Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bis(dipyrrinato)metal(II) coordination polymers: crystallization, exfoliation into single wires, and electric conversion ability†
Ryota
Matsuoka
a,
Ryojun
Toyoda
a,
Ryota
Sakamoto
*a,
Mizuho
Tsuchiya
a,
Ken
Hoshiko
a,
Tatsuhiro
Nagayama
a,
Yoshiyuki
Nonoguchi
b,
Kunihisa
Sugimoto
c,
Eiji
Nishibori
d,
Tsuyoshi
Kawai
b and
Hiroshi
Nishihara
*a
aDepartment of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: sakamoto@chem.s.u-tokyo.ac.jp; nisihara@chem.s.u-tokyo.ac.jp
bGraduate School of Materials Science, Nara Institute of Science and Technology (NAIST), 8916-5 Takayama, Ikoma, Nara 630-0192, Japan
cJapan Synchrotron Radiation Research Institute (JASRI), 1-1-1, Kouto, Sayo-cho, Sayo-gun, Hyogo 679-5198, Japan
dDivision of Physics, Faculty of Pure and Applied Sciences, Tsukuba Research Center for Interdisciplinary Materials Science (TIMS), and Center for Integrated Research in Fundamental Science and Engineering (CiRfSE), University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8571, Japan
First published on 26th February 2015
Abstract
One-dimensional coordination polymers (1D-CPs) tend either to dissociate into constitutive ligands and metals readily in solution, or to aggregate randomly and amorphously, which prevents them from widespread application. In the present research, 1D-CPs comprising bridging dipyrrin ligands and divalent metal ions (Zn2+, Ni2+, and Cu2+) are synthesized. A liquid/liquid interfacial reaction gives rise to single crystals suitable for X-ray diffraction analysis: A dichloromethane solution of the ligand is layered with aqueous metal(II) acetate, such that the coordination reaction proceeds at the liquid/liquid interface. Isolated single fibers of the zinc coordination polymer may be exfoliated from the single crystal or bulk solid upon ultrasonication. Atomic force microscopy (AFM) detects the isolated fibers with lengths of more than several μm. The exfoliated 1D-CP wires feature good processability, realizing a conjugate with single-wall carbon nanotubes (SWCNTs), and a thin film on a transparent SnO2 electrode. The processed materials show electric conversion ability: For example, the modified SnO2 electrode serves as a photoanode for a photoelectric conversion system. The designability and tunability of the present 1D-CPs is demonstrated by a ligand modification, affording a luminescent property and an extension of the photoelectric conversion response to longer wavelengths.
Introduction
Two- and three-dimensional coordination polymers such as nanosheets,1–10 metal–organic frameworks (MOFs),11–13 and porous coordination polymers (PCPs)14–16 have attracted intense interest. On the other hand, the physical flexibility of one-dimensional coordination polymers (1D-CPs) makes them useful for conjugation with micro and nano-sized functional materials such as carbon nanotubes,17–19 although difficulties in handling have prevented 1D-CPs from widespread application. Many 1D coordination chains are stable only in the solid phase, and they dissociate into constitutive ligands and metals readily in solution. Furthermore, 1D-CPs tend to aggregate randomly and amorphously, and there are few examples of 1D-CPs where both crystalline phase and isolated single chain phase coexist.20,21The dipyrrin-metal complex is an attractive molecular motif for coordination polymers. Dipyrrin ligands accept various metal ions, and in most cases the complexation reaction proceeds spontaneously even in the absence of a base.22,23 This feature is desirable for synthesizing supramolecules24,25 and coordination polymers.26–28 However, no 1D-CP based on the dipyrrin–metal complex has demonstrated crystallinity, single-chain isolation, and potential applicability at the same time.
In the present work, we synthesize 1D-CP M1 featuring the bis(dipyrrinato)metal(II) complex motif, which is composed of bridging dipyrrin ligand L1 and divalent metal ion M2+ (M = Zn, Ni, and Cu, Fig. 1a). A liquid/liquid interfacial reaction is effective for ordering M1, giving rise to single crystals suitable for X-ray diffraction analysis (XRD). Single fibers of Zn1 are isolated from the single crystal upon ultrasonication, and then visualized by atomic force microscopy (AFM). The dispersibility of the exfoliated fibers of Zn1 affords good processability, giving rise to a conjugate with single-wall carbon nanotubes (SWCNTs), and a thin film of Zn1 on a transparent SnO2 electrode. These processed materials may be applied in thermo and photoelectric conversion systems, thereby demonstrating the utility of Zn1. The designability and tunability of the present 1D-CP system are illustrated with analogue Zn2 comprising π-extended ligand L2 (Fig. 1b), which shows luminescence in the exfoliated fibrous form, and an extension of the photoelectric conversion response to longer wavelengths.
