An epistemological inquiry into organic chemistry education: exploration of undergraduate students' conceptual understanding of functional groups
Nalan
Akkuzu
* and
Melis Arzu
Uyulgan
Dokuz Eylul University, Department of Chemistry Education, Izmir, Turkey. E-mail: nalan.akkuzu@gmail.com
First published on 24th September 2015
Abstract
This study sought to determine the levels of conceptual understanding of undergraduate students regarding organic compounds within different functional groups. A total of 60 students who were enrolled in the Department of Secondary Science and Mathematics Education of a Faculty of Education at a state university in Turkey and who had followed an Organic Chemistry Laboratory Course participated in the study. The data were collected using two tools: functional group worksheets and concept maps. Both qualitative and quantitative evaluations were used in analyzing the data. The findings showed that the students generally had low levels of understanding of concepts relating to functional groups, and they exhibited a considerable number of misconceptions. Considering the findings more fully, it was determined that students have remarkable misconceptions and low levels of understanding about certain topics regarding functional groups, including the physical properties of functional groups, intermolecular bonds, acidity and basicity, reduction and oxidation, stereoisomerism and structural isomerism, and aromaticity and aliphaticity, as well as other organic chemistry concepts (e.g. decarboxylation, oxyacide, and phenol). The results suggest that since students are unable to adequately comprehend the subjects of general chemistry, such as intramolecular and intermolecular bonds, acidity and basicity, oxidation and reduction and determination of molecular structures, they fail to accurately transfer their knowledge to learning about organic chemistry. In order to promote students' understanding of functional groups and prevent related misconceptions, it is recommended that basic chemistry topics should be reinforced, and the relationship between students' current knowledge and the new information to be learned in organic chemistry should be emphasized with the help of activities in which various forms of thinking are used.
Introduction
Learning is an active configuration process involving a dynamic interaction between a number of factors (Piaget, 1964; Vosniadou and Ioannides, 1998). Conceptions are the central form of knowledge in this process. As Schwartz (1993) states, an important aspect of acquiring new knowledge is understanding the relationships between various concepts. Likewise, Deci et al. (1991) define learning as a mixture of conceptual understanding and flexible use of knowledge. In this sense, modern teaching approaches indicate that permanent learning depends on conceptual understanding (Driver and Erickson, 1983), in line with Ausubel's (1968) emphasis on the necessity for individuals to correlate the concepts they encounter for meaningful learning to take place. When we use the concepts accurately and establish convenient, hierarchical relationships between the concepts being learned, this will lead to the effective structuring of knowledge. In his film Minds of our Own, Sadler (1997) made the following comment regarding conceptual understanding: “If the meaning of the term is not fully understood, then everything involving that term that the subject encounters tends to have problems” (as cited in Meyer, 2005). The problems that emerge when a relationship is not established between the concepts that are the units of thoughts and the constituents of knowledge cause not only a failure in learning, but also the formation of misconceptions (Nakhleh, 1992).Misconceptions are encountered in a number of areas. One area in which concepts often cannot be learned effectively, and where misconceptions are encountered at the highest rate is the physical sciences. Studies carried out on science learning in the last several decades have revealed that students fail to comprehend many concepts within the physical sciences, and that misconceptions are frequently detected (Bodner, 1991; Mintzes et al., 1997; Galley, 2004; Coştu et al., 2010; Wasacz, 2010). It is asserted in the literature that misconceptions in chemistry are mainly caused by lack of information, incorrect strategies being applied during instruction, misconceptions of teachers, and in the prior understanding of students, as well as past experiences and memorization-oriented learning (Taber, 2001). Misconceptions formed for all or any of these reasons may negatively affect subsequent learning (Palmer, 2001). Thus, it is essential to determine which incorrect ideas are held by students in order to enable them to reach scientifically accepted understandings and learn the related concepts effectively (Osborne and Freyberg, 1985). As Case and Fraser (1999) also indicate, it is necessary to determine the existing misconceptions in order to remove them and enable conceptual learning. Unless this process is carried through, it is thought that the teaching strategies being applied and students' new experiences will not be sufficient to reach the desired outcomes. In this respect, it is very important to understand the conceptual learning and misconceptions that may occur in courses in organic chemistry, which is considered one of the physical sciences and has a broad subject area (Driver and Easley, 1978; Duffy, 2006; Duis, 2011).
Organic chemistry, which was believed to be presented in the structure of living organisms and was known as “carbon compounds” until the beginning of the 19th century, has always had an important place in our daily lives. According to Yong (1994), organic chemistry is an indispensable part of daily life. This entwinement of organic chemistry in our lives and the need for scientific progress necessitate constant attention to education in the field. However, researchers have made it clear that students have great difficulty in understanding organic chemistry (Birk and Kurtz, 1999; Taagepera and Noori, 2000; Gaddis, 2001; Childs and Sheehan, 2009; Wasacz, 2010; O'Dwyer and Childs, 2011; Graulich, 2015). In fact, students have attributed different meanings to this academic course due to its difficulties, and it has gained a reputation as a “rite of passage” (Pungente and Badger, 2003), a “killer course” (Grove et al., 2008), “gatekeeper” (McClary, 2010) and “dreaded wash-out” (Katz, 1990, as cited in O'Dwyer and Childs, 2011). When students were asked why they found organic chemistry to be difficult, they answered that it was an abstract, onerous task and a memorization-oriented subject with many details to be learnt (Bhattacharyya and Bodner, 2005; O'Dwyer and Childs, 2011).
On the other hand, a number of studies have shown that students fail to learn organic chemistry primarily due to their lack of conceptual understanding, as in other areas of chemistry (Duffy, 2006). Bhattacharyya and Bodner (2005) demonstrated that even with high performance in terms of solving problems in organic chemistry, graduate students have very low levels of conceptual understanding, which has been mainly associated with a memorization-oriented approach to learning. It has also been observed that a reason they are unable to learn these concepts is that they apply an algorithmic approach, using a limited number of problems with gradually-progressing solutions, to understand organic chemistry; and they are thus not able to generate alternative ways of thinking (Nakhleh, 1993; Sanger, 2005). Ferguson (2003) contends that rather than answering the questions of “how” and “why” in interpreting reactions in organic chemistry, students focus solely on initial products and the products that may be formed.
In order to address this issue, researchers have drawn attention to the teaching of the granular structure of matter, conducting studies aimed at the development of spatial and reasoning ability in students (Fleming et al., 2000; Liu, 2005; Nandagopal, 2008). Bhattacharyya (2004) emphasizes the necessity for students to focus on the visualization of molecules, reactions and even ‘non-visual’ particles in order to understand the concepts involved in organic chemistry. Some researchers have determined that students are able to understand basic concepts in organic chemistry through macro-micro-symbol and sub-micro representations (Nakhleh, 1993; Reid and Yang, 2002; Ferguson and Bodner, 2008). Additionally, to counteract misconceptions Kozma et al. (2000) emphasize that it is possible to make a connection between these representations with the help of alternative ways of thinking. The relationships between representations are not algorithmic; while using these four representations, students tend to apply alternative ways of thinking between each of them without pursuing an order. Duffy (2006) notes that conceptual learning depends on the ways of thinking deployed. According to her, conceptual understanding occurs when the students create viable and scientific pathways in order to obtain the correct answers. She suggests that terminological representation plays an active role in developing conceptual understanding and increases the comprehensibility of organic chemistry. Since the students may misuse or misunderstand the specific chemical concepts, or use vocabulary either non-scientifically or inconsistent with the chemical definitions, she denotes the presence of a non-referential use of terminology (NRT) way of thinking. Robinson and Nurrenbern (n.d.) have also touched upon the types of problem related to organic chemistry in conceptual learning and emphasized the necessity for using algorithmic-A and recall-R methods, as well as the conceptual-C method (Raker, 2011).
In light of all these studies, it can be understood that organic chemistry may only be learned through acquisition of conceptual knowledge. We think that conceptual understanding is not merely about knowing the definition of the concepts or defining them, but also about seeing the relationship between concepts and the ways in which concepts are constructed in the minds of students. Moreover, conceptual understanding occurs when new knowledge is connected with existing knowledge using alternative ways of thinking in a logical way. Thus, it is essential to determine the misconceptions of students in order to promote meaningful learning and to plan instruction accordingly (Driver and Easley, 1978). Accordingly, the first step in improving the teaching of these concepts is to carry out an analysis regarding what misconceptions students have (Tennyson, 1983, as cited in Ülgen, 2004).
According to Ausubel et al. (1978), the most important factor affecting learning is students' available knowledge (as cited in Köseoğlu and Tümay, 2013). However, in terms of national and international research, only a limited number of studies have been conducted regarding students' misconceptions in the field of organic chemistry. In one of the existing studies, Rushton et al. (2008) examined the conceptual understandings of fourth-year university chemistry students regarding the basic concepts of organic chemistry. In another study, Cruz-Ramirez de Arellano and Towns (2014) investigated students' understanding of alkyl halide reactions in undergraduate organic chemistry. In this interview study, the questions related to predicting the product that might be formed depending on certain reactivities, writing the best mechanism to describe the organic reactions, and explaining the basic concepts of organic chemistry. Likewise, in his exploratory study, Duis (2011) conducted interviews with 23 organic chemistry educators; their answers revealed that students have misconceptions in the basic concepts of organic chemistry. In particular, her participants reported that students had difficulty with reaction mechanisms, acid–base chemistry, synthesis reactions, stereochemistry, and resonance and functional groups, as well as their properties.
Another study relating to the determination of misconceptions concerning organic chemistry was conducted with 60 students from a junior college in Singapore (Bryan, 2007). According to the results, the students had learning difficulties in determining the presence of chiral carbon atoms, cis–trans isomerism, optical isomerism, reactivity of alkenes, and activity of organic compounds consisting of functional groups like alcohols and carboxylic acids. Likewise, as a result of a diagnostic test applied with 276 university students, O'Dwyer and Childs (2011) determined that students had an average success rate of 53% in organic chemistry. The researchers concluded that students primarily had difficulties in understanding the subjects of reaction and mechanism, as well as in classifying organic compounds and understanding their properties.
