4TA compound would probably also be AIE-active on considering that tetraphenylethene is the most frequently used AIE unit. To confirm this probability, a tetrahydrofuran (THF) solution of P4TA was titrated with water and the change in the fluorescence emission was monitored. P4TA exhibited an extremely weak emission in the THF solution, where it was well dissolved (Fig. 2a). Hence, almost no PL signal was recorded. However, when 90% (v/v) water was present, a strong green emission that peaked at 499 nm was observed and the corresponding intensity (∼705 a. u.) dramatically increased by up to ∼100 times compared to that at the 0% water fraction (∼7 a. u.). Adding water to the THF solution of P4TA significantly induced the formation of nanoparticles because P4TA molecules contain highly hydrophobic aromatic rings. In other words, the emission enhancement was caused by molecule aggregation which suggests that P4TA is AIE-active.12
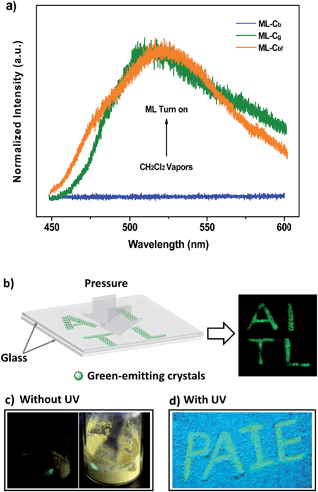
The prominent AIE character of P4TA motivated the application of the material in the solid state. Accordingly, block-like crystals (Cg-form) were achieved through solvent evaporation of P4TA in a mixed solvent of n-hexane and DCM (Fig. S2a†). Compared to the emissive nanoaggregates in THF–H2O, the as-prepared sample Cg exhibited an even stronger green-light emission, centered at 498 nm [ΦF,s = 52%] (Fig. 2b). By grinding the Cg crystals with a pestle or shearing them with a spatula, a very bright green light emission peaking at 517 nm was observed in the dark without UV irradiation (Fig. 3a and c and Video S1†). This experiment unambiguously illustrated that P4TA in the Cg-form was ML-active. The strong ML of Cg was indeed clearly seen even under daylight at room temperature and was maintained while the crystals were crushed (Fig. 3c and Video S2†). Certain organic materials, such as coumarin, phenanthrene, N-acetyl anthranilic acid, N-isopropyl carbazole and N-phenyl imides, have also been reported to show mechanoluminescence activities. However, none of these materials could emit a ML strong enough to be observed with the naked eye under daylight at room temperature.5,13 The poor performance of the conventional organic ML materials should be attributed to their intrinsic ACQ property, which leads to the low emitting efficiency in the solid state. By contrast, the unique AIE feature of P4TA perfectly surmounted the ACQ effect and exhibited a positive effect on luminescence enhancement, thereby giving the brilliant ML of Cg. To further demonstrate the ML characteristics of Cg, a simple device was made by sandwiching the sample between two pieces of pre-sculptured glass. The capital letters ‘AITL’ were clearly displayed when pressure was used as the driving force, which suggests the ML ‘display capability’ of Cg (Fig. 3b). As such, the extraordinary AIE-active ML material P4TA will be a promising candidate for displays and optical recording.