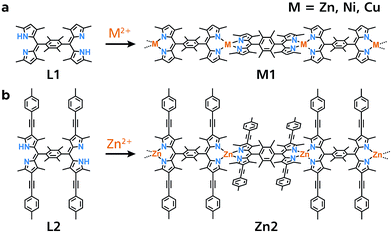 | ||
| Fig. 1 Chemical structures of bridging dipyrrin ligands and corresponding 1D-CPs based on bis(dipyrrinato)metal(II) complexes. (a) L1 and M1 (M = Zn, Ni, Cu). (b) L2 and Zn2. | ||
Results and discussion
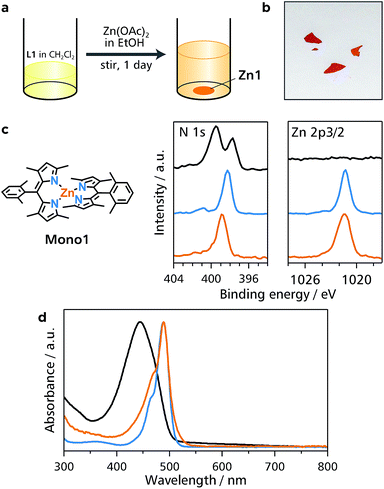
Initially, a conventional single-phase reaction was performed to synthesize Zn1 (Fig. 1a). Equimolar amounts of bridging dipyrrin ligand L1 and zinc(II) acetate were reacted in a mixture of dichloromethane and ethanol (Fig. 2a and b). The resultant dark-orange powder was subjected to X-ray photoelectron spectroscopy (XPS) using L1 and mononuclear bis(dipyrrinato)zinc(II) complex Mono1 as references (Fig. 2c). The Zn 2p 3/2 peak is only visible in Mono1 and Zn1, which is consistent with the presence of Zn ions. Another distinctive fingerprint of the coordination between the dipyrrin ligand and zinc(II) ions occurs in the N 1s region. Free base L1 features two peaks (397.5 and 399.1 eV) arising from the iminic and pyrrolic nitrogens that are chemically different from one another.29 In contrast, Mono1 shows a single N 1s peak, which stems from the homogenization of the two nitrogen atoms upon coordination to the zinc center. A solitary N 1s peak is also observed for Zn1. In addition, the nitrogen-to-zinc abundance ratio calculated from the peak area corrected by the photoionization cross-section is consistent with the ideal value of N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn = 4
Zn = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (79.8
1 (79.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.2 and 79.5
20.2 and 79.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.5 for Zn1 and Mono1, respectively, Fig. S1a and b†). These results indicate the formation of the desired coordination polymer Zn1 consisting of the bis(dipyrrinato)zinc(II) complex. The authors note that Ni1 and Cu1 synthesized by means of the single-phase method also displayed nitrogen-to-metal abundance ratios of ca. 4
20.5 for Zn1 and Mono1, respectively, Fig. S1a and b†). These results indicate the formation of the desired coordination polymer Zn1 consisting of the bis(dipyrrinato)zinc(II) complex. The authors note that Ni1 and Cu1 synthesized by means of the single-phase method also displayed nitrogen-to-metal abundance ratios of ca. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in XPS (80.2
1 in XPS (80.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.8 and 80.3
19.8 and 80.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.7 for Ni1 and Cu1, respectively, Fig. S1c and d†).
19.7 for Ni1 and Cu1, respectively, Fig. S1c and d†).
Zn1 is dispersible in dimethylformamide, and was characterized by UV/vis spectroscopy (Fig. 2d). An intense absorption with a maximum at 445 nm in L1 stems from the 1π–π* transition of the free-base dipyrrin moiety.30 However, the 1π–π* band is red-shifted by 44 nm in Mono1 (489 nm), which is typical of zinc(II) complexation with a dipyrrin ligand.30 The 1π–π* band of Zn1 has the same absorption maximum (489 nm) as that of Mono1, which also shows that Zn1 formed the bis(dipyrrinato)zinc(II) coordination polymer.