Aside from the studies related to determining students' misconceptions, the literature regarding organic chemistry includes studies that were primarily aimed at teaching approaches and reaction mechanisms as a subject (Bauer, 1998; Gaddis, 2001; Shatila, 2007; Nandagopal, 2008; Schroeder, 2008; McClary, 2010; Pakhira, 2012; Evans, 2013; Cruz-Ramirez de Arellano and Towns, 2014). However, there is a very limited number of studies concerning students' level of understanding of functional groups, which are among the most important subjects related to organic chemistry. In fact, functional groups play an important role in both the classification of organic compounds according to their reactivities and in the characteristic chemical reactions of molecules. Therefore, it is necessary to investigate the extent to which students learn the concepts of organic compounds with functional groups and the extent of the convenient, hierarchical relationships between these concepts, and to reveal their degree of understanding. In this study the authors wish to foster an awareness of misconceptions regarding functional groups. Developing and promoting such an awareness may be useful for teachers and educators in structuring their teaching more productively. Additionally, the results of this research may contribute to subsequent studies. Accordingly, this study aims to determine the learning levels of undergraduate students regarding organic compounds with different functional groups, as well as their misconceptions, if present. Thus, answers have been sought for the following questions:
• What are the undergraduate students' levels of conceptual understanding regarding functional groups?
• What are the undergraduate students' misconceptions regarding functional groups?
Method
Research design
This study aims to determine the misconceptions concerning functional groups of undergraduate students followed in the Organic Chemistry Laboratory Course in the spring semester. A case study design was used to draw attention to the specific circumstances related to the participants' understanding of functional groups. The study was carried out over 12 weeks in the spring semester. During this period various experiments relating to the subject of functional groups were performed. Following each experiment, worksheets related to the concepts mentioned in the experiment were given to the students, and the students were asked to complete them. At the end of the 12-week period of study, the researchers instructed the students in concept mapping techniques during 1 week's class hours (4 hours weekly). The content of this program included types of concept maps, the methods for writing propositions between concepts, and the directions in which arrows should be drawn. Following this instruction, each student was asked to build a concept map related to the subject of functional groups.The study group
The total of 60 students who cooperated with this research were enrolled in the Department of Secondary Science and Mathematics Education of a Faculty of Education at a state university in Turkey. Among these students, 32 were female and 28 were male. Students took the Organic Chemistry Laboratory Course in the spring term; they had also followed the Organic Chemistry I and Organic Chemistry II during two previous academic years. The subject of functional groups was taught within the scope of the Organic Chemistry II course, and these theoretical lessons were conducted in parallel with laboratory lessons. Throughout the study, the students carried out experiments regarding functional groups (molecule models of alcohols and ethers, aldehydes and ketones, carboxylic acids and esters, maleic and fumaric acid output, and iodoform output) under the guidance of the researchers. Furthermore, the authors of this article, who were also the lead researchers, have a combined experience of 8 years in teaching the Organic Chemistry Laboratory courses.The researchers explained the research procedures and potential consequences accurately and in sufficient detail to the students in the laboratory environment. All the students were asked to participate in the study and gave their informed consent before participating in research and the researchers use individuals' existing data for research purposes. Students had a right to make a free choice over whether to contribute to the study or not (British Educational Research Association, 2011; as cited in Taber, 2014). Data were obtained solely by two researchers and they were stored in a secure location. All data and the identities of the individuals were kept anonymous. In order to maintain confidentiality, the participants' names were not revealed; the students were referenced using the code “S” and a number (e.g. S1).
Data collection and instruments
Two data collection tools were used in the study: functional group worksheets and concept maps. All of the data were collected in Turkish. The study data were translated into English by two English experts, and finally, the researchers checked the translations for the accuracy of chemistry-related terminology.| Name of the experiment | Goal of the experiment | Concepts |
|---|---|---|
| Molecule models of alcohols and ethers | Examine the physical and chemical properties of the 3-dimensional structures of alcohols and ethers with the help of the molecule modeling method | Alcohol, phenol, primary alcohol, secondary alcohol, tertiary alcohol, monoalcohol, dialcohol, trialcohol, hydrogen bond, hydroxyl group, number of branchings, polarity, structural isomer, ether, dipole–dipole, van der Waals, boiling point, solubility, structural formula |
| Molecule models of aldehydes and ketones | Examine the physical and chemical properties of the 3-dimensional structures of aldehydes and ketones with the help of the molecule modeling method | Aldehyde, ketone, aromatic, aliphatic, carbonyl group, carbaldehyde, nucleophile, electrophile, hemiacetal, acetal, structural isomer, van der Waals, hydrogen bond, dipole–dipole, tautomeri |
| Molecule models of carboxylic acids and esters | Examine the physical and chemical properties of the 3-dimensional structures of carboxylic acids and esters with the help of the molecule modeling method | Carboxylic acid, ester, dimeric structure, oxyacide, dicarboxylic acid, tricarboxylic acid, amphoteric properties, structural formula, mono carboxylic acid, dipole–dipole, van der Waals, acidic properties, amino acid, decarboxylation, aliphatic, aromatic, anhydride, hydrogen bond, lactone, saponification |
| Maleic and fumaric acid output | Learn the physical properties of the concept of isomery and isomer types in functional groups, as well as organic molecules in the experiment | Geometrical isomer, substitution reaction, elimination, physical properties, melting point, chiral, carboxylic acid, dipole–dipole, van der Waals, hydrolysis, nucleophile, crystal, solubility, acid regulation, structural isomer, enantiomer, diastereomer, optical isomer |
| Iodoform output | Learn the oxidation and reduction reactions in functional groups | Oxidation, reduction, oxidizer, reducer, primary alcohol, secondary alcohol, tertiary alcohol, aldehyde, ketone, carboxylic acid, ester, haloform, nucleophile |
The worksheets included the concepts mentioned in the experiments, as well as the concepts that were thought to be related to those concepts; that is to say, a concept mentioned in one experiment that could also be related to another experiment was mentioned in the worksheet related to that experiment as well. For instance, the dipole–dipole bond, van der Waals bond, hydrogen bond, nucleophile, aromatic and aliphatic could be given as examples of the common concepts that were used in this way.
As shown in Table 2, the students were asked to define the concept and make statements about the relationships between the subjects studied with regard to the concepts in the worksheets. Open-ended questions regarding the concepts were prepared in a manner that enabled the students to freely express how they had structured the concepts and their relationship in their minds, and thus to reveal their misconceptions (Palmer, 1998). The worksheets also included the option “I don't know”. This option was offered in order to assist in determining whether the students had remembered the concepts mentioned in the subject.
| Name of the experiment | Alcohols and ethers | |||
|---|---|---|---|---|
| Way of answering | Concept | I don't know | What does it mean to you? | What is the relation to the subject? |
| Example 1 | Structural isomer | X | ||
| Example 2 | Structural isomer | Compounds with the same closed formula and a different structural formula are called structural isomers of each other. | ||
| Example 3 | Structural isomer | Compounds with the same closed formula and different structural formula are called the structural isomers of each other. | Alcohols and ethers are structural isomers of each other. | |
Table 2 shows examples of 3 different ways of answering about the concept of the structural isomer in the worksheets. Among these examples, the student in this case marked the option “I don't know” in the first example, as he had no understanding of the concept. In the second example, the student only wrote the definition of the concept and was unable to indicate its relation to the subject; and in the third example, he defined and associated the concept accurately. The definitions and associations of students regarding the concepts may not always include accurate statements; thus, the researchers attempted to reveal the levels of understanding and misconceptions of the students with the aid of any incorrect statements written on the worksheets.
| Categories | Coding criteria |
|---|---|
| Sound understanding (SU) | Responses that include all components of the acceptable responses |
| Partial understanding (PU) | Responses that include at least one of the components of the acceptable response |
| Specific misconception (SM) | Responses that include descriptive, incorrect or illogical information |
| No understanding (NU) | Repeats part or all of the question and uncodable responses |
| No response (NR) | Blank or I don't know |
In order to establish the reliability of the coding process, the researchers coded the data in the worksheets separately at different times. Following this coding process, the researchers checked the coherence between them and determined the match percentage as 90% (0.90). The fact that this value was higher than 0.75 shows that the analyses were reliable and demonstrated “excellent coherence” (Fleiss et al., 2003).
In this study, the students were instructed in concepts relating to the functional groups and were asked to form a concept map using these concepts. The process of preparing the concept maps provided the students with cognitive freedom and enabled them to visualise the associations they had formed. A total of 50 concepts were selected relating to the subject of functional groups. These concepts are displayed in Table 4. Otherwise, the students were exposed to no influence and were free to use any concepts in order to demonstrate their structures of cognition. Accordingly, they used other concepts and examples in addition to the concepts given in the maps.
| Concepts | Acetal | Carboxylic acid | Ester | Mono-alcohol | Saponification |
| Achiral | Chiral | Ether | Nucleophile | Secondary alcohol | |
| Alcohol | Decarboxylation | Geometrical isomer | Optical isomer | Solubility | |
| Aldehyde | Dialcohol | Haloform | Oxidation | Stereoisomerism | |
| Aliphatic | Diastereomer | Hemiacetal | Oxidizer | Structural isomer | |
| Amino acid | Dimeric structure | Hydrogen bond | Oxyacide | Structural formula | |
| Anhydride | Dipole–dipole | Hydroxyl group | Phenol | Substitution reaction | |
| Aromatic | Electrophile | Isomery | Polyalcohol | Tautomeri | |
| Boiling point | Elimination | Ketone | Primary alcohol | Tertiary alcohol | |
| Carbonyl group | Enantiomer | Lactone | Reducer | van der Waals |
The concept maps were prepared in approximately 60–90 minutes. In order to encourage the students to individually reflect their knowledge about the concepts, they were prohibited from communicating with each other or using outside resources in the process of preparation. The students were permitted to give different concepts or examples aside from the concepts given in the maps they prepared. At the end of this process, a total of 60 concept maps were collected.
The scales developed by McClure and Bell (1990) and adapted by McClure et al. (1999) were taken into consideration in scoring the concept maps. The propositional statements among the concepts were taken into consideration during the qualitative evaluation of the concept maps. The propositions where the relationship between the concepts was indicated with arrows were evaluated with the following scoring criteria: scientifically accurate propositions and the direction of the arrow, accurate propositions, incorrect propositions, and those involving no scientific statements were assigned a value of 3 points, 2 points, 1 point and 0 points respectively. The findings used in this study consist of the incorrect propositions acquired from the qualitative analysis of the concept maps. These incorrect propositions involve statements with misconceptions.
The Pearson product-moment correlation coefficient of the scorers was calculated as 0.976 (p < 0.01) via the SPSS 15.0 program. The high correlation value does not signify that both researchers had given the same score to the same map; the scores given to the concept maps show some variation. However, the correlation coefficient shows that there was a significant, high and positive relationship between the scorers; therefore, it can be asserted that the evaluations of the concept maps were both valid and reliable.
