Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Conducting polyfurans by electropolymerization of oligofurans†‡
Dennis
Sheberla§
 *,
Snehangshu
Patra
,
Yair H.
Wijsboom
,
Sagar
Sharma
,
Yana
Sheynin
,
Abd-Elrazek
Haj-Yahia
,
Adva Hayoun
Barak
,
Ori
Gidron
and
Michael
Bendikov¶
*,
Snehangshu
Patra
,
Yair H.
Wijsboom
,
Sagar
Sharma
,
Yana
Sheynin
,
Abd-Elrazek
Haj-Yahia
,
Adva Hayoun
Barak
,
Ori
Gidron
and
Michael
Bendikov¶
Department of Organic Chemistry, Weizmann Institute of Science, Rehovot, 76100, Israel
First published on 17th October 2014
Abstract
Polyfurans have never been established as useful conjugated polymers, as previously they were considered to be inherently unstable and poorly conductive. Here, we show the preparation of stable and conducting polyfuran films by electropolymerization of a series of oligofurans of different chain lengths substituted with alkyl groups. The polyfuran films show good conductivity in the order of 1 S cm−1, good environmental and electrochemical stabilities, very smooth morphologies (roughness 1–5 nm), long effective conjugation lengths, well-defined spectroelectrochemistry and electro-optical switching (in the Vis-NIR region), and have optical band-gaps in the range of 2.2–2.3 eV. A low oxidation potential needed for polymerization of oligofurans (compared to furan) is a key factor in achievement of improved properties of polyfurans reported in this work. DFT calculations and experiments show that polyfurans are much more rigid than polythiophenes, and alkyl substitution does not disturb backbone planarity and conjugation. The obtained properties of polyfuran films are similar or superior to the properties of electrochemically prepared poly(oligothiophene)s under similar conditions.
Introduction
The field of conjugated and electrically conducting polymers continues to attract much attention both scientifically and technologically.1 Among them, an important class of five-membered unsaturated heterocyclic polymers, such as polypyrrole (PPy),2 polythiophene (PT),3 polyselenophene (PSe)4 as well as their derivatives, have been extensively studied and applied. Polyfuran (PFu), on the other hand, has scarcely been explored and has not been established as a conductive polymer. This is because of the difficulty in polymerization, the low stability and the poor properties of the polyfuran samples that have been obtained.5 The reported electrical conductivities of doped polyfurans were measured in the range of 10−5 to 10−2 S cm−1,5,6 which is significantly lower than the conductivity of polythiophenes electrochemically prepared from a terthiophene (PT3) (100 S cm−1)3b,7 and thiophene monomers (102 S cm−1).3b,8 Polyfurans have also been reported as environmentally and electrochemically unstable. For example, the authors noticed that upon exposure to ambient light and air, films of chemically prepared alkylated polyfuran bleached over a period of hours to days.9 Furthermore, the electrochemical redox activity of polyfuran was shown to degrade during cycling in acetonitrile (ACN) solution.10Many researchers have tried to overcome preparation problems by varying the conditions of polymerization.5,11 The main problem associated with electrochemical polymerization is the high oxidation potential required to polymerize furan, which results in irreversible over-oxidation of the resulting polyfurans.5,12 A mixture of trifluoride diethyl etherate and ethyl ether (BFEE/EE) as a solvent was used to reduce this electropolymerization potential.11c,d,13 However, the reported conductivities of the resulting polyfurans were small (up to 10−2 S cm−1), and their absorption spectra (λmax = 420 nm)11d reveal a short conjugation length of about eight furan rings.14 Kanatzidis et al., used terfuran as the starting monomer to lower the potential needed for polymerization to less than 1.0 V (vs. SCE).11b The resulting polyfuran was characterized by a variety of techniques and showed a red shifted absorbance maximum centered at 468 nm. Nevertheless, the polyfuran produced had a low conductivity of 2 × 10−3 S cm−1, which is probably due to defects in the polymer, as were observed by IR spectroscopy. Chemically synthesized polyfuran did not appear to be highly conjugated because of a significant degree of furan ring-opening defects.9,15 It is noteworthy that in contrast to polyfurans, the incorporation of furan blocks in conjugated polymers resulted in high quality materials.16 Overall, despite numerous reports on the preparation of polyfuran (usually lacking full characterization),5 it appears that there are no reports of polyfurans with properties such as high conductivity, stability, high conjugation length and small number of defects, all of which are required for their application as organic electronic materials.
We previously reported the preparation of long unsubstituted α-oligofurans (Fu5-Fu9) and recently alkyl substituted α-oligofurans (Fun-2C6 (n = 4, 6, 8) and Fu16-6C6) that exhibit higher fluorescence, better packing, greater rigidity, and higher solubility than the corresponding oligothiophenes.14,17 We also showed that these oligofurans display good electronic properties, e.g., field effect mobilities and on-off ratios, similar to those of the corresponding oligothiophene analogues.18 Thus, we surmised that polyfurans would not only possess similar properties, but would have advantages over other conductive polymers. The rigidity of their backbone is expected to enable the introduction of large side substituents without breaking planarity and π-conjugation. Moreover, polyfurans can be synthesized from renewable resources19 and may be biodegradable.20 Thus, we believe that the reported poor properties, such as low conductivity,5 blue-shifted absorption,11d and instability,9 of polyfurans were due to defects produced during their preparation rather than intrinsic properties of polyfurans.
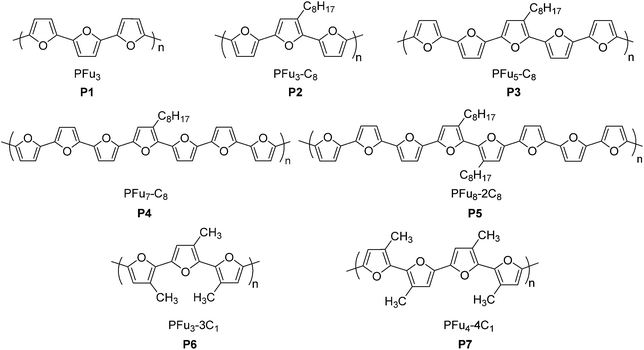
Here we report the first electrochemically-prepared stable and conducting polyfuran films P1–P7 (Chart 1), which possess all the properties necessary to be considered as conducting polymers suitable for applications in organic electronics. These polymers have several advantages over other conjugated polymers, such as rigidity of the backbone and film smoothness. The use of long oligofurans with solubilizing groups, which have relatively low oxidation potential, as the starting monomers for polymerization was the key factor in obtaining polyfuran with improved properties. The electrochemical and spectroelectrochemical behavior, conductivity, electrochemical stability in air under ambient conditions, as well as the morphology of the prepared polyfuran films were studied and compared with corresponding polythiophene analogues. We also report the development of a synthetic methodology for the preparation of long oligofurans 1–7 substituted with solubilizing groups.