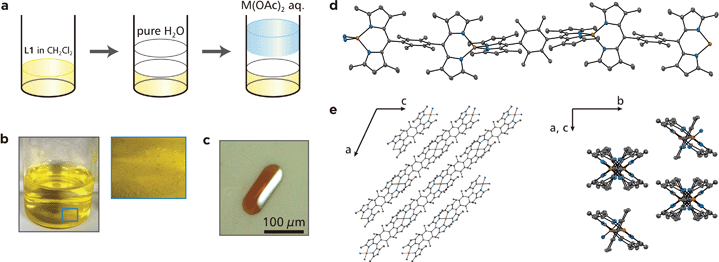
We have demonstrated previously that liquid/liquid interfacial synthesis—where organic ligand molecules in an organic solvent and aqueous metal ions are layered to form a liquid/liquid interface—is effective for synthesizing low-dimensional CPs with ordered secondary structures.4,5,31,32 For example, a liquid/liquid interfacial synthesis using 1,2,4,5-benzenetetrathiol and nickel(II) ions produced a nickel bis(dithiolene) 1D-CP that aligns to form two-dimensional ordered structures.31 In the present work, the coordination reaction between bridging dipyrrin ligand L1 in dichloromethane and divalent metal ions M2+ (M = Zn, Ni, and Cu) in water was carried out at the liquid/liquid interface, and it produced crystals of M1 that floated on the interface or sank to the bottom of the reaction container (Fig. 3a and b). A photograph of a typical single crystal for Zn1 is shown in Fig. 3c, and those for Ni1 and Cu1 are placed in Fig. S2.† This series of crystals was analyzed by synchrotron radiation X-ray diffraction (Fig. 3d and e and S3,† and Table S1† for Zn1, Fig. S3 and Table S2† for Ni1, and Fig. S3 and Table S3† for Cu1).33 The crystal structures are almost identical to each other for the three metal centers. They show the desired 1D polymeric chains, propagating along the [1 0 −1] crystallographic axis. The metal centers adopt slightly distorted tetrahedral coordination spheres with dihedral angles of 81.29°, 81.11°, and 87.36° for Zn, Ni, and Cu, respectively. The average metal–nitrogen bond lengths are, respectively, 1.9181, 1.9151, and 1.9032 Å for Zn, Ni, and Cu. This series of dihedral angles and metal–nitrogen bond lengths are typical of bis(dipyrrinato)metal(II) complexes bearing α-methylated dipyrrin ligands.30,34 Divalent nickel and copper generally prefer square-planar coordination spheres, though, the methyl group at the α-position induces tetrahedral coordination spheres even to the Ni and Cu centers because of steric hindrance. The dipyrrinato ligand is orthogonal to the bridging 2,3,4,5-tetramethylphenyl group with dihedral angles of 101.18°, 100.02°, and 98.93° for Zn, Ni, and Cu, respectively, reflecting steric hindrance between the two moieties.
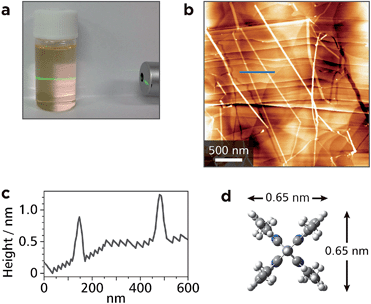
Upon ultrasonication, the single crystal of Zn1 could be dispersed in dichloromethane, and showed Tyndall scattering (Fig. 4a). The dispersion was cast onto highly ordered pyrolytic graphite (HOPG), which was then subjected to AFM. A representative height image shows several straight white lines with lengths of >3 μm traversing the steps of the HOPG substrate (Fig. 4b). The cross-sectional analysis shows that the height of the lines is ca. 0.7 nm (Fig. 4c), which is consistent with the size of Mono1 estimated by means of DFT calculation (Fig. 4d). This indicates that Zn1 is stable enough both chemically and physically to be isolated as single chains. We note that the width of Zn1 is overestimated by AFM. This is common in 1D polymer systems and AFM resolution.35–37