Results
Results acquired from the analysis of the worksheets
The concepts relating to each experiment were analyzed separately in the worksheets that were prepared to determine the level of understanding and misconceptions of students regarding functional groups. The analysis was evaluated according to five categories: Sound understanding (SU), Partial understanding (PU), Specific misconception (SM), No understanding (NU) and No response (NR). Table 6 shows the findings acquired from the analyses of the worksheets.| Concept number | Concepts involved in worksheets | SU | PU | SM | NU | NR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| f | % | f | % | f | % | f | % | f | % | ||
| a f: Number of students, %: percentage value of students. | |||||||||||
| 1 | Alcohol | 51 | 85.0 | 6 | 10.0 | 1 | 1.7 | 1 | 1.7 | 1 | 1.7 |
| 2 | Phenol | 30 | 50.0 | 13 | 21.7 | 4 | 6.7 | 5 | 8.3 | 8 | 13.3 |
| 3 | Primary alcohol* | 34 | 56.6 | 18 | 30.0 | 3 | 5.0 | 2 | 3.3 | 3 | 5.0 |
| 4 | Secondary alcohol* | 33 | 55.0 | 16 | 26.7 | 5 | 8.3 | 3 | 5.0 | 3 | 5.0 |
| 5 | Tertiary alcohol* | 32 | 53.3 | 17 | 28.3 | 5 | 8.3 | 3 | 5.0 | 4 | 6.7 |
| 6 | Mono-alcohol | 52 | 86.6 | 5 | 8.3 | 0 | 0 | 1 | 1.7 | 2 | 3.3 |
| 7 | Dialcohol | 50 | 83.3 | 3 | 5.0 | 3 | 5.0 | 1 | 1.7 | 3 | 5.0 |
| 8 | Trialcohol | 51 | 85.0 | 1 | 1.7 | 2 | 3.3 | 1 | 1.7 | 5 | 8.3 |
| 9 | Hydrogen bond* | 18 | 30.0 | 25 | 41.7 | 13 | 21.7 | 2 | 3.3 | 5 | 8.3 |
| 10 | Hydroxyl group | 43 | 71.7 | 14 | 23.3 | 1 | 1.7 | 0 | 0 | 2 | 3.3 |
| 11 | Number of branchings | 18 | 30.0 | 24 | 40.0 | 10 | 16.7 | 5 | 8.3 | 3 | 5.0 |
| 12 | Polarity | 12 | 20.0 | 36 | 60.0 | 5 | 8.3 | 3 | 5.0 | 4 | 6.7 |
| 13 | Structural isomer* | 12 | 20.0 | 38 | 63.3 | 1 | 1.7 | 5 | 8.3 | 4 | 6.7 |
| 14 | Ether | 47 | 78.3 | 9 | 15.0 | 0 | 0 | 1 | 1.7 | 3 | 5.0 |
| 15 | Dipole–dipole interaction* | 15 | 25.0 | 30 | 50.0 | 7 | 11.7 | 2 | 3.3 | 6 | 10.0 |
| 16 | van der Waals interaction* | 13 | 21.7 | 30 | 50.0 | 9 | 15.0 | 3 | 5.0 | 5 | 8.3 |
| 17 | Boiling point* | 25 | 41.7 | 27 | 45.0 | 4 | 6.7 | 2 | 3.3 | 2 | 3.3 |
| 18 | Solubility* | 10 | 16.7 | 32 | 53.3 | 4 | 6.7 | 11 | 18.3 | 3 | 5.0 |
| 19 | Structural formula* | 21 | 35.0 | 27 | 45.0 | 1 | 1.7 | 4 | 6.7 | 7 | 11.7 |
| 20 | Aldehyde* | 32 | 53.3 | 18 | 30.0 | 2 | 3.3 | 3 | 5.0 | 5 | 8.3 |
| 21 | Ketone* | 33 | 55.0 | 16 | 26.7 | 2 | 3.3 | 3 | 5.0 | 6 | 10.0 |
| 22 | Aromatic* | 13 | 21.7 | 38 | 63.3 | 3 | 5.0 | 2 | 3.3 | 4 | 6.7 |
| 23 | Aliphatic* | 13 | 21.7 | 35 | 58.3 | 3 | 5.0 | 2 | 3.3 | 7 | 11.7 |
| 24 | Carbonyl group | 30 | 50.0 | 17 | 28.3 | 3 | 5.0 | 3 | 5.0 | 7 | 11.7 |
| 25 | Carbaldehyde | 26 | 43.3 | 3 | 5.0 | 2 | 3.3 | 8 | 13.3 | 21 | 35.0 |
| 26 | Nucleophile* | 9 | 15.0 | 32 | 53.3 | 7 | 11.7 | 1 | 1.7 | 11 | 18.3 |
| 27 | Electrophile | 6 | 10.0 | 25 | 41.7 | 13 | 21.7 | 3 | 5.0 | 13 | 21.7 |
| 28 | Hemiacetal | 21 | 35.0 | 7 | 11.7 | 2 | 3.3 | 8 | 13.3 | 22 | 36.7 |
| 29 | Acetal | 21 | 35.0 | 5 | 8.3 | 2 | 3.3 | 6 | 10.0 | 26 | 43.3 |
| 30 | Tautomeri | 15 | 25.0 | 25 | 41.7 | 0 | 0 | 3 | 5.0 | 17 | 28.3 |
| 31 | Carboxylic acid* | 28 | 46.7 | 24 | 40.0 | 3 | 5.0 | 0 | 0 | 5 | 8.3 |
| 32 | Ester* | 26 | 43.3 | 21 | 35.0 | 3 | 5.0 | 4 | 6.7 | 6 | 10.0 |
| 33 | Dimeric structure | 21 | 35.0 | 12 | 20.0 | 0 | 0 | 4 | 6.7 | 23 | 38.3 |
| 34 | Oxyacide | 21 | 35.0 | 11 | 18.3 | 6 | 10.0 | 4 | 6.7 | 18 | 30.0 |
| 35 | Dicarboxylic acid | 49 | 81.7 | 4 | 6.7 | 2 | 3.3 | 0 | 0 | 5 | 8.3 |
| 36 | Tricarboxylic acid | 50 | 83.3 | 3 | 5.0 | 2 | 3.3 | 0 | 0 | 5 | 8.3 |
| 37 | Amphoteric properties | 6 | 10.0 | 38 | 63.3 | 3 | 5.0 | 3 | 5.0 | 10 | 16.7 |
| 38 | Mono-carboxylic acid | 45 | 75.0 | 6 | 10.0 | 0 | 0 | 1 | 1.7 | 8 | 13.3 |
| 39 | Acidic properties | 19 | 31.7 | 34 | 56.7 | 0 | 0 | 2 | 3.3 | 5 | 8.3 |
| 40 | Amino acid | 25 | 41.7 | 23 | 38.3 | 2 | 3.3 | 7 | 11.7 | 3 | 5.0 |
| 41 | Decarboxylation | 20 | 33.3 | 8 | 13.3 | 7 | 11.7 | 4 | 6.7 | 21 | 35.0 |
| 42 | Anhydride | 21 | 35.0 | 14 | 23.3 | 1 | 1.7 | 6 | 10.0 | 18 | 30.0 |
| 43 | Lactone | 28 | 46.7 | 7 | 11.7 | 0 | 0 | 6 | 10.0 | 19 | 31.7 |
| 44 | Saponification | 28 | 46.7 | 19 | 31.7 | 0 | 0 | 5 | 8.3 | 8 | 13.3 |
| 45 | Geometrical isomer | 21 | 35.0 | 33 | 55.0 | 0 | 0 | 4 | 6.7 | 2 | 3.3 |
| 46 | Substitution reaction | 20 | 33.3 | 31 | 51.7 | 0 | 0 | 2 | 3.3 | 7 | 11.7 |
| 47 | Elimination | 18 | 30.0 | 25 | 41.7 | 1 | 1.7 | 3 | 5.0 | 13 | 21.7 |
| 48 | Physical properties | 7 | 11.7 | 37 | 61.7 | 1 | 1.7 | 10 | 16.7 | 5 | 8.3 |
| 49 | Melting point | 12 | 20.0 | 29 | 48.3 | 1 | 1.7 | 5 | 8.3 | 13 | 21.7 |
| 50 | Chiral | 19 | 31.7 | 21 | 35.0 | 7 | 11.7 | 3 | 5.0 | 10 | 16.7 |
| 51 | Hydrolysis | 12 | 20.0 | 25 | 41.7 | 6 | 10.0 | 5 | 8.3 | 12 | 20.0 |
| 52 | Crystal structure | 7 | 11.7 | 34 | 56.7 | 0 | 0 | 5 | 8.3 | 14 | 23.3 |
| 53 | Acid regulation | 2 | 3.3 | 20 | 33.3 | 0 | 0 | 11 | 18.3 | 27 | 45.0 |
| 54 | Enantiomer | 13 | 21.7 | 19 | 31.7 | 4 | 6.7 | 3 | 5.0 | 21 | 35.0 |
| 55 | Diastereomer | 7 | 11.7 | 20 | 33.3 | 1 | 1.7 | 7 | 11.7 | 25 | 41.7 |
| 56 | Optical isomer | 5 | 8.3 | 34 | 56.7 | 6 | 10.0 | 3 | 5.0 | 12 | 20.0 |
| 57 | Oxidation | 10 | 16.7 | 40 | 66.7 | 6 | 10.0 | 1 | 1.7 | 3 | 5.0 |
| 58 | Reduction | 8 | 13.3 | 41 | 68.3 | 6 | 10.0 | 2 | 3.3 | 3 | 5.0 |
| 59 | Oxidizer | 6 | 10.0 | 37 | 61.7 | 12 | 20.0 | 2 | 3.3 | 3 | 5.0 |
| 60 | Reducer | 4 | 6.7 | 39 | 65.0 | 12 | 20.0 | 2 | 3.3 | 3 | 5.0 |
| 61 | Haloform | 19 | 28.3 | 19 | 31.7 | 0 | 0 | 6 | 10.0 | 18 | 30.0 |
As shown in Table 6, the worksheets involved the understanding levels and misconceptions of students regarding a total of 61 concepts. The concepts indicated with an asterisk (*) in the table were associated with each of the experiments and were therefore included in the worksheets for all of the experiments. An examination of the table reveals that the students had the highest levels of understanding of the concepts of alcohol and its various types, such as monoalcohol, dialcohol, trialcohol, hydroxyl groups, and ether; as well as carboxylic acid types, such as monocarboxylic acid, dicarboxylic acid and tricarboxylic acid. A level of partial understanding was observed in relation to the concepts such as polarity, structural isomer, dipole–dipole interaction, solubility, boiling point, aromatic, nucleophile, amphoteric properties, geometrical isomer, and reduction. On the other hand, there were some concepts, such as tautomeri, oxyacide, etc. that were generally left blank by students, as they had no connotation or correlation regarding the functional groups (see Table 6). Specific misconceptions as related to these concepts are given in Table 7 in detail.