Results
Synthesis of oligofurans
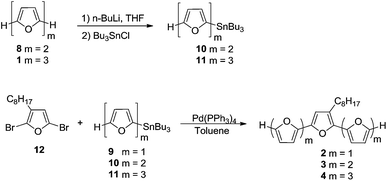
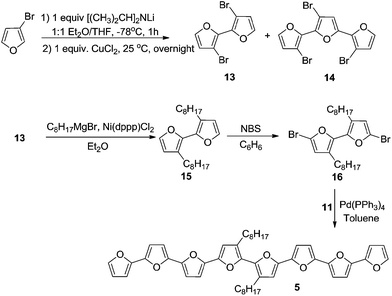
Oligomers 1–5 (Schemes 1 and 2) were synthesized by Stille coupling, oligomer 6 (Scheme 3) was synthesized by Kumada coupling, and oligomer 7 (Scheme 3) was obtained by a combination of Kumada and Ullmann couplings of suitable precursors. Synthesis of compounds 1, 10 and 11 was reported previously.14,21 Synthesis of oligomers 2, 3, and 4 (Scheme 1) was achieved by Stille coupling of 2,5-dibromo-3-octylfuran (12) with tributyl(furan-2-yl)stannane (9), [2,2′-bifuran]-5-yltributylstannane (10), and [2,2′:5′,2′′-terfuran]-5-yltributylstannane (11), respectively. The precursor 2,5-dibromo-3-octylfuran (12) was prepared by bromination of 3-octylfuran with N-bromosuccinimide (NBS) with a 68% yield.For the synthesis of octafuran 5 (Scheme 2), dibromo derivative 13 was prepared using the procedure described in the literature.22 Interestingly, during the course of the reaction, terfuran derivative 14 was also obtained with a 6% yield. Subsequent reaction of compound 13 and octylmagnesium bromide with Ni(dppp)Cl2 gave 3,3′-dioctyl-2,2′-bifuran (15) with a 74% yield. Stille coupling of dibromo derivative 16 (obtained from the bromination of 15 using NBS) with [2,2′:5′,2′′-terfuran]-5-yltributylstannane (11) yielded dioctyl-substituted octafuran 5.
Oligomer 2 has the highest octyl chain to furan ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), while oligomer 4 has the lowest octyl chain to furan ratio (1
3), while oligomer 4 has the lowest octyl chain to furan ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). In order to synthesize polymers having at least a 1
7). In order to synthesize polymers having at least a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
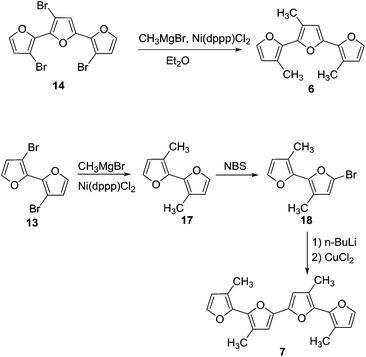
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of alkyl chain to furan ring, bromo groups present in compound 14 were replaced with alkyl chains. Kumada coupling of 14 with methylmagnesium bromide gave compound 6 (Scheme 3) with a moderate yield of 51%. The tetramethyl quaterfuran 7 (Scheme 3) was synthesized by lithiation of the monobromo precursor 18 followed by coupling with CuCl2.
1 ratio of alkyl chain to furan ring, bromo groups present in compound 14 were replaced with alkyl chains. Kumada coupling of 14 with methylmagnesium bromide gave compound 6 (Scheme 3) with a moderate yield of 51%. The tetramethyl quaterfuran 7 (Scheme 3) was synthesized by lithiation of the monobromo precursor 18 followed by coupling with CuCl2.
Electrochemical polymerization and characterization
Electropolymerization of furan monomer could be achieved only at high potentials (ca. 1.8 V vs. SCE),5,12a at which point ring opening and irreversible degradation leads to non-conjugated and low quality polyfurans. Thus, the electropolymerization potential needs to be lowered. This is achieved by using oligofurans rather than furan monomer as the repeat units.3b,11b,23 Starting from terfuran, the oxidation potential of oligofurans drastically decreases, and the polymerization process proceeds below 1.0 V vs. SCE.11b Such low oxidation potentials allowed the preparation of polyfurans reported herein under air at ambient conditions, which can be beneficial for their possible applications.The electrodeposition of polymers P1–P7 has been performed both potentiodynamically and potentiostatically. The best polyfuran films were obtained using a 0.1–1 mM concentration of the oligomer in an ACN solution containing 0.1 M TBACF3SO3 as the electrolyte. In general, for potentiodynamic polymerization, the potential was scanned at 50 mV s−1 from −0.2 V to 0.8 V vs. Ag/AgCl-wire and for potentiostatic polymerization, a potential of 0.75 V vs. Ag/AgCl-wire was applied. Further optimization of polymerization conditions is possible for each of the oligomers (see Table S2, ESI‡). Smooth electrodeposition and the growth of insoluble, highly adhesive continuous polymeric films were observed on different types of working electrodes (i.e., ITO-coated glass, Pt-disk, Au-coated quartz crystal and IDA). It is worth noting that the electrodes were not modified and specially cleaned as they were in the preparation of smooth polythiophenes.24 Representative cyclic voltammograms (CV) for the polymerization of P3 (PFu5-C8) on ITO electrode, showing stepwise increases in the current over the course of polymerization, are displayed in Fig. 1 (for other polymers see Fig. S3, ESI‡).
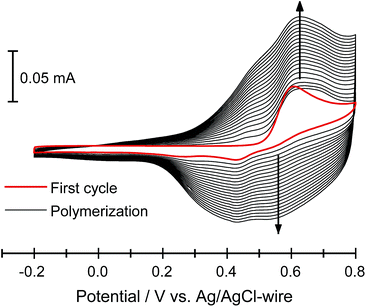 | ||
| Fig. 1 Electrochemical deposition of 3 (0.1 mM) in 0.1 M TBACF3SO3/ACN at 50 mV s−1 on an ITO-coated glass slide. | ||
Oxidation peak potentials are poorly resolved for oligomers 1, 2, 4 and 5 (Fig. S3‡). Therefore, the onset potentials for oligomer oxidation (Eox), measured from the first CV curve of the polymerization, were used for comparison between different oligomers (Table 1). As expected, both the introduction of an alkyl group into an oligomer and the elongation of the oligomer decrease the oxidation potential. For example, the Eox for P1 (PFu3) is 0.62 V, whereas for P2 (PFu3-C8) and P3 (PFu5-C8), it is 0.51 V and 0.43 V, respectively. Furthermore, polyfurans are obtained at lower oxidation potentials than polythiophenes, as shown by the oxidation onset potential of terfuran, which is 0.33 V lower than that of terthiophene.
It is well known that the electrolyte, solvent, oligomer concentration, and method of electropolymerization drastically affect the quality of the resulting polymeric films.1d,23,25 We found that TBACF3SO3/ACN electrolyte (see Fig. S4‡ for others) and concentrations of monomers lower than usually used3b,11b produced better polyfurans. Electrolyte anions and solvent could affect the stability of intermediate σ-dimers, while lower concentrations could reduce π-dimerization of cation radicals that are produced during electropolymerization.1d
CVs of polyfurans in monomer-free TBACF3SO3/ACN electrolyte showing broad oxidation and reduction peaks (Fig. 2 and S5‡), are similar to those of other conducting polymers.1d,3b It is difficult to determine accurate oxidation potentials for polyfurans from the obtained CVs. Nevertheless, oxidation potentials could be estimated from in situ conductivity measurements (see below).