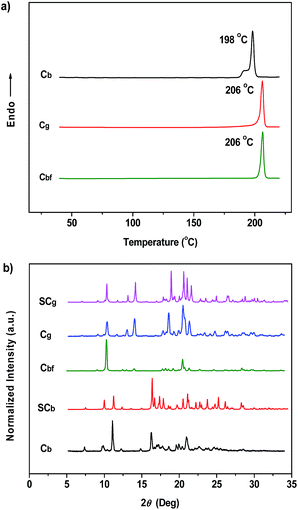
Interestingly, another type of prism-like crystal (Cb-form) was observed while exploring different processing conditions. The Cb-form crystals, which showed an intense blue-light emission that peaked at 476 nm (ΦF,s = 36%), could be obtained by adding ethanol into a P4TA/DCM solution under the action of ultrasound (Fig. S2b†). However, in contrast to the vivid Cg phenomenon, the P4TA sample completely lost its ML activity when aggregated in the Cb-form (Fig. 3a). The Cb crystals showed a small melting endothermic shoulder peak at 191 °C and a sharp peak at 198 °C in the first heating curve of differential scanning calorimetry (DSC) (Fig. 4a), indicating that Cb was mainly composed of microcrystals that melt at 198 °C. This result is different from that of Cg, which melts at 206 °C. Moreover, the powder X-ray diffraction (XRD) spectra also exhibited distinctly different patterns for the two samples (Fig. 4b). These results imply that the different ML activities of Cg and Cb could be attributed to their dissimilar molecular packing modes. A single crystal X-ray analysis was thus performed for the P4TA crystals to obtain more insight into this aspect. Single crystals of the two polymorphs (i.e., SCg and SCb) suitable for X-ray structural analysis were isolated through slow solvent evaporation of P4TA in mixtures of ethanol and CHCl3 of different concentrations.
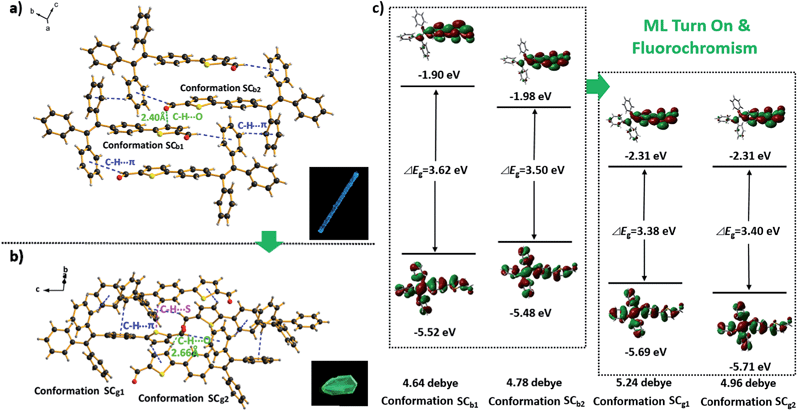
The SCg and SCb samples emitted intense green or blue light peaked at 499 and 476 nm, respectively (Fig. 2b and 5a and b). These light emissions are similar to those of the as-prepared crystals of Cg and Cb. The main peaks of the simulated XRD patterns of SCg and SCb also agree well with those in the patterns obtained from P4TA in the Cg and Cb phases, which suggests that the initial powders were mainly composed of P4TA microcrystals in the polymorphs of SCg and SCb (Fig. 4b). Further systematic analysis revealed that both SCg and SCb belong to the non-centrosymmetric polar space group of P(2)1 (Table S1†). Some previous reports have shown that dipolar structures and non-centrosymmetric molecular arrangements are favorable for obtaining piezoelectric properties, which were closely pertinent to the ML activities of the crystals.14 In principle, the fracture of crystals with a strong piezoelectric effect will lead to electronic discharge at the crack surface, which would result in dye excitation and generation of ML for the crystals.5,15 The molecular structure of P4TA and the crystalline symmetry of SCg and SCb also meet the requirements for piezoelectric properties, thus making the as-prepared crystals of Cg and Cb more active and more likely to achieve the ML character. However, the dipole moments and the HOMO–LUMO band gaps (ΔEg) of the molecules in the SCg and SCb polymorphs were different. These differences were caused by their distinct molecular conformations and packing characteristics. The asymmetric units in both polymorphs (i.e., SCg and SCb) were composed of two crystallographically independent molecules (i.e., SCg1 and SCg2 for SCg, and SCb1 and SCb2 for SCb). Each molecule showed the formation of a C–H⋯O intermolecular hydrogen bond (Fig. 5a and b, S3 and S4†). Compared with SCb, the most notable conformational difference of P4TA in the SCg polymorph was the dihedral angle θ between the thiophene and the adjacent phenyl ring. While the conformations of P4TA were twisted (θ = 18.9° for SCg1 and 6.1° for SCg2) in polymorph SCg, the two aromatic rings were nearly coplanar in polymorph SCb (θ = 2.5° for SCb1 and 4.7° for SCb2) (Table S2†). In the case of SCg, the two thiophene rings of SCg1 and SCg2 were almost perpendicular to each other, showing a dihedral angel of 85.8°. By contrast, an anti-parallel packing mode was observed between the thiophene rings of SCb1 and SCb2 (θ = 3.7°) in SCb.