| Concept | Misconception | f | % | |
|---|---|---|---|---|
| Molecule models of alcohols and ethers | Boiling point | As the number of branchings increases, the boiling point increases. | 4 | 6.7 |
| Number of branchings | As the number of branching increases in alcohols, the solubility decreases. | 10 | 16.7 | |
| As the number of branchings increases, the boiling point increases, since the molecules grow. | ||||
| Polarity | No polarity due to the ether structure. | 5 | 8.3 | |
| There is polarity between metal atoms. | ||||
| Primary alcohol | Only one carbon (C) atom in the primary alcohol structure. | 3 | 5.0 | |
| Secondary alcohol | Alcohols containing two hydrogens in the centre carbon atom. | 5 | 8.3 | |
| Alcohols containing two hydroxyl groups (–OH). | ||||
| Tertiary alcohol | Alcohols containing three hydroxyl groups (–OH). | 5 | 8.3 | |
| Solubility | The process where the bonds of a molecule are torn with the help of another solvent. | 4 | 6.7 | |
| Homogeneous distribution of compounds within one another. | ||||
| Since the molecular structure of ether resembles the water molecule, it dissolves in water better. Alcohol, on the other hand, dissolves less. | ||||
| Phenol | It is an alcohol type which has an aromatic ring. | 4 | 6.7 | |
| Hydrogen bond | Intramolecular bonds. Since alcohol contains the OH group, they form intramolecular hydrogen bonds. | 10 | 16.7 | |
| Dipole–dipole interaction | Alcohols contain higher rates of dipole–dipole interactions compared to ethers. Because ethers are more apolar. | 9 | 15.0 | |
| van der Waals interaction | Since alcohols contain the hydroxyl group, they have a stronger van der Waals interaction compared to ethers. | 11 | 18.3 | |
| Dialcohol | Alcohols with two hydroxyl groups (OH) bonded to the same carbon atom. | 3 | 5.0 | |
| Molecule models of aldehydes and ketones | Carbonyl group | Compounds containing the COOH group. | 3 | 5.0 |
| Hydrogen bond | Attraction force between efficient particles in aldehydes. | 23 | 38.3 | |
| The first four members of aldehydes and ketones contain a hydrogen bond. | ||||
| Since aldehydes contain a hydrogen atom, they have intermolecular hydrogen bonds. | ||||
| Nucleophile | In the carbonyl group, a carbon atom with a relatively positive charge is called nucleophile. | 10 | 16.7 | |
| Electrophile | Structures showing a tendency to give electrons. | 13 | 21.7 | |
| Electron-rich molecules. | ||||
| Negatively (−) charged parts. | ||||
| An oxygen atom with a negative charge in the carbonyl group is called an electrophile. | ||||
| van der Waals interaction | Since aldehydes and ketones have a straight chain structure, they contain van der Waals. | 11 | 18.3 | |
| Aliphatic | Aldehydes and ketones are aliphatic compounds. | 6 | 10.0 | |
| Molecule models of carboxylic acid and esters | Oxyacide | Carboxylic acids contain an oxygen atom | 6 | 10.0 |
| Hydrogen bond | Esters are polar and form hydrogen bonds. | 5 | 8.3 | |
| Decarboxylation | An event occurring when the carboxyl group (–COOH) goes out. | 7 | 11.7 | |
| Amphoteric properties | Carboxylic acids have an amphoteric property. | 3 | 5.0 | |
| Both metal and non-metal elements. | ||||
| van der Waals interaction | Very weak intermolecular interaction observed on apolar molecules. It is not observed on carboxylic acids. | 8 | 13.3 | |
| Dipole–dipole interaction | It is observed on esters and not on carboxylic acids as they contain hydrogen bonds. | 7 | 11.7 | |
| Maleic–fumaric acid output | Chiral | Structures with conflicting mirror images. | 7 | 11.7 |
| If four different atoms are bonded to the carbon atom, then it is chiral. | ||||
| Dipole–dipole interaction | Maleic acid contains the dipole–dipole interaction, whereas the fumaric acid does not. | 8 | 13.3 | |
| Solubility | Since fumaric acid is stable, it has a higher solubility than maleic acid. | 4 | 6.7 | |
| Optical isomer | Both maleic and fumaric acids displaying a geometrical isomer are also optical isomeries of each other. | 6 | 10.0 | |
| A type of isomer formed by molecules with a plane of symmetry. | ||||
| Enantiomer | An isomer caused by the cis–trans property. | 4 | 6.7 | |
| Maleic acid is the enantiomer of the fumaric acid. | ||||
| Hydrolysis | A reaction causing water (H2O) in the environment as a result of a reaction. | 6 | 10.0 | |
| Iodoform output | Secondary alcohol | Aldehydes are formed when it is oxidised. | 4 | 6.7 |
| Tertiary alcohol | Ketones are formed when it is oxidized. | 4 | 6.7 | |
| Carboxylic acids are formed when it is oxidized. | ||||
| Ester | Ketones are formed when the ester is reduced. | 3 | 5.0 | |
| Carboxylic acid | Carboxylic acids are formed when the ketone is oxidized. | 3 | 5.0 | |
| Oxidation | An event where the ion receives electrons during the reaction. | 6 | 10.0 | |
| Oxidation occurs by giving Hydrogen atom. | ||||
| Reduction | An event where the ion gives electrons during the reaction. | 6 | 10.0 | |
| Reduction occurs by receiving Hydrogen atom. | ||||
| Oxidizer | Atoms that give electrons. | 12 | 20.0 | |
| A structure being oxidized by giving Hydrogen atom. | ||||
| Reducer | Atoms that receive electrons. | 12 | 20.0 | |
| A structure being reduced by receiving Hydrogen atom. | ||||
| Nucleophile | The electron-rich carbon atom in iodoform compound displays a nucleophilic property. | 7 | 11.7 | |
| Common concepts | Hydrogen bond | The bond formed by a carbon (C) atom with the hydrogen (H) atom is called hydrogen bond. | 13 | 21.7 |
| A bond formed between hydrogen atoms. | ||||
| Strong intramolecular bonds. | ||||
| Dipole–dipole interaction | Intramolecular bonds. | 7 | 11.7 | |
| Bonds caused by electron exchange. | ||||
| Bonds that are formed by electron sharing. | ||||
| van der Waals interaction | Short-duration intramolecular bonds. | 9 | 15.0 | |
| Bonds that are formed by electron sharing. | ||||
| Attraction force between apolar molecules. | ||||
| Established between non-metal atoms. | ||||
| The weakest intramolecular bond. | ||||
| Nucleophile | Structures showing a tendency to receive electrons. | 7 | 11.7 | |
| Positively (+) charged parts. | ||||
| Electron-poor groups. | ||||
| Aromatic | Cyclical structures are aromatic compounds. | 7 | 11.7 | |
Table 7 shows the concepts that had the greatest misconceptions in terms of experimental subjects in the worksheets according to their frequencies and percentages. The table also illustrates on a separate line the misconceptions regarding common concepts in the worksheets. The reason for including these common concepts separately was that the same misconceptions were encountered on each worksheet. The frequency and percentage values of the errors relating to the common concepts in worksheets were attained by averaging the frequency and percentage values in all worksheets. While the frequency values indicate the number of students expressing the misconception, the percentage values indicate the percentage value among the 60 students who participated in the study. Concepts involving the misconceptions were not evaluated for frequency values less than 3.
Table 7 also shows the statements of students regarding concepts in relation to which they had the greatest number of errors. According to these statements, they confused the van der Waals and dipole–dipole interactions and both intramolecular and intermolecular bonds in the concepts of the hydrogen bond; they were also unable to associate the bond structures of the molecules. In addition, they confused concepts that are usually used together, such as oxidizer and reducer and nucleophile and electrophile, and they were unable to define them accurately. The students also made erroneous statements about concepts such as optical isomers, chirality and enantiomers in the subject of isomery.
Quantitative results acquired from the analysis of the concept maps
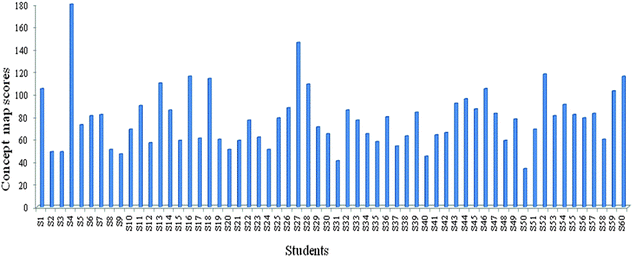
The data in the study were acquired from a total of 60 concept maps that were prepared by the students. The concept maps were scored by the researchers according to the relational scoring method. Fig. 1 shows the distribution of findings that were acquired as a result of the scoring.The scores obtained from the concept maps varied between 34 and 180. In order to interpret these scores, the scores were categorized equally in order to have an interval value of 36 using the SPSS 15.0 program. Table 8 shows the frequency and percentage distribution of the numbers of students in 4 categories (excellent, good, average, and poor) that were obtained as a result of the process of categorization. Examples of two students' concept maps categorized good and poor are given in the Appendix.
| Scale codes | Intervals of score | f | % |
|---|---|---|---|
| a f: Number of students, %: percentage value of students. | |||
| Excellent | 143–180 | 2 | 3.3 |
| Good | 107–142 | 6 | 10.0 |
| Average | 71–106 | 26 | 43.3 |
| Poor | 34–70 | 26 | 43.3 |
| Total | 60 | 100 | |
According to Table 8, it can be seen that the students had low levels of conceptual understanding regarding functional groups.