Among bis(dipyrrinato)metal(II) complexes, zinc-centered ones are known to possess luminescence ability. Nevertheless, Zn1 did not show detectable fluorescence at room temperature. This situation was improved by one of the virtues of 1D-CPs, designability and tunability: The authors also synthesized π-extended bridging dipyrrin ligand L2, and prepared another 1D-CP Zn2 (Fig. 1b). In XPS, Zn2 showed a nitrogen-to-metal abundance ratio of 79.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.6, almost consistent with the ideal value (4
20.6, almost consistent with the ideal value (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
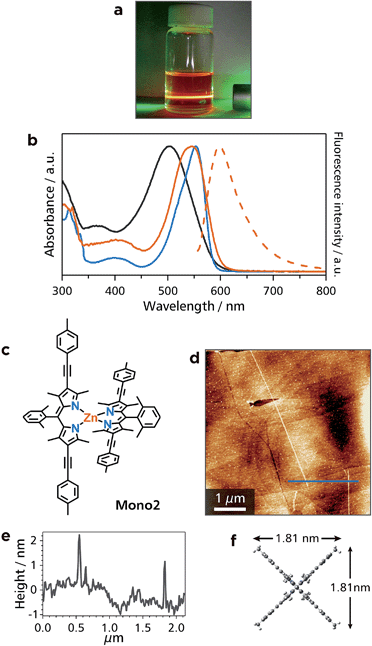
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. S1e†). Thanks to the (4-methylphenyl)ethynyl group at the β-position of the dipyrrin ligand, the dispersibility of Zn2 was better than that of Zn1. In addition, Zn2 featured orange emission in a dichloromethane dispersion, so that Tyndall scattering was concealed (Fig. 5a). In UV/vis and luminescence spectroscopy in toluene (Fig. 5b), Zn2 featured the 1π–π* absorption with a maximum at 547 nm, which is similar to that of corresponding mononuclear complex Mono2 (λmax = 553 nm, Fig. 5b and c). These bands are red-shifted relative to that of L2 (λmax = 505 nm), indicative of the complexation with zinc(II) ions. In contrast to non-fluorescent Zn1, the fluorescence of Zn2 was observed at the maximum wavelength of 597 nm (quantum efficiency: 8%): therefore, the fluorescence ability revived upon the ligand modification in L2. AFM for a HOPG substrate modified with a dispersion of Zn2 disclosed straight white lines with a length of more than 4.5 μm (Fig. 5d and e). The height of the lines (1.9 nm) is in good agreement with the size of Mono2 estimated by DFT calculation (Fig. 5f).
1) (Fig. S1e†). Thanks to the (4-methylphenyl)ethynyl group at the β-position of the dipyrrin ligand, the dispersibility of Zn2 was better than that of Zn1. In addition, Zn2 featured orange emission in a dichloromethane dispersion, so that Tyndall scattering was concealed (Fig. 5a). In UV/vis and luminescence spectroscopy in toluene (Fig. 5b), Zn2 featured the 1π–π* absorption with a maximum at 547 nm, which is similar to that of corresponding mononuclear complex Mono2 (λmax = 553 nm, Fig. 5b and c). These bands are red-shifted relative to that of L2 (λmax = 505 nm), indicative of the complexation with zinc(II) ions. In contrast to non-fluorescent Zn1, the fluorescence of Zn2 was observed at the maximum wavelength of 597 nm (quantum efficiency: 8%): therefore, the fluorescence ability revived upon the ligand modification in L2. AFM for a HOPG substrate modified with a dispersion of Zn2 disclosed straight white lines with a length of more than 4.5 μm (Fig. 5d and e). The height of the lines (1.9 nm) is in good agreement with the size of Mono2 estimated by DFT calculation (Fig. 5f).