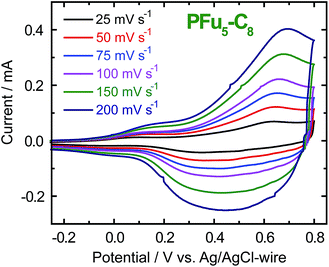 | ||
| Fig. 2 CV of P3 film on an ITO coated glass electrode in monomer-free 0.1 M TBACF3SO3/ACN electrolyte at different scanning rates. | ||
Optical and structural properties
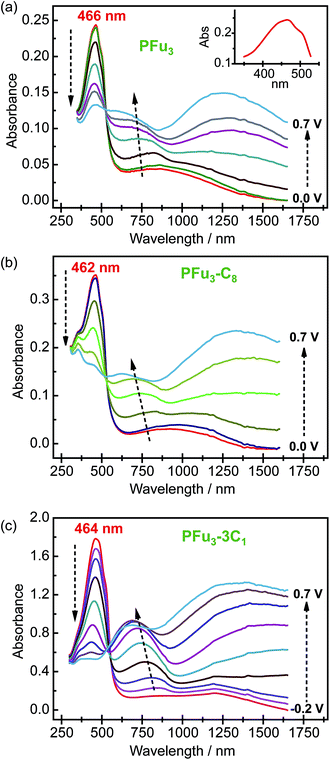
Spectroelectrochemical studies of the electrochemically produced P1–P7 films on ITO electrodes were performed to evaluate their electronic structure. All prepared polymers exhibited well-defined spectroelectrochemical responses, namely, a reversible and distinct change in the absorption spectrum during the redox processes (doping/dedoping). Fig. 3 shows representative spectroelectrochemical spectra for P1, P2, and P6 films (for other polymers see Fig. S8‡). During polymer oxidation, the absorption peak of the neutral polymer film at around 460 nm decreases and two peaks at around 750 nm and 1250 nm develop. In addition, the isosbestic point is observed at ca. 530 nm for P1–P5 and at ca. 550 nm for P6 and P7. Importantly, the presence of one isosbestic point indicates interconversion between neutral and cation-radical (polaron) states.26 Only the 0.7 V spectral trace of P6 (Fig. 3c) shows beginning of bipolaron formation, which is consistent with the decrease in electrical conductivity (see below).The measured λmax values of neutral polyfurans are in the range of 456–466 nm (Table 2). This range is red-shifted compared with most of previously reported values of <420 nm.10c,11a,c,d,12b This suggests that much longer effective conjugation lengths are obtained in the current work. We note that Kanatzidis et al. reported11b an absorbtion maximum of 468 nm for PFu3. However, their spectrum lacks vibronic shoulders, which are observed in all our spectra. We estimate the effective conjugation length in prepared polyfurans to be more than 25 furan units, as can be deduced from extrapolation of the λmax values of α-oligofurans (see Fig. S25‡).
| Polymer | λ max, nm | Optical band gapa, eV | E g (calc)b, eV | HOCO (calc)b, eV | LUCO (calc)b, eV |
|---|---|---|---|---|---|
| a The optical band gaps were obtained from the extrapolation of the linear part of the (αhν)2vs. hν plot to (αhν)2 = 0, where α is the absorption coefficient. b Calculated using the PBC/B3LYP/6-31G(d) level. c For comparison, PT3 was prepared and measured in this work at same conditions as polyfurans. | |||||
| PFu3 (P1) | 466 | 2.31 | 2.41 | −4.36 | −1.95 |
| PFu3-C8 (P2) | 462 | 2.31 | 2.35 | −4.20 | −1.85 |
| PFu5-C8 (P3) | 463 | 2.32 | 2.37 | −4.23 | −1.88 |
| PFu7-C8 (P4) | 458 | 2.29 | 2.38 | −4.29 | −1.90 |
| PFu8-2C8 (P5) | 460 | 2.31 | 2.36 | −4.23 | −1.88 |
| PFu3-3C1 (P6) | 464 | 2.22 | 2.25 | −4.02 | −1.78 |
| PFu4-4C1 (P7) | 456 | 2.22 | 2.26 | −4.03 | −1.77 |
| PT3c | 475 | 2.02 | 2.05 | −4.61 | −2.56 |
The optical band gaps of polyfurans estimated from the absorption spectra (Table 2) for unsubstituted P1 and partially alkyl substituted P2–P5 are ca. 2.3 eV. By contrast, for polyfurans P6 and P7 substituted with one methyl group per furan ring, the band gaps are decreased to ca. 2.2 eV. This observation is consistent with DFT calculations, which showed that alkyl substitution reduces band gaps (Table 2 and S3‡). Moreover, calculations (Table 2) showed that this reduction in band gap occurs because of the increase in the highest occupied crystal orbital (HOCO) energy (i.e. the energy of the valence band), which is partially offset by an increase in the lowest occupied crystal orbital (LUCO) energy (i.e. the energy of the conduction band). Consequently, the oxidation potential of alkyl-substituted polyfurans decreased. The increase of the HOCO energy is consistent with the electron donating property of alkyl groups.
Interestingly, the peak of neutral polyfuran films shows red-shifted shoulders (Fig. 3 and S8‡), probably because of the vibronic coupling.27 The presence of vibronic coupling may indicate that the polymer backbone is highly rigid, planar and effectively π-conjugated. We have shown that oligofurans exhibit high rigidity,14,28 as evident from calculated twisting potentials which are higher than for oligothiophenes. Similar computational results have been recently shown for polyfurans.29 Consequently, the π-conjugation and planarity of polyfurans should not be distorted by various substitution of the polyfuran backbone as strongly as it is in the polythiophene family. Indeed, DFT geometry optimization (PBC/B3LYP/6-31G(d)) of polyfurans P1–P7 shows that the polyfuran backbone remains planar upon alkyl substitution (Fig. 4a and S10‡). In contrast, in polythiophene PT4-4C1, which is a thiophene analogue of P7, the polymer backbone deviates strongly from planarity (Fig. 4b), distorting the π-conjugation. As a consequence, the calculated band gap for PT4-4C1 increases to 2.9 eV from 2.0 eV for planar unsubstituted polythiophene.29,30 Whereas measured optical and calculated band gaps for polyfurans P1–P7 are not increased upon alkyl substitution.
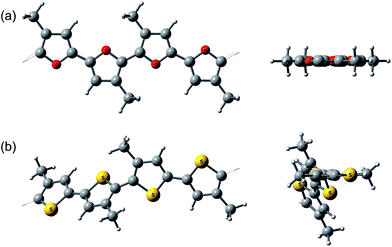 | ||
| Fig. 4 Calculated (PBC/B3LYP/6-31G(d)) geometry of (a) P7 and (b) polythiophene analog of P7, PT4-4C1. A side view and a view along the polymer backbone. | ||
IR spectroscopy
Structural defects in the conjugated backbone of polyfuran can be revealed by IR spectroscopy.32 Previously reported polyfurans showed defects, such as ring-opening and β-coupling, which were observed by stretching vibrational modes of the aliphatic C–H bond (∼2900 cm−1), carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (∼1720 cm−1), and O–H bond (∼3500 cm−1).10b,11a–c The measured ATR-FTIR spectra of P1 and P3 films on Pt-foil (Fig. 5a and b) show little structural defects for P1 and almost none for P3 and are in good agreement with the calculated spectrum of model Fu20 (Fig. 5c, Table S4 and Fig. S12‡) and with previous IR data.10b,11c,33 The IR spectra of P1 and P3 are similar, showing the same bands except for the aliphatic C–H stretching mode of the octyl substituents at around 2900 cm−1.