Table 9 illustrates in detail the levels of understanding of the students regarding the concepts in the concept maps. The data from the concept maps are presented separately as the number of concepts, total number of propositions used, number of accurate propositions used and number of incorrect propositions. The relationships between the concepts included in the column of concepts in incorrect propositions (IPC) is shown in Table 9; however, there is an error concerning the propositions between these concepts that were written by the students. The incorrect propositions among these concepts are specified in the qualitative analysis of the concept maps.
| SN | TCN | Score | TPN | CPN | IPN | IPC |
|---|---|---|---|---|---|---|
| a SN: Student number, TCN: Used total concept number, TPN: Used total proposition number, CPN: Used correct proposition number, IPN: Used incorrect proposition number, IPC: Concepts used in incorrect propositions. | ||||||
| S1 | 47 | 105 | 40 | 36 | 4 | Aldehyde → Hydroxyl group; Secondary alcohol → Ether; Primary alcohol → Boiling point; Phenol → Secondary alcohol |
| S2 | 29 | 49 | 19 | 17 | 2 | Alcohol → Solubility; Anhydride → Nucleophile |
| S3 | 36 | 49 | 18 | 15 | 3 | Carboxylic acid → Hydroxyl group; Alcohol → Haloform; Ether → Solubility |
| S4 | 48 | 180 | 68 | 62 | 6 | Alcohol → Carboxylic acid; Ether → Boiling point; Ester → Aromatic; Anhydride → Nucleophile; Anhydride → Elimination; Aldehyde → Tautomerizm |
| S5 | 19 | 73 | 32 | 27 | 5 | Anhydride → Nucleophile; Geometric isomer → Chiral; Alcohol → Primary alcohol; Alcohol → Secondary Alcohol; Alcohol → Tertiary alcohol |
| S6 | 38 | 81 | 35 | 27 | 8 | Carboxylic acid → Carbonyl group; Isomery → Geometrical isomer; Primary, secondary, tertiary alcohol → Structural isomer; Ketone → Ether; Aldehyde → Aldol; Aldehyde → Anhydride |
| S7 | 32 | 82 | 32 | 27 | 5 | Isomery → Dicarboxylic acid; Anhydride → Carboxylic acid; Aldehyde → Aldol; Ketone → Ether; Anhydride → Nucleophile |
| S8 | 23 | 51 | 17 | 15 | 2 | Alcohol → Primary alcohol; Carboxylic acid → Oxyacide |
| S9 | 25 | 47 | 20 | 17 | 3 | Alcohol → Aldehyde; Alcohol → Carboxylic acid; Aldehyde → Aliphatic |
| S10 | 28 | 69 | 25 | 22 | 3 | Carboxylic acid → H bond; Stereoisomer → Diastereomer; Ether → Carbonyl compound |
| S11 | 30 | 90 | 35 | 26 | 9 | Alcohol → Mono-alcohol, Dialcohol, Trialcohol; Alcohol → Haloform; Ether → Carbonyl compound; Optical isomer → Chiral, Achiral; Stereoisomer → Geometrical isomer, Optical isomer |
| S12 | 35 | 57 | 33 | 27 | 6 | Enantiomer → Achiral; Diastereomer → Chiral; Carboxylic acid → Decarboxylation; Aldehyde → Aliphatic; Ketone → Aliphatic; Ketone → Dialcohol |
| S13 | 45 | 110 | 43 | 35 | 8 | Hydroxyl group → Oxidizer; Tertiary alcohol → Reduction; Ketone → Carboxyl group; Carbonyl group → hydrolysis; Carboxyl group → Decarboxylation; Aldehyde → Acetal; Aldehyde → Aliphatic; Carboxylic acid → Aliphatic |
| S14 | 32 | 86 | 34 | 28 | 6 | Stereoisomerism → Chiral; Ketone → Ester; Ketone → Dialcohol; Alcohol → Primary alcohol, Secondary alcohol, Tertiary alcohol |
| S15 | 37 | 59 | 24 | 18 | 6 | Achiral → Geometrical isomer; Ether → Solubility; Alcohol → Phenol; Alcohol → Secondary alcohol; Alcohol → Tertiary alcohol; Carboxylic acid → Hydroxyl group |
| S16 | 46 | 116 | 42 | 36 | 6 | Ketone → Dialcohol; Dialcohol → Ether; Geometrical isomer → Chiral; Chiral → Diastereomer; Chiral → Enantiomer; Haloform → hydrolysis |
| S17 | 41 | 61 | 27 | 17 | 10 | Ketone → Ether; Alcohol → Primary alcohol; Alcohol → Dialcohol; Alcohol → Phenol; Carboxylic acid → Carbonyl group; Carbonyl compound → Hemiacetal; Carboxylic acid → Oxyacide; Carboxylic acid → Dipole–dipole, van der Waals, H bond |
| S18 | 41 | 114 | 41 | 37 | 4 | Ether → Solubility; Aliphatic → Ketone; Aliphatic → Aldehyde; Ketone → Ester |
| S19 | 22 | 60 | 21 | 19 | 2 | Ether → Solubility; Isomery → Geometrical isomer |
| S20 | 30 | 51 | 22 | 16 | 6 | Diastereomer → Chiral; Enantiomer → Chiral; Ester → H bond; Alcohol → Primary alcohol, Secondary alcohol, Tertiary alcohol |
| S21 | 35 | 59 | 27 | 16 | 11 | Carboxylic acid → van der Waals, Dipole–dipole, H bond; Carboxylic acid → Oxyacide; Isomery → Geometrical isomer; Structural isomer → Primary, secondary, tertiary alcohol; Ketone → Ether; Ketone → Decarboxylation; Hydroxyl group → H bond |
| S22 | 41 | 77 | 33 | 22 | 11 | Carboxylic acid → Oxyacide; Carboxylic acid → van der Waals, dipole–dipole, H bond; Carbonyl compound → Hemiacetal; Alcohol → Dialcohol; Alcohol → Phenol; Structural isomer → Primary, secondary, tertiary alcohol; Hydroxyl group → H bond |
| S23 | 37 | 62 | 29 | 16 | 13 | Carboxylic acid → Carbonyl group; Alcohol → Dialcohol; Hydroxyl group → H bond; Ketone → Ether; Carboxylic acid → Decarboxylation; Carboxylic acid → van der Waals, dipole–dipole; Anhydride → Nucleophile; Aromatic → Electrophile; Diastereomer → Enantiomer; Structural isomer → Primary, secondary, tertiary alcohol |
| S24 | 35 | 51 | 26 | 23 | 3 | Aldehyde → Aliphatic; Carboxylic acid → Aliphatic; Ether → Carbonyl group |
| S25 | 32 | 79 | 30 | 26 | 4 | Aldehyde → H bond; Secondary alcohol → Tertiary alcohol; Ester → Aromatic; Isomery → Geometric isomer |
| S26 | 37 | 88 | 34 | 24 | 10 | Alcohol → Dialcohol; Hydroxyl group → H bond; Carboxylic acid → Oxyacide; Carboxylic acid → van der Waals, dipole–dipole; Isomery → Geometrical isomer; Ketone → Ether; Structural isomer → Primary, secondary, tertiary alcohol |
| S27 | 46 | 146 | 49 | 45 | 4 | Mono-alcohol → Dimeric structure; Aldehyde → H bond; Aldehyde → van der Waals; Alcohol → Phenol |
| S28 | 43 | 109 | 38 | 35 | 3 | Enantiomer → Achiral; Enantiomer → Chiral; Polyalcohol → Ketone |
| S29 | 28 | 71 | 27 | 24 | 3 | Amino acid → Decarboxylation; Enantiomer → Achiral; Diastereomer → Chiral |
| S30 | 27 | 65 | 25 | 20 | 5 | Alcohol → Phenol; Aldehyde → H bond; Structural isomer → Primary, secondary, tertiary alcohol |
| S31 | 20 | 41 | 15 | 9 | 6 | Carboxylic acid → Decarboxylation; Alcohol → Ether; Carboxylic acid → Oxyacide; Aldehyde → H bond; Diastereomer → Chiral; Enantiomer → Achiral |
| S32 | 34 | 86 | 30 | 25 | 5 | Mono-alcohol → Dimeric structure; Alcohol → Phenol; Anhydride → Nucleophile; Aldehyde → Aliphatic; Ketone → Ether |
| S33 | 31 | 77 | 26 | 19 | 7 | Dialcohol → Ether; Alcohol → Phenol; Enantiomer → Achiral; Diastereomer → Chiral; Structural isomer → Primary, secondary, tertiary alcohol |
| S34 | 31 | 65 | 22 | 17 | 5 | Dialcohol → Ether; Carboxylic acid → Ester; Ketone → Tautomerism; Hydroxyl group → Electrophile; Anhydride → Nucleophile |
| S35 | 27 | 58 | 21 | 16 | 5 | Phenol → Secondary alcohol; Primary, secondary, tertiary alcohol → Structural isomer; Ester → Aromatic |
| S36 | 33 | 80 | 26 | 21 | 5 | Carboxylic acid → Decarboxylation; Geometrical isomer → Chiral; Chiral → Diastereomer; Alcohol → Phenol; Aldehyde → H bond |
| S37 | 19 | 54 | 14 | 12 | 2 | Hydroxyl group → H bond; Dialcohol → Ketone |
| S38 | 29 | 63 | 31 | 26 | 5 | Mono-Alcohol → Phenol; Aliphatic → Ketone; Aliphatic → Aldehyde; Stereoisomerism → Geometrical isomer, Optical isomer |
| S39 | 43 | 84 | 41 | 31 | 10 | Primary, secondary, tertiary alcohol → Structural isomer; Diastereomer → Chiral; Enantiomer → Achiral; Alcohol → Phenol; Ester → H bond; Anhydride → Nucleophile; Carboxylic acid → Oxyacide; Hydroxyl group → H bond |
| S40 | 19 | 45 | 17 | 13 | 4 | Aldehyde → van der Waals, dipole–dipole; Polyalcohol → Ketone; Isomer → Geometrical isomer |
| S41 | 25 | 64 | 20 | 16 | 4 | Enantiomer → Achiral; Enantiomer → Chiral; Aliphatic → Ketone; Aliphatic → Aldehyde |
| S42 | 22 | 66 | 19 | 16 | 3 | Ester → Aromatic; Hydroxyl group → Oxidizer; Carboxylic acid → Oxyacide |
| S43 | 34 | 92 | 36 | 28 | 8 | Hydroxyl group → H bond; Geometrical isomer → Chiral; Chiral → Diastereomer; Structural isomer → Primary, secondary, tertiary alcohol; Ketone → Ether; Ketone → Dialcohol |
| S44 | 34 | 96 | 39 | 35 | 4 | Alcohol → Phenol; Carboxylic acid → van der Waals, Dipole–dipole; Ether → Carbonyl group |
| S45 | 37 | 87 | 34 | 29 | 5 | Alcohol → Phenol; Aldehyde → Hydroxyl group; Ester → Aromatic; Isomery → Geometrical isomer; Ketone → Dialcohol |
| S46 | 43 | 105 | 47 | 43 | 4 | Ketone → Ether; Carboxylic acid → Oxyacide; Enantiomer → Achiral; Diastereomer → Chiral |
| S47 | 32 | 83 | 27 | 22 | 5 | Aldehyde → Ketone; Carboxylic acid → Ester; Carboxylic acid → Decarboxylation; Aldehyde, Ketone → Aliphatic |
| S48 | 20 | 59 | 23 | 20 | 3 | Alcohol → Secondary alcohol; Phenol → Secondary alcohol; Hydroxyl group → Electrophile |
| S49 | 22 | 78 | 30 | 25 | 5 | Dialcohol → Ketone; Aldehyde → Anhydride; Isomery → Geometrical isomer; Aliphatic → Ketone, Aldehyde |
| S50 | 19 | 34 | 14 | 12 | 2 | Alcohol → Carboxylic acid; Ether → Solubility |
| S51 | 23 | 69 | 33 | 31 | 2 | Hydroxyl group → H bond; Mono-Alcohol → Phenol |
| S52 | 45 | 118 | 44 | 36 | 8 | Structural isomer → Primary, secondary, tertiary alcohol; Alcohol → Secondary alcohol; Anhydride → Nucleophile; Carboxylic acid → Oxyacide; Hydroxyl group → H bond; Enantiomer → Achiral |
| S53 | 32 | 81 | 28 | 26 | 2 | Aldehyde → Aliphatic; Ketone → Aliphatic |
| S54 | 33 | 91 | 40 | 37 | 3 | Carboxylic acid → van der Waals, dipole–dipole, H bond |
| S55 | 28 | 82 | 31 | 24 | 7 | Alcohol → Phenol; Structural isomer → Primary, secondary, tertiary alcohol; Ketone → Ether; Hydroxyl group → Electrophile; Ester → H bond |
| S56 | 33 | 79 | 27 | 22 | 5 | Polyalcohol → Ketone; Enantiomer → Achiral; Enantiomer → Chiral; Dialcohol → Ether; Ester → Aromatic |
| S57 | 31 | 83 | 30 | 25 | 5 | Aldehyde → Ketone; Ether → Boiling point; Ether → Solubility; Carboxylic acid → Decarboxylation; Carboxylic acid → Oxyacide |
| S58 | 26 | 60 | 30 | 26 | 4 | Alcohol → Phenol; Aldehyde → Boiling point; Ester → Aromatic; Ketone → Ester |