The most popular B3LYP density functional theory was then used to calculate the dipole moments and the ΔEg of P4TA in the four conformations at the 6-31G(d, p) level based on their ground state geometries in the single crystals. Fig. 5c presents the results. The dipole moments of SCg1 and SCg2 in the SCg polymorph were 5.24 and 4.96 debye (D), respectively. Both values were larger than those of SCb1 (4.64 D) and SCb2 (4.78 D) in SCb. The larger dipole moments of the molecules combining the non-centrosymmetric molecular arrangement may result in a larger net-dipole moment of the crystalline structure, and would subsequently lead to a stronger piezoelectric effect in the SCg polymorph when breaking the crystals. The theoretical calculation results also suggest that the molecules in both SCg and SCb have ICT characteristics: the electronic transitions (mainly from HOMO to LUMO for all the four conformations in SCg and SCb) from the occupied orbitals delocalized over the TPE (donor) moiety to the thiophenaldehyde (acceptor) moiety made major contributions to the excited states (Fig. 5c and Table S4†).16 SCg1 and SCg2 showed even smaller ΔEg (HOMO → LUMO) values of 3.38 and 3.40 eV, respectively, as compared to those of SCb1 (3.62 eV) and SCb2 (3.50 eV) in the SCb polymorph. The calculations showed good agreement with the solid state UV-visible spectra of Cg and Cb, which absorbed at 373 nm and 367 nm, respectively (Fig. S5†). The preceding results thus indicate that the electrons of the molecules in SCg can be excited with a lower energy. Hence, the stronger piezoelectric effect and the lower electronic transition energy resulted in the excitation of P4TA molecules and the generation of ML in the SCg phase by breaking the crystals. Meanwhile, all the molecules adopted a highly twisted propeller-like conformation in the SCg polymorph, which prevented the formation of detrimental species, such as excimers or exciplexes, caused by π–π stacking interactions. Furthermore, numerous intermolecular interactions such as C–H⋯π and C–H⋯S might also exist in the crystals aside from the C–H⋯O hydrogen bonding (Fig. 5b and Table S3†). These multiple interactions had rigidified the molecular conformations and impeded intramolecular rotation, which largely reduced the energy loss via non-radiative relaxation channels, and subsequently resulted in a notable AIE effect and high ΦF,s value for P4TA. The preceding factors consequently made the ML of sample Cg, which was mainly composed of P4TA microcrystals in the SCg polymorph, highly emissive under the stimulus of mechanical force. By contrast, the weaker piezoelectric effect in the SCb polymorph seemed not to reach the higher energy requirement for the electronic excitation although SCb also exhibited strong photoluminescence. Consequently, P4TA lost its ML activity when aggregated in the Cb phase. These results also suggest a feasible design direction for the development of efficient ML materials through combining the prominent piezoelectric property for molecular excitation and the abnormal AIE character for emission.
The PL of Cb was remarkably changed when the sample was exposed to DCM or acetone vapors for about 10 min, passing from an initial blue to green light at 499 nm (Cbf-form). The resulting spectrum of Cbf was superimposable on that of Cg (Fig. 2b). The coincidence of the PL emissions suggested that the fumed sample of Cbf probably had the same molecular arrangement as that of the Cg polymorph. Further evidence for this standpoint was provided by their similar XRD patterns and their overlapping DSC curves with the same melting point at 206 °C (Fig. 4a and b). As mentioned in the preceding discussion, sample Cg was ML-active. And expectantly, the ML activity of Cb could be tuned by simply altering the molecular packing mode upon fumigation. To verify this hypothesis, the ML spectrum of Cb was collected after exposure to DCM vapor (Cbf). As anticipated, Cbf also exhibited a strong green light emission without UV irradiation using pressure, which revealed that the ML of Cb could be facilely turned on with the aid of DCM vapor. The ML emission maximum of Cbf was located at 520 nm, which is close to that of Cg (Fig. 3a).