In order to demonstrate the processability of Zn1, the authors fabricated a conjugate with SWCNTs (Zn1-SWCNT). A mixture of Zn1 and SWCNTs with a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
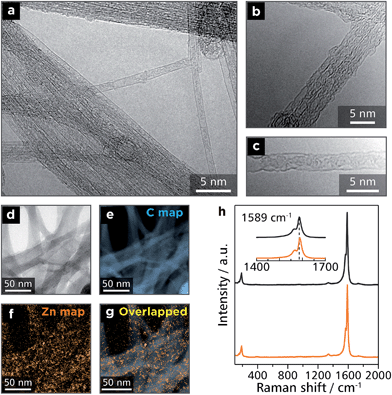
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 was dispersed in DMF by ultrasonication for 90 min, and the dispersion was then shaken for 1 d. This process resulted in the disappearance of the orange color of Zn1 from DMF, which indicated that Zn1 was adsorbed onto SWCNTs. Upon filtration, the conjugate spontaneously assembled into a free-standing membrane with a thickness of 64 μm (Fig. S4†). The conjugate was then subjected to spectroscopic analyses. Fig. 6a–c shows TEM images of bundles of SWCNTs with some exfoliated tubes. On the other hand, electron energy-loss spectroscopy (EELS) reveals the presence of zinc, which is scattered uniformly across the carbon scaffold derived from the SWCNT skeleton (Fig. 6d–g). Raman spectroscopy shows an intense G band (1590 cm−1) and a weak D band (1350 cm−1), which stem from intact and damaged SWCNTs, respectively (Fig. 6h).38 These results indicate that Zn1 wraps around the SWCNT uniformly without destroying the SWCNT structure. A preliminary experiment disclosed the thermoelectric conversion ability of Zn1-SWCNT (Fig. S5†). Zn1-SWCNT has a power factor of 33 μW m−1 K−2, which is greater than that of pristine SWCNTs (9.3 μW m−1 K−2), and those derived from conjugates between SWCNTs and small organic molecules (26 μW m−1 K−2 at most).39
10 was dispersed in DMF by ultrasonication for 90 min, and the dispersion was then shaken for 1 d. This process resulted in the disappearance of the orange color of Zn1 from DMF, which indicated that Zn1 was adsorbed onto SWCNTs. Upon filtration, the conjugate spontaneously assembled into a free-standing membrane with a thickness of 64 μm (Fig. S4†). The conjugate was then subjected to spectroscopic analyses. Fig. 6a–c shows TEM images of bundles of SWCNTs with some exfoliated tubes. On the other hand, electron energy-loss spectroscopy (EELS) reveals the presence of zinc, which is scattered uniformly across the carbon scaffold derived from the SWCNT skeleton (Fig. 6d–g). Raman spectroscopy shows an intense G band (1590 cm−1) and a weak D band (1350 cm−1), which stem from intact and damaged SWCNTs, respectively (Fig. 6h).38 These results indicate that Zn1 wraps around the SWCNT uniformly without destroying the SWCNT structure. A preliminary experiment disclosed the thermoelectric conversion ability of Zn1-SWCNT (Fig. S5†). Zn1-SWCNT has a power factor of 33 μW m−1 K−2, which is greater than that of pristine SWCNTs (9.3 μW m−1 K−2), and those derived from conjugates between SWCNTs and small organic molecules (26 μW m−1 K−2 at most).39
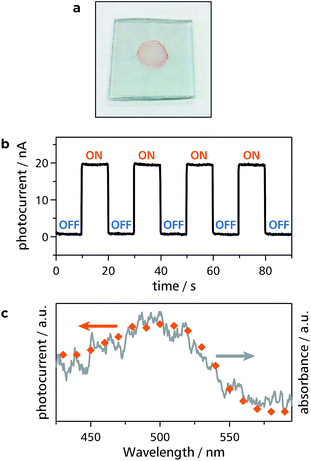
Thanks to intense light absorption disclosed in Fig. 2d and 5b, Zn1 and Zn2 are expected to show photofunctionality. As one of such demonstrations, Zn1 and Zn2 were built into a photoelectric conversion system. Dispersions of Zn1 and Zn2 were deposited onto transparent SnO2 electrodes, such that thin films of Zn1 and Zn2 were formed (Fig. 7a and S6a†): This also ensures the good processability of Zn1 and Zn2. A three-electrode electrochemical cell was then set up (Fig. S7†), where the modified SnO2 electrode was employed as a photoanode. An anodic photocurrent was observed only when the Zn1-modified SnO2 electrode was illuminated with 500 nm light (Fig. 7b). In addition, the action spectrum for the photocurrent coincided with the absorption spectrum of Zn1 on a SnO2 electrode (Fig. 7c). This series of facts indicates that Zn1 functions as an active layer for the photoelectric conversion system. The authors then investigated the photoelectric conversion efficiency (Fig. S8 and S9†). As shown in Fig. S9a,† the photocurrent reaches the maximal value with the optical density of the film at 500 nm of ∼0.005. On the other hand, the quantum yield for the photoelectric conversion decreased as the growth of the film, showing the maximal value of 1.0% in an acetonitrile medium (Fig. S9b†). To demonstrate the predominance of Zn1, the authors also prepared mononuclear bis(dipyrrinato)zinc(II) complex Mono3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 with carboxy groups, which forms a self-assembled monolayer (SAM) on a SnO2 electrode upon chemisorption (Fig. S10a†). The SAM of Mono3 performed a conversion efficiency of 0.069% in an acetonitrile medium (Fig. S10b and c†), which is much lower than that of Zn1 with the same optical density. The Zn2-modified SnO2 electrode also served as a photoanode in the same manner as Zn1, except that the photoresponse is red-shifted, which is esteemed in photoelectric conversion applications (Fig. S6b and c†). The red-shift is induced by the π-extension of dipyrrin ligand L2, thereby demonstrating the designability and tunability of the present 1D-CPs. The highest conversion efficiency of Zn2 (0.027% in an aqueous medium, Fig. S6d and S11†) is far greater than that of Mono3 in the same medium (∼0%, Fig. S10d and e†). The series of comparative experiments displays that the polymer structure of Zn1 and Zn2 is advantageous for photoelectric conversion applications.