| S59 | 36 | 103 | 38 | 35 | 3 | Alcohol → Ether; Hydroxyl group → Electrophile; Anhydride → Nucleophile |
| S60 | 42 | 116 | 47 | 42 | 5 | Aldehyde → Aliphatic; Carboxylic acid → Aliphatic; Structural isomer → Primary, secondary, tertiary alcohol |
Qualitative findings acquired from the analysis of the concept maps
Table 10 involves the misconceptions acquired from incorrect propositions that were displayed by the majority of the students. Table 10 shows the frequency and percentage distribution of these misconceptions according to students.| Misconceptions acquired from concept maps | f | % |
|---|---|---|
| a f: Number of students, %: percentage value. | ||
| Primary, secondary and tertiary alcohols are structural isomers of each other. | 11 | 18.3 |
| Phenol is a secondary alcohol. | 3 | 5.0 |
| Phenol is a cyclical structure of mono-alcohols. | 16 | 26.7 |
| The boiling point increases in parallel with the increase of the number of branchings in functional groups. | 3 | 5.0 |
| Carboxylic acids are formed when all the alcohols are oxidized. | 3 | 5.0 |
| A structure with conflicting mirror images is chiral. | 12 | 20.0 |
| Ether is formed by removing C from the ketone. | 11 | 18.3 |
| An isomer containing a double bond is a geometrical isomer. | 10 | 16.7 |
| A carboxylic acid containing an oxygen molecule (O2) is an oxyacide. | 11 | 18.3 |
| Ethers contain the carbonyl group. | 4 | 6.7 |
| Chiral molecules with non-conflicting mirror images are the diastereomers of each other. | 10 | 16.7 |
| Achiral molecules with conflicting mirror images are the enantiomers of each other. | 10 | 16.7 |
| When the carboxyl group (–COOH) goes out of the carboxylic acid, this event is called decarboxylation. | 7 | 11.7 |
| The OH group is an oxidizer (electrophile) group. | 6 | 10.0 |
| Esters are formed when ketones are oxidized. | 3 | 5.0 |
| Ethers have a high solubility in water. | 6 | 10.0 |
| Diols are formed when ketones are reduced. | 7 | 11.7 |
| Dialcohols and ethers are structural isomers of each other. | 4 | 6.7 |
| van der Waals, dipole–dipole interactions and hydrogen bonds are intramolecular bonds. | 11 | 18.3 |
| Ketones are formed when polyalcohols are oxidized. | 4 | 6.7 |
| Alcohols are separated as primary-secondary-tertiary according to the number of OH. | 9 | 15.0 |
| Anhydride molecule is an electron-rich nucleophile. | 9 | 15.0 |
| Aldehydes contain hydrogen bonds. | 5 | 8.3 |
| Hydroxyl group contains hydrogen bond between O–H atoms. | 9 | 15.0 |
| Aldehydes, ketones and carboxylic acids are aliphatic compounds. | 13 | 21.7 |
| The dialcohol structure is the bicarbureted form of alcohol. | 6 | 10.0 |
| The functional group of carboxylic acids is the carbonyl group. | 3 | 5.0 |
As shown in Table 10, as a result of the analysis of the students' concept maps, the students were observed to have various misconceptions regarding concepts in the functional groups, particularly with regard to the structural isomer, phenol, chiral, achiral, geometrical isomer, oxyacide, aliphatic, intermolecular bonds (van der Waals, dipole–dipole, and H bonds), diastereomer, enantiomer, optical isomer, oxidizer, nucleophile and solubility (see Table 10). In addition, the students were observed to have problems in determining the oxidation and reduction products of functional groups, classifying mono-alcohols and determining the electrophile or nucleophile groups.
Discussion
This study has attempted to reveal students' levels of understanding and their misconceptions regarding the concepts concerning functional groups, which are among the most important subjects of organic chemistry. The understanding levels and misconceptions of students were identified from worksheets that were prepared by the researchers and from concept maps that were prepared by the students. All of the findings indicate that the students generally had low levels of understanding of concepts regarding functional groups, and they had a considerable number of related misconceptions.Considering the findings acquired from the worksheets, it was observed that the students had good levels of understanding of concepts of “alcohol, ether, aldehyde, ketone, ester, carboxylic acid and its variations” (see Table 6). This indicates that students are able to discern the compounds within this functional group. However, it was also observed that the students had low levels of understanding of concepts relating to “solubility, nucleophile, electrophile, melting point, acid regulation, diastereomer, optical isomer, reduction and oxidizer.” On the other hand, the students had the greatest misconceptions regarding “hydrogen bond, number of branchings, dipole–dipole interaction, van der Waals interaction, nucleophile, electrophile, decarboxylation, chiral, oxidizer and reducer.” Considering these findings, it can be determined that the students generally had misconceptions about certain topics regarding functional groups, as well as low levels of understanding in relation to “physical properties of functional groups, intermolecular bonds, acidity and basicity, reduction and oxidation, stereoisomerism and structural isomerism, aromaticity and aliphaticity, as well as other organic chemistry concepts being encountered (such as decarboxylation, oxyacide, and phenol)”.
Physical properties of functional groups of organic compounds
Examination of the findings shows that students had difficulty in understanding the concepts relating to solubility, amphoteric properties, melting point, crystal structure and acid regulation. These concepts are related to the physical properties of functional groups of organic compounds. Even though students were able to discern the functional groups, it can be seen that they were not able to sufficiently understand and correlate the concepts regarding their properties. Misconceptions concerning solubility and boiling point were also observed in the concept maps. For instance, some students (16.7% in the worksheets and 5.0% in the concept maps – see Tables 7 and 10) associated the increase of the boiling point in compounds comprising the functional groups such as alcohol with an increase in the number of branchings. This misconception could have been caused by the students confusing the number of branchings with the size of the molecules. An example relating to solubility is that, since the molecular structure of ether resembles water, its solubility is higher than that of alcohol (6.7% in the worksheets – see Table 7). This condition indicates that students also had problems with the intermolecular hydrogen bonds that exist in alcohol. In their study conducted with students enrolled in a department of organic chemistry, Henderleiter et al. (2001) determined that students could form definitions about the hydrogen bond and explain the formation of the hydrogen bond; however, they could not make interpretations regarding how the hydrogen bond would affect the physical properties of molecules, and they had misconceptions regarding boiling point and solubility.Intermolecular bonds in functional groups
One of the most important findings of the current study is that the students had particular misconceptions regarding the definitions of the concepts of hydrogen bonds, van der Waals interaction and dipole–dipole interaction, which are among the intermolecular bonds, as well as their association with functional groups. The majority of the students (18.3% on the concept maps and 21.7%, 11.7% and 15.0%, respectively, among the common concepts in the worksheets – see Tables 7 and 10) defined these bonds as intramolecular bonds and failed to consider intermolecular bonds. Examining the literature, it is observed that students have a number of misconceptions regarding chemical bonds (Peterson and Treagust, 1989; Birk and Kurtz, 1999; Nicoll, 2001; Taber and Coll, 2002; Othman et al., 2008).The results obtained from the concept maps in the present case show that 15% of the students had misconceptions regarding “the existence of a hydrogen bond between the oxygen and hydrogen atoms in a hydroxyl group”. This result is in parallel with the results obtained by Gaddis (2001) and Meyer (2005). In a study performed in an organic chemistry laboratory with organic chemistry students, Gaddis (2001) posed a question about intermolecular forces. According to the answers, 32% (N = 22) of students confused the hydrogen bond with the intramolecular covalent bond.
Similarly, Meyer (2005) determined that the majority of students were unable to understand the nature of intermolecular forces and that they confused them with intramolecular covalent bonds. For instance, in one of the interview questions, the students were asked to show the intermolecular bond on the structures of water molecules; some of the students pointed to the covalent bond between the hydrogen and oxygen atom in the structure of the water molecule. Moreover, in their study conducted with college students in an organic chemistry course, Taagepera and Noori (2000) observed an error regarding the fact that the “hydrogen bonds contain the covalent bond”. In this present case, some of the students (38.3% in the worksheets – see Table 7) also defined the hydrogen bond as “the attraction force between the efficient particles in aldehydes”. Accordingly, they stated: “as aldehydes contain the hydrogen atom, they provide an intermolecular hydrogen bond”. Furthermore, the students noted that “the first four members of aldehydes and ketones make up the hydrogen bond”. This error could have been caused by confusion with the following information: “In particular, the first four members of aldehydes and ketones that have a small molecular mass make up the hydrogen bond with the help of water molecules when they dissolve in water”.