Noticeably, the ML maxima of Cg and Cbf were both significantly red-shifted (Δλem,max ≈ 21 nm) as compared to their PL spectra. This result shows a special mechanofluorochromic effect. To gain an understanding of this, the influence of applied pressure on the luminescence was investigated. The PL maximum of the pristine P4TA in the Cg-form shifted from 498 nm to 521 nm (Gg) after pressing or grinding (Fig. 2a), agreeing well with its ML emission (Fig. S6†), thereby confirming that the bathochromic shift between the ML and PL of Cg was caused by its intrinsic mechanochromic properties. The phase characteristics of the ground sample Gg were determined by XRD to decipher further the relationship between the ML and the mechanochromism of Cg. Most of the diffraction peaks were diffuse or even disappeared, although some resolvable peaks of Gg were consistent with those of their original crystals (Fig. 6a). This revealed that the ground sample was partially in a metastable amorphous state. Accordingly, DSC was performed for the sample after grinding (Fig. 6b). Compared with Cg, an additional exothermal peak around 86 °C was observed in the DSC thermogram of Gg, which demonstrated that the Cg crystals were partially destroyed and converted to an amorphous state by the grinding or pressing treatment. The P4TA molecules in the Cg phase adopt twisted conformations in the crystalline state to fit into the crystalline lattice, and the crystalline lattices may collapse when triggered with mechanical force. The dye molecules also then relaxed to a more planar conformation, thereby emitting redder ML and PL. In other words, the bathochromic shift of the ML for Cg originates from the microcrystal amorphization and the extension of molecular conjugation, which were believed to be the main reasons for the Cg mechanochromism.16,17 Unlike other conventional stimuli-responsive materials, the Cg-form of P4TA can show a luminescence response and luminescence color change simultaneously and respectively both without and with UV irradiation under a force stimulus. This new kind of force-responsive material with AIE properties has not yet been achieved before, and would facilitate applications of ML materials in the field of sensors.11c In addition, fluorescence spectroscopy was also performed to evaluate the mechanochromic behavior of P4TA in the Cb phase, and the Cb sample exhibited a more remarkable emission wavelength change of 47 nm upon grinding. Furthermore, the corresponding PL spectrum (Gb) with λem,max = 523 nm fitted well with that for the powder ground from Cg (Fig. 2b), which indicated that P4TA could switch to the same emission under a force stimulus regardless of its initial state. Also the fluorescence ‘writability’ of Cb can be verified by writing on a piece of filter paper as shown in Fig. 3d. The mechanofluorochromism of Cb should occur by a similar mechanism to that proposed for Cg.
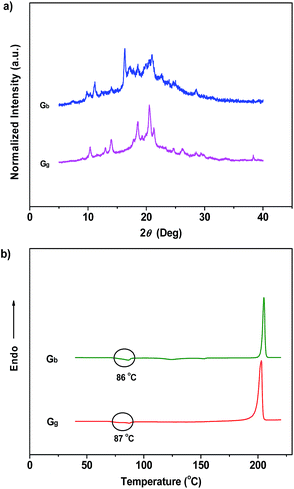 | ||
| Fig. 6 XRD patterns (a) and DSC curves (b) of the ground samples of P4TA: (Gb) ground sample from the blue-light crystals; (Gg) ground sample from the green-light crystals. | ||
Conclusions
Based on AIE-active P4TA molecules, two photoluminescent polymorphs (i.e., Cg and Cb) with multiple molecular conformations were achieved. They can show opposing mechanoluminescence activities by tuning the molecular assembly structures in the crystals. The block-like crystals of Cg exhibited a very bright green color ML upon pressing or grinding under daylight at room temperature. This unique property should be attributed to the strong piezoelectric effect of the crystals and the positive effect of the AIE property on luminescence enhancement. Moreover, with UV irradiation, the Cg of P4TA showed mechanofluorochromism under a mechanical stimulus. This new kind of force-responsive compound with an AIE property could facilitate the application of ML materials in the display and sensor fields. This work may provide a feasible design direction for the development of more efficient ML materials by combining the prominent piezoelectric property for molecular excitation and the unique AIE character for emission.Acknowledgements
The authors gratefully acknowledge financial support from the NSF of China (51173210, 51073177), the Fundamental Research Funds for the Central Universities and NSF of Guangdong (S2011020001190).Notes and references
- (a) N. C. Eddingsaas and K. S. Suslick, Nature, 2006, 444, 163 CrossRef CAS PubMed; (b) N. C. Eddingsaas and K. S. Suslick, J. Am. Chem. Soc., 2007, 129, 6718 CrossRef CAS PubMed.