32 with carboxy groups, which forms a self-assembled monolayer (SAM) on a SnO2 electrode upon chemisorption (Fig. S10a†). The SAM of Mono3 performed a conversion efficiency of 0.069% in an acetonitrile medium (Fig. S10b and c†), which is much lower than that of Zn1 with the same optical density. The Zn2-modified SnO2 electrode also served as a photoanode in the same manner as Zn1, except that the photoresponse is red-shifted, which is esteemed in photoelectric conversion applications (Fig. S6b and c†). The red-shift is induced by the π-extension of dipyrrin ligand L2, thereby demonstrating the designability and tunability of the present 1D-CPs. The highest conversion efficiency of Zn2 (0.027% in an aqueous medium, Fig. S6d and S11†) is far greater than that of Mono3 in the same medium (∼0%, Fig. S10d and e†). The series of comparative experiments displays that the polymer structure of Zn1 and Zn2 is advantageous for photoelectric conversion applications.
Conclusions
The authors determined the structural and functional features of 1D-CPs comprising the bis(dipyrrinato)metal(II) complex motif. Layering bridging dipyrrin ligand L1 in dichloromethane and metal(II) ions in water produced single crystals of 1D-CP M1 through a spontaneous coordination reaction at the liquid/liquid interface. X-ray diffraction analysis showed that the fibers of M1 were propagated along the [1 0 −1] crystallographic axis. Isolated fibers of Zn1 could be exfoliated from the single crystal upon ultrasonication in dichloromethane, and AFM confirmed that the fibers were more than several μm long. Zn1 did not show detectable fluorescence, whereas 1D-CPs Zn2 comprising π-extended bridging dipyrrin ligand L2 and zinc(II) ions featured orange fluorescence with the maximum wavelength of 597 nm in a toluene dispersion: this demonstrated the designability and tunability of the present 1D-CP. The dispersibility of Zn1 and Zn2 was sufficient enough to afford a significant advantage in processing them for applications. Zn1 formed a conjugate with SWCNTs, where the fibers of Zn1 wrapped around SWCNTs uniformly, and which featured thermoelectric conversion. In addition, Zn1 and Zn2 could be deposited onto transparent SnO2 electrodes as thin films, which served as photoanodes for a photoelectric conversion system. The photoelectric conversion response could be extended to longer wavelengths by a dipyrrin ligand modification. Our present results herein highlight the utility of 1D-CPs in materials science.Acknowledgements
The authors acknowledge Grants-in-Aid from MEXT of Japan (nos 21108002, 24750054, 25107510, 26708005, 26107510, 26620039, 26220801, 26248017, 26110505, areas 2107 [Coordination Programming], 2406 [All Nippon Artificial Photosynthesis Project for Living Earth], 2506 [Science of Atomic Layers], 2509 [Molecular Architectonics]), and JSPS fellowship for young scientists. R.S. is grateful to The Japan Prize Foundation, Iketani Science and Technology Foundation, The Murata Science Foundation, Tokuyama Science Foundation, Ogasawara Foundation for the Promotion of Science & Engineering, The Kao Foundation for Arts and Sciences, The Asahi Glass Foundation, The Noguchi Institute, Japan Association for Chemical Innovation, The MIKIYA Science and Technology Foundation, Yazaki Memorial Foundation for Science and Technology, Shorai Foundation for Science and Technology, The Kurata Memorial Hitachi Science and Technology Foundation, and Kumagai Foundation for Science and Technology for financial supports. The synchrotron radiation experiment was performed at the BL02B1 (ref. 40) of SPring-8 (Proposal no. 2014A1355) with the approval of the Japan Synchrotron Radiation Research Institute (JASRI). The authors acknowledge the Research Hub Advanced Nano Characterization (Graduate School of Engineering, The University of Tokyo) for the XPS study.Notes and references
- D. Sheberla, L. Sun, M. A. Blood-Forsythe, S. Er, C. R. Wade, C. K. Brozek, A. Aspuru-Guzik and M. Dincă, J. Am. Chem. Soc., 2014, 136, 8859–8862 CrossRef CAS PubMed.