O bond (∼1720 cm−1), and O–H bond (∼3500 cm−1).10b,11a–c The measured ATR-FTIR spectra of P1 and P3 films on Pt-foil (Fig. 5a and b) show little structural defects for P1 and almost none for P3 and are in good agreement with the calculated spectrum of model Fu20 (Fig. 5c, Table S4 and Fig. S12‡) and with previous IR data.10b,11c,33 The IR spectra of P1 and P3 are similar, showing the same bands except for the aliphatic C–H stretching mode of the octyl substituents at around 2900 cm−1.
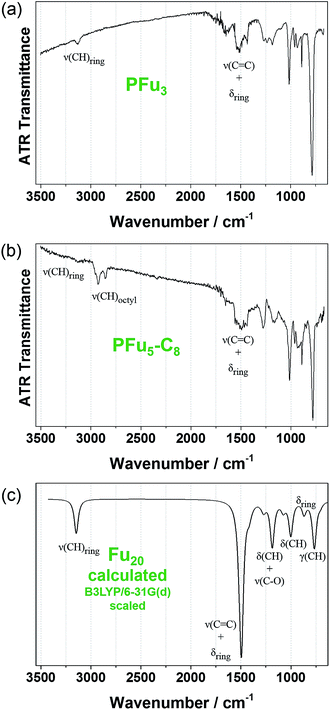 | ||
| Fig. 5 Experimental FTIR-ATR spectra: (a) of P1 and (b) of P3 films electrodeposited on platinum foil. (c) DFT B3LYP/6-31G(d) calculated IR spectrum of Fu20 (frequencies were scaled by 0.96 (ref. 31)). | ||
In situ conductivity
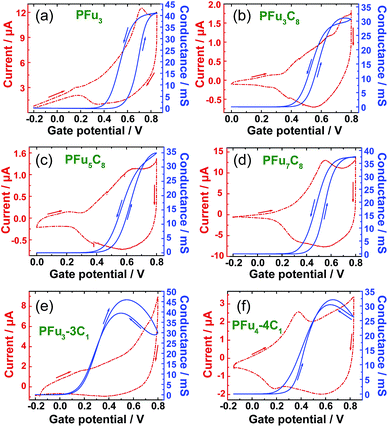
The conductivity of conjugated polymers strongly depends on many factors, particularly the method of preparation, the presence of impurities, humidity, and the measurement technique.1b Owing to their simplicity as well as their ability to obtain a potentiodynamic response, in situ conductivity measurements using an interdigitated array (IDA) microelectrode34 or two-band electrode35 have found wide use.36 However, obtaining the absolute value of polymer film conductivity is not straightforward because it depends on many factors that are hard to define, such as non-trivial geometrical factor of the electrode, non-linear dependence of the conductance on film thickness, and undefined contact resistances. Thus, we report conductance values in comparison to that of poly(terthiophene) (PT3), which was electrochemically prepared and measured under the same setup. Additionally, we compare the conductance values of our polyfurans to those of drop-cast commercial poly(3,4-ethylenedioxythiophene) poly(styrenesulfonate) (PEDOT:PSS) film on the same type of electrode. The conductivity of electrochemically prepared PT3 film7a and that obtained from commercial PEDOT:PSS37 is estimated to be on the order of 1 S cm−1.Fig. 6 shows simultaneous measurements of CV and conductance of P1–P4, P6 and P7 films. All obtained polyfurans show maximum conductances comparable to those of PT3 and PEDOT:PSS films (Table 3, Fig. S15‡). Thus electrical conductivities of doped polyfurans are in the order of 1 S cm−1. This conductivity is much higher than most published values, which range from 10−2 to 10−5 S cm−1.5
| Polymer | Conductance (max), mS | E hc , V |
|---|---|---|
| a Half-maximal conductance potential (vs. Ag/AgCl-wire) for the forward scan. b Limited by the resistance of the IDA electrode contacts (Fig. S15). c Drop-cast film. | ||
| PFu3 (P1) | 43 | 0.69 |
| PFu3-C8 (P2) | 32 | 0.58 |
| PFu5-C8 (P3) | 38 | 0.62 |
| PFu7-C8 (P4) | 37 | 0.55 |
| PFu3-3C1 (P6) | 46 | 0.30 |
| PFu4-4C1 (P7) | 32 | 0.44 |
| PT3 | 42 | 0.87 |
| PEDOT | >72b | −0.21 |
| PEDOT:PSSc | 50 | — |
Each polymer undergoes transition to conductive state at different potentials (Fig. 6 and S15‡). We used the potential at half-maximal conductance (for the forward scan) (Ehc) for comparison between polymers. As evident from Table 3, Ehc depends on the degree of alkyl substitution, i.e., Ehc decreases with increasing degree of alkyl substitution (Table 3). For example, the value of Ehc for fully methylated polyfuran P6 is 0.39 V is lower than that of the unsubstituted P1. Since, the transition to conductive state is associated with the oxidation of a conjugated polymer, we can conclude that the oxidation potential of polyfurans is also decreased by alkyl substitution. This is in the agreement with DFT calculations, which show increase in energy of the HOCO as the degree of alkyl substitution increases (Table 2 and S3‡). Furthermore, for polyfurans, the Ehc potentials are lower than that of PT3 but higher than that of PEDOT, which is consistent with the higher oxidation potential of PT3 (ref. 38) and the lower oxidation potential of PEDOT.39
We observe that the conductivity of fully methylated polyfurans P6 and P7 reaches maximum at 0.5 V and 0.6 V, respectively, and decreases at higher potentials. This observation may suggest that the charge carriers are polarons. Upon further electrochemical oxidation, their conductivity decreases (reversibly) as bipolarons are formed. However, this behavior is not observed in polyfurans P1–P4 due to degradation of the polyfuran films at potentials above 0.8 V, where their conductivity decreases irreversibly.
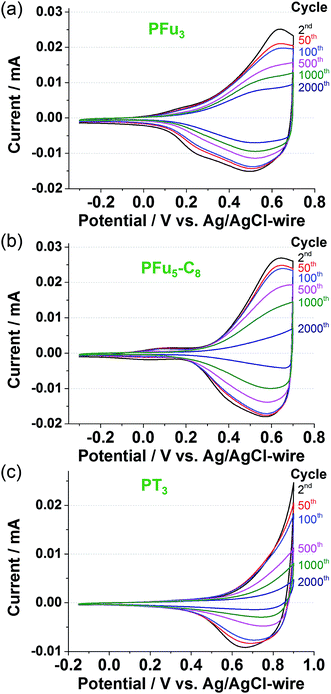
Electrochemical stability
The electrochemical stability of conducting polymers is of utmost importance for their use in electrochemical systems, such as batteries, electrocatalytic materials, sensor devices, and other electronic components.40 Electrochemical degradation of conductive polymers, such as polypyrrole, polythiophene, and polyaniline, has been studied extensively.40 However, very little has been reported on the electrochemical degradation of polyfuran.10b,11c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Fig. S14a and c‡).