- S. M. Jeong, S. Song, K. I. Joo, J. Kim, S. H. Hwang, J. Jeong and H. Kim, Energy Environ. Sci., 2014, 7, 3338 CAS.
- (a) S. Moon Jeong, S. Song, S. K. Lee and B. Choi, Appl. Phys. Lett., 2013, 102, 051110 CrossRef PubMed; (b) S. M. Jeong, S. Song, S. K. Lee and N. Y. Ha, Adv. Mater., 2013, 25, 6194 CrossRef CAS PubMed; (c) D. O. Olawale, T. Dickens, W. G. Sullivan, O. I. Okoli, J. O. Sobanjo and B. Wang, J. Lumin., 2011, 131, 1407 CrossRef CAS PubMed; (d) I. Sage and G. Bourhill, J. Mater. Chem., 2001, 11, 231 RSC; (e) Y. Tsuboi, T. Seto and N. Kitamura, J. Phys. Chem. B, 2003, 107, 7547 CrossRef CAS.
- (a) G. E. Hardy, J. C. Baldwin, J. I. Zink, W. C. Kaska, P. H. Liu and L. Duboisi, J. Am. Chem. Soc., 1997, 99, 3552 CrossRef; (b) L. M. Sweeting, A. L. Rheingold, J. M. Gingerich, A. W. Rutter, R. A. Spence, C. D. Cox and T. J. Kim, Chem. Mater., 1997, 9, 1103 CrossRef CAS; (c) L. M. Sweeting, Chem. Mater., 2001, 13, 854 CrossRef CAS.
- H. Nakayama, J. I. Nishida, N. Takada, H. Sato and Y. Yamashita, Chem. Mater., 2012, 24, 671 CrossRef CAS.
- (a) G. E. Hardy, J. I. Zink, W. C. Kaska and J. C. Baldwin, J. Am. Chem. Soc., 1978, 100, 8001 CrossRef CAS; (b) G. E. Hardy, W. C. Kaska, B. P. Cbandra and J. I. Zink, J. Am. Chem. Soc., 1981, 103, 1074 CrossRef CAS; (c) E. Boldyreva, Chem. Soc. Rev., 2013, 42, 7719 RSC.
- (a) H. Y. Zhang, Z. L. Zhang, K. Q. Ye, J. Y. Zhang and Y. Wang, Adv. Mater., 2006, 18, 2369 CrossRef CAS; (b) K. Wang, H. Zhang, S. Chen, G. Yang, J. Zhang, W. Tian, Z. Su and Y. Wang, Adv. Mater., 2014, 26, 6168 CrossRef CAS PubMed.
- L. M. Sweeting and A. L. Rheingoldf, J. Am. Chem. Soc., 1987, 109, 2652 CrossRef CAS.
- (a) R. Jakubiak, C. J. Collison, W. C. Wan, L. J. Rothberg and B. R. Hsieh, J. Phys. Chem. A, 1999, 103, 2394 CrossRef CAS; (b) K. C. Wu, P. J. Ku, C. S. Lin, H. T. Shih, F. I. Wu, M. J. Huang, J. J. Lin, I. C. Chen and C. H. Cheng, Adv. Funct. Mater., 2008, 18, 67 CrossRef CAS; (c) P. Galer, R. C. Korošec, M. Vidmar and B. Šket, J. Am. Chem. Soc., 2014, 136, 7383 CrossRef CAS PubMed.