Apart from this, while some students (18.3% in the worksheets – see Table 7) were observed to “ground the existence of the van der Waals interaction in aldehydes and ketones on the aliphatic structure”, others (13.3% in the worksheets – see Table 7) stated that “the van der Waals interaction exists in apolar molecules”. Regarding the dipole–dipole interaction, some students (15.0% in the worksheets – see Table 7) stated: “alcohols have higher dipole–dipole interactions compared to ethers, which is caused by the apolar structure of ethers”. Such a misconception could be caused by failure to understand the concept of polarity in alcohol and ether, as well as failure to comprehend the fact that alcohols have stronger bonds, since they involve each intermolecular bond in comparison to ethers.
In fact, the findings acquired from the worksheets suggest a failure of the students to sufficiently understand the concept of polarity. Accordingly, it is observed that while some students (8.3% in the worksheets – see Table 7) had misconceptions regarding polarity, others (20.0% in the worksheets – see Table 6) had lower levels of sound understanding. Students with misconceptions regarding polarity (8.3% in the worksheets – see Table 7) state in particular that “ethers that are the compounds of functional groups are not polar due to their molecular structure”. At this point, it can be observed that students also experienced problems concerning molecular structures outside of bonds and molecular polarity. From this point of view, it is possible to assert that students had either deficiencies or errors in their prior chemistry knowledge. When students with such deficiencies encounter more complex molecules bearing a functional group in organic chemistry, they are unable to determine the molecular polarity and have greater difficulty with this subject.
The related literature indicates that students are generally unable to recognize intramolecular electron distributions and use non-bonding “lone pair” electrons in determining molecular structure, and that therefore they fail to understand molecular polarity (Peterson et al., 1989; Harrison and Treagust, 1996; Birk and Kurtz, 1999; Taagepera et al., 2002). As a result of interviews conducted with 56 university students, Nicoll (2001) likewise concluded that students have a number of misconceptions, especially with regard to bonds and polarity, in organic chemistry.
Considering other misconceptions regarding the dipole–dipole interaction, which is among the intermolecular bonds, some students (11.7% in the worksheets – see Table 7) noted that “while the esters involve the dipole–dipole interaction, the carboxylic acids do not involve the dipole–dipole interaction, as they have a hydrogen bond”. This error could be caused by the failure of students to consider other intermolecular forces, as the hydrogen bond is the strongest intermolecular bond. Apart from this, some students (13.3% in the worksheets – see Table 7) stated that “while the maleic acid, which is among the carboxylic acids, involves the dipole–dipole interaction, the fumaric acid does not”; this could indicate students' confusion about the dipole–dipole interaction and the dipole moment.
Acidity and basicity in functional groups
Another finding of the study relates to misconceptions about the “electrophile” and “nucleophile”. For instance, while some of the students (16.7% in the worksheets – see Table 7) used the statement, “a carbon atom with a relatively positive charge” regarding the concept of the nucleophile, others (11.7% in the worksheets – see Table 7) used the statement, “electron-rich carbon atom in the iodoform compound”. In addition, the students (11.7% among the common concepts in the worksheets – see Table 7) defined the concept of nucleophiles as “structures showing a tendency to receive electrons”, “positive-end parts” and “electron-poor groups”. Considering these errors, it can be seen that the students confused the concept of the nucleophile with the concept of the electrophile. Similarly, considering the misconceptions of students regarding the electrophile, some of them (21.7% in the worksheets – see Table 7) gave the following definitions: “structures showing a tendency to give electrons”, “electron-rich molecules” and “negatively-charged parts”. Considering the findings from the concept maps, on the other hand, some of the students (10.0% in the concept maps – see Table 10) exhibited a misconception that “the group OH is electrophilic”; others (15.0% in the concept maps – see Table 10) had a misconception that “the maleic anhydride molecule being used in the formation of maleic acids is nucleophilic”. In this respect, the students were observed to have problems with both defining and discerning the processes of developing these concepts.Within the secondary school curriculum in Turkey, students are acquainted with the concepts of the nucleophile and the electrophile in organic chemistry for the first time in the 12th grade. Although the students in this case realized the properties of these two concepts in the reactions of displacement and participation on the theoretical level, as well as in a few examples, they were unable to learn the reaction mechanism and its functions and properties in detail. At the university level, on the other hand, the students were taught the concepts of the nucleophile and the electrophile in the organic chemistry course on the basis of reaction mechanisms. However, the past misunderstanding of students affected their subsequent learning. Moreover, students may fail to sufficiently understand these concepts due to the fact that the substances of the nucleophile and the electrophile are associated with the concepts of acid and base, and this relation cannot be transferred from previous learning experiences. Considering the findings derived from the worksheets, it can be observed that the level of sufficient understanding of these concepts was very low (See Table 6), which could be caused by the failure of students to precisely understand the concepts of acid–base at the level of general chemistry.
According to the related literature, the subjects of acidity and basicity are very important in understanding reaction mechanisms in organic chemistry; comprehending the behaviour of reactive substances in a reaction; estimating the products in chemical reaction and understanding the concepts of the nucleophile and the electrophile (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Fishback, 2010; McClary, 2010). As a result of interviews conducted with 14 students, Cartrette and Mayo (2011) identified the relationship between the concepts of acid and base and the concepts of the nucleophile and the electrophile in organic chemistry, as well as the levels of understanding of students. Although some of the students could explain the concepts of the nucleophile and the electrophile, they remained unable to explain the relationship of these to acids and bases. Similarly, the students also had difficulties in determining the nucleophile and electrophile substances in organic chemistry reactions. According to Ferguson and Bodner (2008), students are unable to estimate the flow of electrons during reaction mechanisms in solving problems about reaction mechanisms; they contend that this is caused by students' deficient conceptual understanding regarding acidity, acidity strength and acidity constant. In their qualitative investigation with university students in an organic chemistry course, Cruz-Ramirez de Arellano and Towns (2014) observed that students confused the concepts of base and/or nucleophile and were unable to determine the strengths of basic and/or nucleophilic substances such as iodide and methanol or the strengths of basic and/or electrophilic substances. Therefore, the students could not determine how to assess electron-deficient and electron-rich areas in molecules. This inability was associated with their failure to understand bonds and molecular polarity. In this respect, McClary (2010) points out that students need to have a deeper conceptual understanding regarding acids and bases in order to understand the relationship between the nucleophilic and basicity.
Similarly, Anderson (2009) reported that university students interpreted the concepts of the nucleophile and the electrophile in reactions as a result of activities that were performed to develop their mechanistic problem-solving skills, and they were able to achieve conceptual understanding as a result of this process. Thus, it can be concluded that it is important to clearly present the subjects of acid and base types and properties, as well as acidity and basicity strengths, in order for students to understand the concepts of the nucleophile and the electrophile. It is also recommend that the concepts of acid and base be taught through various learning techniques by associating them with the concepts of the nucleophile and the electrophile with respect to the subject of reaction mechanisms.
Reduction and oxidation in functional groups
Another important finding of this study concerns the concepts of “oxidation”, “reduction”, “oxidizer” and “reducer”. It was observed that the students had a partial understanding in explaining these concepts in general (see Table 6). They also had misconceptions regarding these concepts, which is highly interesting. Examining Table 7, it can be seen that students confused the definitions of an oxidizer and a reducer through statements such as “atoms receiving electrons” and “a structure that is reduced by receiving hydrogen” for the concept of the “reducer” (20.0% in the worksheets); and the statements “atoms giving electrons” and “a structure that is oxidized by giving hydrogen” for the concept of the “oxidizer” (20.0% in the worksheets). This suggests that the students had a problem with both defining and discriminating in the process of developing these concepts. In addition, considering the concept maps, it can be seen that the students encountered difficulty in explaining the products of oxidation and reduction in the functional groups. For instance, a portion of the students (5.0% in the concept maps – see Table 10) had the misconception that “when all the alcohols are oxidized, a carboxylic acid will be formed”.What is remarkable here is that students were not able to soundly comprehend the oxidation products of primary, secondary and tertiary alcohols, which are among the mono-alcohols. This could be caused by their generalization of alcohol oxidation, as excessive generalization in the process of developing concepts may lead to errors. Another misconception regarding this subject is illustrated by the statements of some of the students (5.0% in the concept maps – see Table 10) that “esters are formed when ketones are oxidized”. This misconception may be caused by the fact that students consider the oxidation of a secondary alcohol, which is the reduction product of ketone, to be similar to the oxidation of a primary alcohol. Apart from this, while some of the students (11.7% in the concept maps – see Table 10) evidenced a misconception that “diols are formed when ketones are oxidized”, others (6.7% in the concept maps – see Table 10) had the misconception that “ketones are formed when polyalcohols are oxidized”. Here, the students confused the oxidation and reduction products of ketone with diol and polyalcohol, instead of mono-alcohol.
Due to these related misconceptions, it may be asserted that students primarily have difficulties in understanding the subject of oxidation and reduction. Similarly, it is reported in the literature that students have very low levels of understanding of the subject, as well as various misconceptions (Garnett and Treagust, 1992; Sanger and Greenbowe, 1997; Lin et al., 2002; Childs and Sheehan, 2009; Silverstein, 2011). When studying organic chemistry, students are taught about oxidation–reduction reactions and organic syntheses via functional groups such as alcohol, aldehyde, ketone, carboxylic acid and ester. When students encounter the subject of oxidation and reduction, which they often do not understand soundly in their general chemistry courses, as well as in their organic chemistry courses, they try to establish a relationship between these concepts via organic molecules. The resulting deficiencies and/or errors regarding the development of relevant concepts in their prior learning affect their subsequent learning experiences as well (Palmer, 2001).
According to the findings of the present study, the students had difficulties in understanding the oxidation and reduction reactions of functional groups, as well as the concepts encountered during the reactions. Anselme (1997) suggests that it has been difficult for students to understand the concepts of oxidation and reduction in organic chemistry for years, and that these concepts occasionally become traumatic experiences. In addition, students fail to gain conceptual understanding due to their failure to transfer these concepts to their courses in organic chemistry. Various scholars have suggested different approaches to increase students' conceptual understanding (Shibley et al., 2010). Menzek (2002) suggested a new approach in teaching the concepts of oxidation and reduction in organic chemistry reactions. According to this approach, the oxidation and reduction of compounds in organic chemistry can be easily understood by assigning average oxidation numbers to carbon atoms in small or conjugate organic molecules. Other studies have emphasized the importance of providing macroscopic, submicroscopic, and symbolic representations in order to increase the conceptual understanding in oxidation–reduction reactions (Nieves et al., 2012; Brandriet and Bretz, 2014). In line with all these studies, it can be argued that it is necessary to use alternative methods in order to develop better understanding of the conceptual process of oxidation–reduction reactions.