000 cycles (Fig. S14a and c‡).
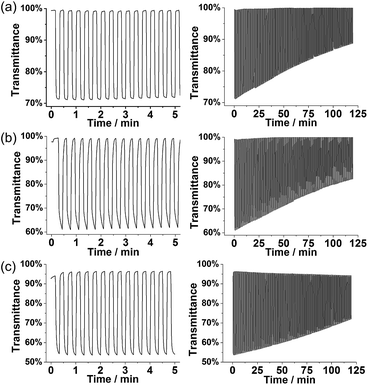
The degraded optical activities (i.e., difference in transmittance of undoped and doped state) obtained after 120 min of cycling (for a total of 360 cycles) were found to be 39%, 45%, and 51% for P1, P3 and PT3 of the starting activity, respectively. Thus, polyfurans P1 and P3 showed only slightly lower stabilities compared with PT3, but are much better than that reported for a PFu/PEDOT device.10c
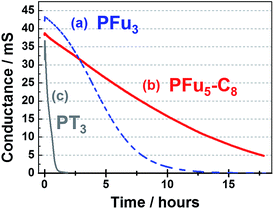 | ||
| Fig. 9 Stability of the conductive state of polymer films: (a) P1 at 0.7 V, (b) P3 at 0.7 V, and (c) PT3 at 0.9 V on an IDA electrode in monomer-free 0.1 M TBACF3SO3/ACN electrolyte. | ||
As shown in Fig. 9, polyfurans P1 and P3 show much higher stabilities toward continuous potential application than PT3, whose lower stability is likely due to the high potential (0.9 V) needed for oxidation (doping), which in turn, is expected to break the conjugation of the backbone via side reactions.42 Thus, the lower potential required for doping the polyfurans is advantageous because it minimizes the occurrence of backbone degradation under ambient conditions.
Morphology
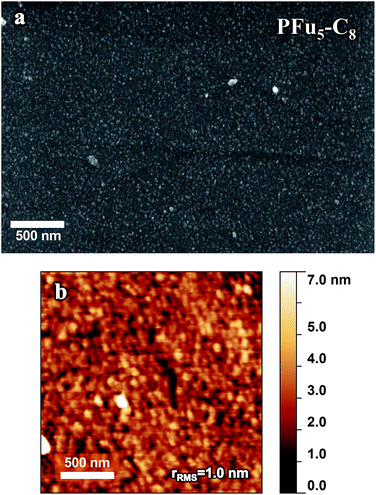
Polymer morphology has a significant effect on polymer charge–transport properties.1b Controlled film thickness, surface smoothness, and uniformity are important considerations for application of conductive polymers in thin film devices (where high surface area is not required, such as sensing), including photovoltaic cells and OFETs. Electrochemical deposition has some advantages over solution processing of conjugated polymers. It combines polymer formation and deposition in one-step and bypasses the need for polymer isolation and purification. As such, it has found use in fabrication of organic electronic devices.43 However, it is difficult to obtain smooth and homogeneous thin films by electropolymerization, necessitating the optimization of electrodeposition.1d For example, the root mean square roughness (rrms) of PT3 on ITO is 173 nm (Fig. S18‡) and PT on ITO is 25–174 nm.24 To the best of our knowledge, the lowest rrms value obtained for electrodeposited PEDOT films in organic media is 46 nm.25c Smooth polythiophene films were obtained after modification of the working electrode,24,44 while smooth electrodeposited PEDOT films were obtained from microemulsions in aqueous solution.45 The polymer precursor approach (i.e., use of inert polymer with pendant electroactive monomer units) was proposed to obtain thin films of less than 100 nm with controllable thickness.46 The drawback of this method, however, is that prior polymer synthesis is necessary.As can be seen in Fig. 10 showing SEM and AFM images of representative example of P3 on HOPG electrode (for the additional images of polyfurans on different electrodes see ESI‡), the prepared polyfuran films show excellent surface smoothness and complete coverage of all working electrodes used. In contrast, polythiophene exhibits separate islands of film and areas of bare electrode surface (see Fig. S17‡ for similarly prepared PT3).47 Additionally, polyfuran films have high adhesion to the electrode surface and are not easily detached. For example, they cannot be pulled off by sticking adhesive tape to the electrode, as in case of poly(3-methylthiophene) films.48 Additionally, films were found to be completely insoluble in organic solvents, such as THF and toluene.
The thickness of prepared polyfuran films can be easily controlled and tailored for the specific application. For instance, the film thickness of P3 linearly depends on the number of CV cycles used in the polymerization (Fig. 11). The thickness of 10 nm on HOPG electrode was obtained after 1 cycle (Fig. 10), 45 nm after 5 cycles (Fig. S19‡) and 90 nm after 10 cycles (Fig. S20‡). The films show full coverage of electrodes with very smooth and homogeneous morphology. Although the rrms value for the 10 nm thick film was only 1 nm, it increased with film growth, as evidenced by the rrms values of 3.5 nm and 5 nm for 45 and 90 nm thick films, respectively (Fig. S20‡). For polyfuran films with thickness above 50 nm, formation of globular structures on the top of smooth film is observed on both HOPG and ITO electrodes (see Fig. S20–S22‡). Polyfuran film roughness on an ITO electrode (Fig. S21–S22‡) is higher than on HOPG and is due to inherent higher roughness of the electrode (i.e., rrms = 4.6 nm for bare ITO, Fig. S23‡).
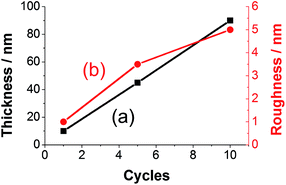 | ||
| Fig. 11 Dependence of (a) thickness and (b) roughness on number of CV cycles used for polymerization of P3 on HOPG electrode. | ||
Discussion
Overall, we have shown that by careful selection of starting oligomers and electropolymerization conditions (i.e., electrochemical method, monomer concentration, electrolyte and solvent), polyfurans of good quality are obtained. By lowering the polymerization potential using long oligofurans as the starting monomers, the polyfurans could be prepared in open air under ambient conditions.The measured high conductivity on the order of 1 S cm−1 for doped polyfuran films may be attributed to better quality of the prepared polyfurans when compared with previous reports.5 This increased quality is manifested in the longer effective conjugation lengths (estimated to be above 25 furan units based on oligomer data, Fig. S25‡) revealed by absorption spectra of the reduced (dedoped) polyfuran films, which show considerable red-shifted (456–466 nm) absorption maxima compared to most reported polyfurans.11a,c,d,12b Additionally, IR spectroscopy showed almost no structural defects, including ring opening and β-coupling in the backbone. Moreover, absorption spectra of the neutral polyfurans show vibronic coupling, indicative of well-ordered, planar, and rigid polymers. Although similar vibronic coupling is also observed in highly conductive samples of PT49 and PEDOT,27b they were not previously observed in electrochemically prepared polyfurans. Furthermore, the measured conductivity of polyfurans is comparable to the conductivity of electrochemically prepared poly(oligothiophene)s.7a Thus, we are convinced that the previously reported low conductivities5 are due to preparation problems, and are not an intrinsic property of polyfurans.