- J. Cui and Z. Xu, Chem. Commun., 2014, 50, 3986–3988 RSC.
- G. Xu, K. Otsubo, T. Yamada, S. Sakaida and H. Kitagawa, J. Am. Chem. Soc., 2013, 135, 7438–7441 CrossRef CAS PubMed.
- T. Kambe, R. Sakamoto, T. Kusamoto, T. Pal, N. Fukui, T. Shimojima, Z. Wang, T. Hirahara, K. Ishizaka, S. Hasegawa, F. Liu and H. Nishihara, J. Am. Chem. Soc., 2014, 136, 14357–14360 CrossRef CAS PubMed.
- T. Kambe, R. Sakamoto, K. Hoshiko, K. Takada, J.-H. Ryu, S. Sasaki, J. Kim, K. Nakazato, M. Takata and H. Nishihara, J. Am. Chem. Soc., 2013, 135, 2462–2465 CrossRef CAS PubMed.
- H. Nishihara, Chem. Lett., 2014, 43, 388–395 CrossRef CAS.
- P. Amo-Ochoa, L. Welte, R. González-Prieto, P. J. S. Miguel, C. J. Gómez-García, E. Mateo-Martí, S. Delgado, J. Gómez-Herrero and F. Zamora, Chem. Commun., 2010, 46, 3262–3264 RSC.
- J.-C. Tan, P. J. Saines, E. G. Bithell and A. K. Cheetham, ACS Nano, 2012, 6, 615–621 CrossRef CAS PubMed.
- T. Bauer, Z. Zheng, A. Renn, R. Enning, A. Stemmer, J. Sakamoto and A. D. Schlüter, Angew. Chem., Int. Ed., 2011, 50, 7879–7884 CrossRef CAS PubMed.
- Z. Zheng, L. Opilik, F. Schiffmann, W. Liu, G. Bergamini, P. Ceroni, L.-T. Lee, A. Schütz, J. Sakamoto, R. Zenobi, J. VandeVondele and A. D. Schlüter, J. Am. Chem. Soc., 2014, 136, 6103–6110 CrossRef CAS PubMed.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- M. Sadakiyo, T. Yamada, K. Honda, H. Matsui and H. Kitagawa, J. Am. Chem. Soc., 2014, 136, 7701–7707 CrossRef CAS PubMed.
- T. Li, M. T. Kozlowski, E. a. Doud, M. N. Blakely and N. L. Rosi, J. Am. Chem. Soc., 2013, 135, 11688–11691 CrossRef CAS PubMed.
- R. Matsuda, R. Kitaura, S. Kitagawa, Y. Kubota, R. V Belosludov, T. C. Kobayashi, H. Sakamoto, T. Chiba, M. Takata and Y. Kawazoe, Nature, 2005, 436, 238–241 CrossRef CAS PubMed.
- H. Deng, S. Grunder, K. E. Cordova, C. Valente, H. Furukawa, M. Hmadeh, F. Gándara, A. C. Whalley, Z. Liu and S. Asahina, Science, 2012, 336, 1018–1023 CrossRef CAS PubMed.
- Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen and M. Fujita, Nature, 2013, 495, 461–466 CrossRef CAS PubMed.
- L. Ren, L. Yang, P. Yu, Y. Wang and L. Mao, ACS Appl. Mater. Interfaces, 2013, 5, 11471–11478 CAS.
- J. T. Culp, L. Sui, A. Goodman and D. Luebke, J. Colloid Interface Sci., 2013, 393, 278–285 CrossRef CAS PubMed.
- J. Liu, M. Chen, D.-J. Qian and M. Liu, RSC Adv., 2014, 4, 5678–5682 RSC.
- D. Olea, S. S. Alexandre, P. Amo-Ochoa, A. Guijarro, F. de Jesús, J. M. Soler, P. J. de Pablo, F. Zamora and J. Gómez-Herrero, Adv. Mater., 2005, 17, 1761–1765 CrossRef CAS.