- (a) J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, B. Z. Tang, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu and D. Zhu, Chem. Commun., 2001, 1740 CAS; (b) B. K. An, S. K. Kwon, S. D. Jung and S. Y. Park, J. Am. Chem. Soc., 2002, 124, 14410 CrossRef CAS PubMed; (c) H. Tong, Y. Hong, Y. Dong, M. Häußler, J. W. Y. Lam, Z. Li, Z. Guo, Z. Guo and B. Z. Tang, Chem. Commun., 2006, 3705 RSC; (d) W. Z. Yuan, P. Lu, S. Chen, J. W. Y. Lam, Z. Wang, Y. Liu, H. S. Kwok, Y. Ma and B. Z. Tang, Adv. Mater., 2010, 22, 2159 CrossRef CAS PubMed; (e) B. Xu, M. Xie, J. He, B. Xu, Z. Chi, W. Tian, L. Jiang, F. Zhao, S. Liu, Y. Zhang, Z. Xu and J. Xu, Chem. Commun., 2012, 49, 273 RSC; (f) C. Li, T. Wu, C. Hong, G. Zhang and S. Liu, Angew. Chem., Int. Ed., 2011, 51, 455 CrossRef PubMed.
- (a) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332 RSC; (b) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC; (c) Z. Chi, X. Zhang, B. Xu, X. Zhou, C. Ma, Y. Zhang, S. Liu and J. Xu, Chem. Soc. Rev., 2012, 41, 3878 RSC; (d) Z. Zhao, J. W. Y. Lam and B. Z. Tang, J. Mater. Chem., 2012, 22, 23726 RSC; (e) Y. Gong, Y. Zhang, W. Z. Yuan, J. Z. Sun and Y. Zhang, J. Phys. Chem. C, 2014, 118, 10998 CrossRef CAS.
- (a) B. Xu, Z. Chi, H. Li, X. Zhang, X. Li, S. Liu, Y. Zhang and J. Xu, J. Phys. Chem. C, 2011, 115, 17574 CrossRef CAS; (b) Z. Yang, W. Qin, J. W. Y. Lam, S. Chen, H. H. Y. Sung, I. D. Williams and B. Z. Tang, Chem. Sci., 2013, 4, 3725 RSC.
- (a) J. I. Zink and W. Klimt, J. Am. Chem. Soc., 1974, 96, 4960 Search PubMed; (b) P. Jha and B. P. Chandra, Luminescence, 2014, 29, 977 CrossRef CAS PubMed.
- (a) S. Biju, N. Gopakumar, J. C. G. Bünzli, R. Scopelliti, H. K. Kim and M. L. P. Reddy, Inorg. Chem., 2013, 52, 8750 CrossRef CAS PubMed; (b) S. Balsamy, P. Natarajan, R. Vedalakshmi and S. Muralidharan, Inorg. Chem., 2014, 53, 6054 CrossRef CAS PubMed.
- L. S. McCarty and G. M. Whitesides, Angew. Chem., Int. Ed., 2008, 47, 2188 CrossRef CAS PubMed.
- R. Misra, T. Jadhav, B. Dhokale and S. M. Mobin, Chem. Commun., 2014, 50, 9076 RSC.
- (a) W. Z. Yuan, Y. Tan, Y. Gong, P. Lu, J. W. Y. Lam, X. Y. Shen, C. Feng, H. H. Y. Sung, Y. Lu, I. D. Williams, J. Z. Sun, Y. Zhang and B. Z. Tang, Adv. Mater., 2013, 25, 2837 CrossRef CAS PubMed; (b) Y. Gong, Y. Tan, J. Liu, P. Lu, C. Feng, W. Z. Yuan, Y. Lu, J. Z. Sun, G. He and Y. Zhang, Chem. Commun., 2013, 49, 4009 RSC; (c) P. Gautam, R. Maragani, S. M. Mobin and R. Misra, RSC Adv., 2014, 4, 52526 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of the synthesis; structural information for the compound (NMR, IR, and mass spectra); Tables S1–S5; Fig. S1–S11. CCDC 1037840 and 1057432. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc00466g |
| ‡ These authors contributed equally to the preparation of this work. |
| This journal is © The Royal Society of Chemistry 2015 |