We have studied the effect of alkyl substitution on the properties of polyfurans. DFT calculations predict reductions of the band gaps, as well as oxidation potentials of polyfurans, with increasing alkyl substitution ratio (see Table S3‡). Indeed, compared to P1, we experimentally observe a reduction of around 0.08 eV (Table 2) in optical band gaps in fully methylated polyfurans P6 and P7. Although we cannot reliably measure the oxidation potentials from cyclic voltammetry, we do qualitatively observe that the onset of the broad oxidation peak is shifted to lower potentials for P6 and P7. Another estimation of oxidation potentials can be obtained from the potential at half-maximum conductance Ehc, which is significantly decreased upon alkyl substitution (e.g., Ehc = 0.69 V vs. Ag/AgCl-wire for PFu3 (P1) and Ehc = 0.30 V for PFu3-3C1 (P6)). Furthermore, Ehc for polyfurans is lower than that of PT3 (Ehc = 0.87 V). Therefore, when compared to polythiophenes, a lower oxidation potential of polyfurans serves as an advantage. The lower doping potential minimizes the occurrence of the destructive side reactions (e.g., with water and oxygen). Moreover, oligofurans show lower oxidation potential compared to oligothiophenes, as evidenced by the onset potentials for oligomer oxidation (0.62 V for terfuran and 0.95 V for terthiophene).
As shown by DFT calculations29 and absorption spectra (Fig. 4 and S11‡), polyfurans have the further advantage of being more rigid than polythiophenes. High rigidity of polyfurans is also consistent with the experimental observation that regioirregular methylated polyfurans P6 and P7 show lower band gaps and oxidation potentials. This is advantageous for future development of furan-based polymers, where regioregular arrangement of different functional groups (otherwise needed in the case of polythiophenes to keep the efficient π-conjugation, e.g., rrP3HT)50 is no longer mandatory.
We have carefully studied the stability of electrochemical, optical, and conductive properties of polyfurans in open air under ambient conditions. The stability of the redox activity and conductivity over time showed that polyfurans are actually more stable than polythiophene PT3 under similar conditions. This stability is attributed to lower potential needed for doping of polyfurans. The optical switching stability of polyfurans is found to be comparable with that of PT3, and it is much better than the previously reported stability of the optical switching of a PFu/PEDOT device at 415 nm. Previous studies showing optical switching degradation after only 90 s then is likely attributable to the polyfuran's poor quality, as suggested by the low absorption maximum (λmax = 420).10c Therefore, we believe that the earlier reported instabilities of polyfurans are also due to problems in their preparation.
We have identified two phases of the polyfuran film growth. Initially (up to ca. 50 nm thickness), we observe a smooth thin film covering the whole electrode surface is formed. As the film continues to grow, globular bumps and aggregates are observed on top of the smooth film surface. As a result, the roughness of the surface increases. For instance, in P3 on HOPG, the rrms value was ca. 5 nm for the underlying film and ca. 17 nm for the full scanned area (Fig. S20‡). The formation of the structures on top of homogenous film is consistent with electrochemical polymerization where a new polymer forms at surface points with a higher conductivity.
It is worth noting that prepared polyfuran films have lower roughness than most other electrochemically-prepared films under similar conditions,24,25c,44–46i.e., in organic media electrolyte, in ambient environment, without electrode surface modifications and extensive electrode cleaning. This is certainly an advantage for technological applications of polyfurans.
Conclusion
Using long oligofurans as precursors, we were able to obtain and thoroughly characterize polyfuran films with different alkyl substitutions that exhibit high stability, are spectroelectrochemically active, show good conductivity in the open air under ambient conditions, and have smooth morphology. These properties are considerably improved from any other known polyfuran prepared electrochemically. Additionally, we showed that polyfurans exhibit high rigidity, which allows many different substituents to be introduced without distorting the planarity and conjugation of the polyfuran backbone. We showed that regioirregular alkyl-substituted polyfurans do not lose their conjugation, and moreover exhibit reduced band gaps.Polymerization of oligofurans using considerable lower potential than parent furan allowed us to obtain stable polyfurans with very good properties. This is in contrast to the previous belief that polyfurans are intrinsically unstable and possess low conductivity. We believe that this work will establish polyfurans as a fundamental series of conductive polymers with important properties, opening up future applications for polyfurans as conductive polymers.
Acknowledgements
We thank the Kamin program administrated by the Ministry of Trade and Industry, the Leona M. and Harry B. Helmsley Charitable Trust, and the Helen and Martin Kimmel Center for Molecular Design for financial support.Notes and references
- (a) H. S. Nalwa, Handbook of organic conductive molecules and polymers, Wiley, Chichester, 1997 Search PubMed; (b) T. A. Skotheim and J. R. Reynolds, Handbook of conducting polymers, CRC Press, Boca Raton, FL, 2007 Search PubMed; (c) G. Inzelt, Conducting Polymers: a New Era in Electrochemistry, Springer, New York, 2008 Search PubMed; (d) J. Heinze, B. A. Frontana-Uribe and S. Ludwigs, Chem. Rev., 2010, 110, 4724 CrossRef CAS PubMed.
- (a) R. McNeill, R. Siudak, J. Wardlaw and D. Weiss, Aust. J. Chem., 1963, 16, 1056 CrossRef CAS; (b) S. J. Higgins, Chem. Soc. Rev., 1997, 26, 247 RSC; (c) S. Sadki, P. Schottland, N. Brodie and G. Sabouraud, Chem. Soc. Rev., 2000, 29, 283 RSC; (d) K. T. Song, S. H. Cho and J. Y. Lee, in Conjugated Polymers, CRC Press, 3rd edn, 2007, ch. 8, p. 87 Search PubMed.
- (a) I. F. Perepichka and D. F. Perepichka, Handbook of thiophene-based materials: applications in organic electronics and photonics, Wiley, Chichester, West Sussex, England, 2009 Search PubMed; (b) J. Roncali, Chem. Rev., 1992, 92, 711 CrossRef CAS; (c) A. Mishra, C.-Q. Ma and P. Bäuerle, Chem. Rev., 2009, 109, 1141 CrossRef CAS PubMed.
- (a) A. Patra, Y. H. Wijsboom, S. S. Zade, M. Li, Y. Sheynin, G. Leitus and M. Bendikov, J. Am. Chem. Soc., 2008, 130, 6734 CrossRef CAS PubMed; (b) M. Heeney, W. Zhang, D. J. Crouch, M. L. Chabinyc, S. Gordeyev, R. Hamilton, S. J. Higgins, I. McCulloch, P. J. Skabara, D. Sparrowe and S. Tierney, Chem. Commun., 2007, 5061 RSC; (c) A. Patra and M. Bendikov, J. Mater. Chem., 2010, 20, 422 RSC; (d) L. Li, J. Hollinger, A. A. Jahnke, S. Petrov and D. S. Seferos, Chem. Sci., 2011, 2, 2306 RSC.