- U. García-Couceiro, D. Olea, O. Castillo, A. Luque, P. Román, P. J. de Pablo, J. Gómez-Herrero and F. Zamora, Inorg. Chem., 2005, 44, 8343–8348 CrossRef PubMed.
- T. E. Wood and A. Thompson, Chem. Rev., 2007, 107, 1831–1861 CrossRef CAS PubMed.
- S. Baudron, Dalton Trans., 2013, 42, 7498–7509 RSC.
- H. Maeda and T. Hashimoto, Chem.–Eur. J., 2007, 13, 7900–7907 CrossRef CAS PubMed.
- H. Maeda, T. Nishimura, R. Akuta, K. Takaishi, M. Uchiyama and A. Muranaka, Chem. Sci., 2013, 4, 1204–1211 RSC.
- H. Maeda, M. Hasegawa, T. Hashimoto, T. Kakimoto, S. Nishio and T. Nakanishi, J. Am. Chem. Soc., 2006, 128, 10024–10025 CrossRef CAS PubMed.
- A. Béziau, S. Baudron, A. Guenet and M. W. Hosseini, Chem.–Eur. J., 2013, 19, 3215–3223 CrossRef PubMed.
- B. Kilduff, D. Pogozhev, S. Baudron and M. W. Hosseini, Inorg. Chem., 2010, 49, 11231–11239 CrossRef CAS PubMed.
- D. H. Karweik and N. Winograd, Inorg. Chem., 1976, 15, 2336–2342 CrossRef CAS.
- S. Kusaka, R. Sakamoto, Y. Kitagawa, M. Okumura and H. Nishihara, Chem.–Asian J., 2012, 7, 907–910 CrossRef CAS PubMed.
- R. Matsuoka, R. Sakamoto, T. Kambe, K. Takada, T. Kusamoto and H. Nishihara, Chem. Commun., 2014, 50, 8137–8139 RSC.
- R. Sakamoto, K. Hoshiko, Q. Liu, T. Yagi, T. Nagayama, S. Kusaka, M. Tsuchiya, Y. Kitagawa, W.-Y. Wong and H. Nishihara, Nat. Commun., 2015, 6, 6713 CrossRef CAS PubMed.
- ESI†.
- Q. Miao, J.-Y. Shin, B. O. Patrick and D. Dolphin, Chem. Commun., 2009, 2541–2543 RSC.
- D. Olea, U. García-Couceiro, O. Castillo, J. Gómez-Herrero and F. Zamora, Inorg. Chim. Acta, 2007, 360, 48–54 CrossRef CAS.
- P. Amo-Ochoa, M. I. Rodríguez-Tapiador, O. Castillo, D. Olea, A. Guijarro, S. S. Alexandre, J. Gómez-Herrero and F. Zamora, Inorg. Chem., 2006, 45, 7642–7650 CrossRef CAS PubMed.
- L. Welte, R. González-Prieto, D. Olea, M. R. Torres, J. L. Priego, R. Jiménez-Aparicio, J. Gómez-Herrero and F. Zamora, ACS Nano, 2008, 2, 2051–2056 CrossRef CAS PubMed.
- M. S. Dresselhaus, G. Dresselhaus, R. Saito and A. Jorio, Phys. Rep., 2005, 409, 47–99 CrossRef.
- Y. Nonoguchi, K. Ohashi, R. Kanazawa, K. Ashiba, K. Hata, T. Nakagawa, C. Adachi, T. Tanase and T. Kawai, Sci. Rep., 2013, 3, 3344 Search PubMed.
- K. Sugimoto, H. Ohsumi, S. Aoyagi, E. Nishibori, C. Moriyoshi, Y. Kuroiwa, H. Sawa and M. Takata, AIP Conf. Proc., 2010, 1234, 887–890 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods; XPS of M1, Mono1, and Zn2; photographs of the single crystals of Ni1 and Cu1; single-crystal XRD data for M1; photograph of a free-standing film of Zn1-SWCNT; thermoelectric conversion of Zn1-SWCNT; photoelectric conversion and its quantitative analysis of Zn1, Zn2, and Mono3; photoelectric conversion setup. CCDC 1012353, 1044669 and 1044670. For ESI and crystallographic data in CIF or other electronic format See DOI: 10.1039/c5sc00273g |
| This journal is © The Royal Society of Chemistry 2015 |