- M. J. González-Tejera, E. Sánchez de la Blanca and I. Carrillo, Synth. Met., 2008, 158, 165 CrossRef PubMed.
- Except for a few, seemly unreliable, high values of conductivity that are discussed in ESI.
- (a) G. Zotti and G. Schiavon, Synth. Met., 1990, 39, 183 CrossRef CAS; (b) J. P. Ferraris and T. R. Hanlon, Polymer, 1989, 30, 1319 CrossRef CAS.
- M.-a. Sato, S. Tanaka and K. Kaeriyama, J. Chem. Soc., Chem. Commun., 1985, 713 RSC.
- J. K. Politis, J. C. Nemes and M. D. Curtis, J. Am. Chem. Soc., 2001, 123, 2537 CrossRef CAS PubMed.
- (a) M. J. González-Tejera and I. Carrillo, J. Appl. Electrochem., 2002, 32, 447 CrossRef; (b) X.-B. Wan, L. Li, J.-B. He, D.-S. Zhou, G. Xue and T.-W. Wang, J. Appl. Polym. Sci., 2002, 86, 3160 CrossRef CAS; (c) S. Tirkeş and A. M. Önal, J. Appl. Polym. Sci., 2007, 103, 871 CrossRef.
- (a) G. Zotti, G. Schiavon, N. Comisso, A. Berlin and G. Pagani, Synth. Met., 1990, 36, 337 CrossRef CAS; (b) S. Glenis, M. Benz, E. LeGoff, J. L. Schindler, C. R. Kannewurf and M. G. Kanatzidis, J. Am. Chem. Soc., 1993, 115, 12519 CrossRef CAS; (c) X. Wan, F. Yan, S. Jin, X. Liu and G. Xue, Chem. Mater., 1999, 11, 2400 CrossRef CAS; (d) C. Liu, J. Zhang, G. Shi and Y. Zhao, J. Phys. Chem. B, 2004, 108, 2195 CrossRef CAS.
- (a) G. Tourillon and F. Garnier, J. Electroanal. Chem., 1982, 135, 173 CrossRef CAS; (b) T. Ohsawa, K. Kaneto and K. Yoshino, Jpn. J. Appl. Phys., 1984, 23, L663 CrossRef.
- (a) G. Shi, S. Jin, G. Xue and C. Li, Science, 1995, 267, 994 CrossRef CAS PubMed; (b) W. Chen and G. Xue, Prog. Polym. Sci., 2005, 30, 783 CrossRef CAS PubMed.
- O. Gidron, Y. Diskin-Posner and M. Bendikov, J. Am. Chem. Soc., 2010, 132, 2148 CrossRef CAS PubMed.
- (a) A. Gandini, Adv. Polym. Sci., 1977, 25, 47 CrossRef CAS; (b) E. T. Kang and K. G. Neoh, Eur. Polym. J., 1987, 23, 719 CrossRef CAS; (c) R. M. McConnell, W. E. Godwin, S. E. Baker, K. Powell, M. Baskett and A. Morara, Int. J. Polym. Mater., 2004, 53, 697 CrossRef CAS.
- (a) P. A. Peart and J. D. Tovar, Macromolecules, 2009, 42, 4449 CrossRef CAS; (b) J. C. Bijleveld, B. P. Karsten, S. G. J. Mathijssen, M. M. Wienk, D. M. de Leeuw and R. A. J. Janssen, J. Mater. Chem., 2011, 21, 1600 RSC; (c) A. T. Yiu, P. M. Beaujuge, O. P. Lee, C. H. Woo, M. F. Toney and J. M. J. Fréchet, J. Am. Chem. Soc., 2012, 134, 2180 CrossRef CAS PubMed; (d) P. Sonar, T. R. B. Foong, S. P. Singh, Y. Li and A. Dodabalapur, Chem. Commun., 2012, 48, 8383 RSC.
- (a) U. H. F. Bunz, Angew. Chem., Int. Ed., 2010, 49, 5037 CrossRef CAS PubMed; (b) X.-H. Jin, D. Sheberla, L. J. W. Shimon and M. Bendikov, J. Am. Chem. Soc., 2014, 136, 2592 CrossRef CAS PubMed; (c) F. P. V. Koch, M. Heeney and P. Smith, J. Am. Chem. Soc., 2013, 135, 13699 CrossRef CAS PubMed; (d) B. Capozzi, E. J. Dell, T. C. Berkelbach, D. R. Reichman, L. Venkataraman and L. M. Campos, J. Am. Chem. Soc., 2014, 136, 10486 CrossRef CAS PubMed.
- (a) O. Gidron, A. Dadvand, Y. Sheynin, M. Bendikov and D. F. Perepichka, Chem. Commun., 2011, 47, 1976 RSC; (b) O. Gidron, A. Dadvand, E. Wei-Hsin Sun, I. Chung, L. J. W. Shimon, M. Bendikov and D. F. Perepichka, J. Mater. Chem. C, 2013, 1, 4358 RSC; (c) L. Zhang, N. S. Colella, F. Liu, S. Trahan, J. K. Baral, H. H. Winter, S. C. B. Mannsfeld and A. L. Briseno, J. Am. Chem. Soc., 2013, 135, 844 CrossRef CAS PubMed.
- (a) M. N. Belgacem and A. Gandini, in Monomers, Polymers and Composites from Renewable Resources, ed. M. N. Belgacem and A. Gandini, Elsevier, Amsterdam, 2008, ch. 6, p. 115 Search PubMed; (b) A. Gandini, Macromolecules, 2008, 41, 9491 CrossRef CAS; (c) J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979 CrossRef CAS PubMed; (d) A. Gandini, Green Chem., 2011, 13, 1061 RSC.
- (a) M. Okada, K. Tachikawa and K. Aoi, J. Appl. Polym. Sci., 1999, 74, 3342 CrossRef CAS; (b) F. Koopman, N. Wierckx, J. H. de Winde and H. J. Ruijssenaars, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 4919 CrossRef CAS PubMed.
- (a) T. Kauffmann, H. Lexy and R. Kriegesmann, Chem. Ber., 1981, 114, 3667 CrossRef CAS; (b) H. Ishida, K. Yui, Y. Aso, T. Otsubo and F. Ogura, Bull. Chem. Soc. Jpn., 1990, 63, 2828 CrossRef CAS.
- T. Kauffmann, B. Greving, R. Kriegesmann, A. Mitschker and A. Woltermann, Chem. Ber., 1978, 111, 1330 CrossRef CAS.
- J. Heinze, J. Mortensen and K. Hinkelmann, Synth. Met., 1987, 21, 209 CrossRef CAS.
- J. F. Kang, J. D. Perry, P. Tian and S. M. Kilbey, Langmuir, 2002, 18, 10196 CrossRef CAS.
- (a) S. Patra, K. Barai and N. Munichandraiah, Synth. Met., 2008, 158, 430 CrossRef CAS PubMed; (b) A. I. Melato, M. H. Mendonça and L. M. Abrantes, J. Solid State Electrochem., 2009, 13, 417 CrossRef CAS; (c) E. Poverenov, M. Li, A. Bitler and M. Bendikov, Chem. Mater., 2010, 22, 4019 CrossRef CAS.
- T. Taerum, O. Lukoyanova, R. G. Wylie and D. F. Perepichka, Org. Lett., 2009, 11, 3230 CrossRef CAS PubMed.
- (a) C. Taliani and W. Gebauer, in Handbook of Oligo- and Polythiophenes, ed. D. Fichou, Wiley-VCH, 1998, ch. 7, p. 361 Search PubMed; (b) J. Roncali, P. Blanchard and P. Frere, J. Mater. Chem., 2005, 15, 1589 RSC.
- (a) O. Gidron, N. Varsano, L. J. W. Shimon, G. Leitus and M. Bendikov, Chem. Commun., 2013, 49, 6256 RSC; (b) O. Gidron, Y. Diskin-Posner and M. Bendikov, Chem.–Eur. J., 2013, 19, 13140 CrossRef CAS PubMed.
- S. Sharma, N. Zamoshchik and M. Bendikov, Isr. J. Chem., 2014, 54, 712 CrossRef CAS.
- S. S. Zade and M. Bendikov, Org. Lett., 2006, 8, 5243 CrossRef CAS PubMed.
- NIST Computational Chemistry Comparison and Benchmark Database, NIST Standard Reference Database Number 101, Release 15b, ed. D. Russell Johnson III, August 2011, http://cccbdb.nist.gov/.
- (a) B. S. Lamb and P. Kovacic, J. Polym. Sci., Polym. Chem. Ed., 1980, 18, 2423 CrossRef CAS; (b) I. Carrillo, E. S. de la Bianca, M. J. González-Tejera and I. Hernández-Fuentes, Chem. Phys. Lett., 1994, 229, 633 CrossRef CAS.
- V. Hernandez, F. J. Ramirez, G. Zotti and J. T. L. Navarrete, J. Chem. Phys., 1993, 98, 769 CrossRef CAS PubMed.
- (a) J. W. Thackeray, H. S. White and M. S. Wrighton, J. Phys. Chem., 1985, 89, 5133 CrossRef CAS; (b) N. F. Sheppard, R. C. Tucker and C. Wu, Anal. Chem., 1993, 65, 1199 CrossRef CAS; (c) M. Nishizawa and I. Uchida, Electrochim. Acta, 1999, 44, 3629 CrossRef CAS.
- G. Schiavon, S. Sitran and G. Zotti, Synth. Met., 1989, 32, 209 CrossRef CAS.
- (a) L. Groenendaal, G. Zotti, P. H. Aubert, S. M. Waybright and J. R. Reynolds, Adv. Mater., 2003, 15, 855 CrossRef CAS; (b) Z. Pomerantz, M. D. Levi, G. Salitra, R. Demadrille, A. Fisyuk, A. Zaban, D. Aurbach and A. Pron, Phys. Chem. Chem. Phys., 2008, 10, 1032 RSC; (c) M. Erginer, E. Sezer, B. Ustamehmetoğlu and J. Heinze, Electrochim. Acta, 2012, 67, 181 CrossRef CAS PubMed.
- Heraeus Clevios™ P standard dispersion, http://clevios.com/.
- J. Roncali, F. Garnier, M. Lemaire and R. Garreau, Synth. Met., 1986, 15, 323 CrossRef CAS.
- C. Kvarnström, H. Neugebauer, S. Blomquist, H. J. Ahonen, J. Kankare and A. Ivaska, Electrochim. Acta, 1999, 44, 2739 CrossRef.
- A. A. Pud, Synth. Met., 1994, 66, 1 CrossRef CAS.
- (a) D. Zhang, J. Qin and G. Xue, Synth. Met., 1999, 100, 285 CrossRef CAS; (b) G. Tsekouras, C. O. Too and G. G. Wallace, Synth. Met., 2007, 157, 441 CrossRef CAS PubMed.
- M. Can, K. Pekmez, N. Pekmez and A. Yildiz, J. Appl. Polym. Sci., 2000, 77, 312 CrossRef CAS.
- (a) M. A. Invernale, Y. Ding, D. M. D. Mamangun, M. S. Yavuz and G. A. Sotzing, Adv. Mater., 2010, 22, 1379 CrossRef CAS PubMed; (b) W. Yu, B. Xu, Q. Dong, Y. Zhou, J. Zhang, W. Tian and B. Yang, J. Solid State Electrochem., 2010, 14, 1051 CrossRef CAS; (c) R. Schroeder, L. A. Majewski, M. Grell, J. Maunoury, J. Gautrot, P. Hodge and M. Turner, Appl. Phys. Lett., 2005, 87, 113501 CrossRef PubMed.
- D. A. Rider, K. D. Harris, D. Wang, J. Bruce, M. D. Fleischauer, R. T. Tucker, M. J. Brett and J. M. Buriak, ACS Appl. Mater. Interfaces, 2008, 1, 279 CrossRef PubMed.
- (a) S.-C. Luo, E. Mohamed Ali, N. C. Tansil, H.-h. Yu, S. Gao, E. A. B. Kantchev and J. Y. Ying, Langmuir, 2008, 24, 8071 CrossRef CAS PubMed; (b) E. Ventosa, J. L. Palacios and P. R. Unwin, Electrochem. Commun., 2008, 10, 1752 CrossRef CAS PubMed.
- C. Xia, X. Fan, M.-k. Park and R. C. Advincula, Langmuir, 2001, 17, 7893 CrossRef CAS.
- (a) W. Yu, T. Yao, X. Li, T. Wang, H. Gao, J. Zhang and B. Yang, J. Appl. Polym. Sci., 2011, 119, 1052 CrossRef CAS; (b) V. Cocchi, L. Guadagnini, A. Mignani, E. Salatelli and D. Tonelli, Electrochim. Acta, 2011, 56, 6976 CrossRef CAS PubMed.
- A. Yassar, J. Roncali and F. Garnier, Macromolecules, 1989, 22, 804 CrossRef CAS.
- P. J. Brown, D. S. Thomas, A. Köhler, J. S. Wilson, J.-S. Kim, C. M. Ramsdale, H. Sirringhaus and R. H. Friend, Phys. Rev. B: Condens. Matter, 2003, 67, 064203 CrossRef.
- (a) R. D. McCullough and R. D. Lowe, J. Chem. Soc., Chem. Commun., 1992, 70 RSC; (b) R. D. McCullough, R. D. Lowe, M. Jayaraman and D. L. Anderson, J. Org. Chem., 1993, 58, 904 CrossRef CAS.
Footnotes |
| † This work is dedicated to the memory of Professor Michael Bendikov. |
| ‡ Electronic supplementary information (ESI) available: Full experimental and computational details, synthesis of 1–18, Fig. S1–S26 and Tables S1–S4. See DOI: 10.1039/c4sc02664k |
| § Current address: Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, US. E-mail: E-mail: denn@mit.edu |
| ¶ Deceased July 2, 2013. |
| This journal is © The Royal Society of Chemistry 2015 |