Stereoisomerism and structural isomerism in functional groups
Some of the other remarkable findings acquired from the concept maps and worksheets include the students' failure to understand the subject of stereoisomers in functional groups. Considering the findings acquired from the worksheets, it can be seen that the students had very low levels of understanding of the concepts of “chiral”, “enantiomer”, “diastereomer” and “optical isomer”; in fact, a great number of students failed to provide definitions for these concepts. The students also demonstrated a number of misconceptions regarding these concepts (see Table 6). For instance, some of the students (11.7% in the worksheets) applied the statement “structures with conflicting mirror images” to the concept of “chiral”, confusing it with the concept of “achiral” (see Table 7). A similar finding could also be found in the concept maps (see Table 10). In teaching the concept of chiral, the verbal use of definitions like “conflicting with mirror images” and “structures that don't conflict” may cause confusion and a failure to soundly understand the subject, as students may be unable to visualize the concept in this way. Another error regarding the concept of “chiral” relates to the following statement: “If four different atoms are bonded to the carbon atom, then it is a chiral”. A similar error was found in the study of Bryan (2007), where he investigated the misconceptions of second-year university students in organic chemistry. In his study, the students revealed the following error: “In determining the presence of chiral carbon atoms, look for carbon atoms that are bonded to four different atoms”.A further misconception was observed in this case in the concept of the “optical isomer”; namely, a portion of the students (10.0% in the worksheets) applied the statement, “maleic acid and fumaric acid that display a geometrical isomer are also the optical isomers of each other” with respect to the maleic and fumaric acid molecules within the carboxyl group. In addition, the error, “a type of isomer formed by molecules with a plane of symmetry” was also encountered in the students' definition of the concept of the “optical isomer”. Likewise, in his study on symmetry and chirality, Klein (1999) suggested that some school books include the following error: “a molecule is required to be an asymmetric molecule in order to be optically active”. Considering these errors, students mistakenly believe that molecules primarily displaying a geometrical isomer may also display an optical isomer. Furthermore, they also believe that there is a plane of symmetry for molecules with an optical isomer, which could be yet another indicator of their difficulty regarding the concepts of chirality and achirality. This condition may be caused by difficulties in visualizing the mirror images of the structures of maleic acid and fumaric acid, as well as their failure to soundly understanding the concepts of the optical isomer and chirals. According to Hassan et al. (2004), students are unable to visualize the concept of the optical isomer, which is due to their failure to perceive the molecules three-dimensionally.
In terms of functional groups, one of the most remarkable findings regarding stereoisomerism is that a number of the students (35.0% in the worksheets – see Table 6) were unable to provide definitions or relations concerning the concept of the “enantiomer” and the concept of the “diastereomer” (41.7% in the worksheets – see Table 7). Moreover, with respect to the concept maps, it can be seen that a number of students confused the concepts of the “enantiomer” and the “diastereomer” (see Table 10). This suggests that students had difficulties with the process of defining, which one of the processes in developing conceptual understanding. Considering their misconceptions, on the other hand, some of the students (6.7% in the worksheets – see Table 7) made the following errors regarding the concept of the “enantiomer”: “An isomer originating from the cis–trans property” and “maleic acid is the enantiomer of the fumaric acid”. Here, the students appeared to believe that molecules displaying the cis–trans isomer are also enantiomers; this may be due to their failure to visualize the organic molecules on a spatial ground. To address this issue, Harrison and Treagust (2000) emphasize the necessity to develop multiple mental models in order to enable students to imagine the three-dimensional structures of organic molecules, and thus to understand the concept of the enantiomer. Written, physical and computer-aided three dimensional models were applied in their study, enabling the students to make interpretations. Taking an alternative approach, in her study on stereochemistry, Strange de Soria (2001) asked students to draw sample molecules with a chiral and achiral atom in order to determine how they understood the molecular structures and achieved their conceptual understandings. She also asked them about the enantiomer and diastereomer structures of molecules. She then observed the methods (e.g. the Spartan computer modeling program, dimensional analysis notation, physical molecular model kits, Fisher Projection, Newman Projection, and Sawhorse Projection) that were used by the students in the process of learning.
A further misconception observed in the concept maps was the statement: “An isomer containing double bonds is a geometrical isomer”. The fact that several of the students (16.7% in the concept maps – see Table 10) have such a misconception could be associated with the fact that they had only been taught using sample molecules containing double bonds in a geometrical isomer. When students encounter such examples in school books, this may lead to incorrect learning, causing them to make the generalization that the concept of the geometrical isomer exists in molecules with double bonds. However, the geometrical isomer can also be realized around a single bond. In this sense, Hassan et al. (2004) reported that a great majority of students in their study had difficulty in finding the geometric isomers of molecules.
It is frequently reported in the literature that students have difficulties in understanding the subject of stereoisomerism (Hassan et al., 2004; Duffy, 2006; Bryan, 2007; Childs and Sheehan, 2009; Duis, 2011). Cruz-Ramirez de Arellano and Towns (2014) and Rushton et al. (2008) consider stereochemistry, which involves stereoisomerism and the three-dimensional structure of molecules, to also be among the subjects where misconceptions are frequently encountered in organic chemistry. In this sense, they believe that stereochemistry is a very important and compelling subject in understanding both isomery and reaction mechanisms. If students improperly assimilate the relevant concepts, it will be difficult for them to understand these concepts in the future. Thus, it is necessary for them to understand the molecular structures in order to comprehend the concepts related to stereoisomerism.
To improve comprehension in this area, researchers have emphasized the positive effects of developing the ability of spatial visualisation (e.g.Collins and Easdon, 2001; Lujan-Upton, 2001; Strange de Soria, 2001; Duffy, 2006), because, conceptual understanding requires students to visualize the three-dimensional shapes of organic compounds. Gaddis (2001) and Abraham et al. (2010) similarly emphasize the importance of computer simulations in achieving conceptual understanding in stereochemistry. Dori and Barak (2001), on the other hand, suggest the use of concrete and visual models in order to increase the comprehension of the concept of isomery in functional groups.
Another misconception of students observed in the concept maps relates to “structural isomer”. A significant proportion of the students (18.3% in the concept maps – see Table 10) suggested that “primary, secondary and tertiary alcohols are the structural isomers of each other”. This misconception may be caused by students' consideration of chain branching isomerism, which is a type of structural isomer. It can be inferred here that the students considered the primary, secondary and tertiary alcohols to be the branched forms of one another, and thus they believed that they are structural isomers of each other. Furthermore, all of the aforementioned alcohols are mono-alcohols. By generalizing about mono-alcohols, students may believe that these molecules have the same structure, and, thus, that they are structural isomers of each other. A further misconception based on students' generalizations was the statement that “dialcohols have structural isomer with ethers”. Some of the students (6.7% in the concept maps – see Table 10) evidenced the misconception that aside from mono-alcohols, alcohols also have structural isomers with ethers, which implies that the students considered every alcohol type to have structural isomers with ethers. This is supported by Hassan et al. (2004), who asked students about the structural isomer of ethyl, methyl and ether molecules and determined that only 33% of students gave correct responses and thus had difficulty with these concepts. Similarly, Schmidt (1992) determined that students had misconceptions regarding the structural isomer of functional groups.
Aromaticity and aliphaticity in functional groups
Some of the other misconceptions encountered in the students concerned “aromaticity” and “aliphaticity”. It was observed that the students had only a partial understanding regarding these concepts, and 11.7% of them were unable to give a response to the concept of “aliphatic”, while 6.7% were unsure of the concept of “aromatic” (see Table 6). With respect to the errors of students regarding the concept of “aliphatic”, the statement that “aldehydes and ketones are aliphatic compounds” was expressed by some of the students (10.0% in the worksheets – see Table 7). In addition, the concept maps show that a considerable number of students (21.7%) made this error. This could be caused by the failure of students to consider functional groups like aldehydes and ketones as aromatic groups, as well as lack of experience with examples of aromatic aldehydes and ketones during their lessons. The misconception regarding “aromaticity” is shown in the statement of some students (11.7% among the common concepts in the worksheets – see Table 7) that “cyclical structures are aromatic compounds”. This error indicates that students perceive organic compounds with a functional group in every cyclical structure as aromatic. In this sense, it may be asserted that the students confused the concept of “aromatic” with the concept of “cyclo”. In addition, the students were observed to fail to comprehend that free electrons moving in a cyclical structure form an aromatic structure.In a study that was conducted with high school and university students using a descriptive survey method, Topal et al. (2007) examined the misconceptions of students regarding aromaticity. The most remarkable misconception found in their study is “every cyclic compound is aromatic”. Their misconception is supported by the misconception seen in the present study. Similarly, as a result of their study on how students classified organic compounds, Domin et al. (2008) determined that students mainly used the concept of “cyclic” in their definitions regarding the concept of aromatic.
There are various other studies on the understanding of students regarding the concept of aromaticity in the literature arguing that students have difficulties and various misconceptions regarding this subject (Gaddis, 2001; Duffy, 2006; Ealy and Hermanson, 2006; Rushton et al., 2008; Fishback, 2010). For example, as a result of quizzes that were prepared for their study, Ealy and Hermanson (2006) determined that students did not have sufficient information about the concept of aromaticity, which could be associated with their failure to apply information learned in general chemistry about the placement of electrons, as well as the placement of electrons in bonds and hybridization according to the law of octet, to aromatic compounds.
Kuwajima (1984) emphasizes the importance of having sufficient knowledge about the valence bond theory encountered in general chemistry in order to soundly understand the concept of aromaticity. These results indicate that the students failed to develop their comprehension of the concept of aromaticity and that there were related misconceptions. In her study on the understanding of students regarding aromaticity and electrophilic aromatic substitution reactions, Duffy (2006) underlines the necessity of redefining their conceptual understanding. Accordingly, she asserts that how terminology is used is also important to students' conceptual understanding.
Other concepts encountered in functional groups
Finally, the findings demonstrate that a significant number of students failed to respond to concepts like “carbaldehyde”, “dimeric structure”, “oxyacide”, “decarboxylation”, “anhydride” and “lactone” in the functional groups (see Table 6). Examining their apparent misconceptions, problems were found in terms of the concepts of “oxyacide”, “decarboxylation” and “phenol”. For instance, with regard to the concept of “oxyacide”, the following misconception: “carboxylic acids containing oxygen atoms” were encountered in both the worksheets and the concept maps (10.0% in the worksheets and 18.3% in the concept maps – see Tables 7 and 10). In terms of the misconceptions regarding “decarboxylation”, the students (11.7% in the worksheets and in the concept maps) were seen to have the following misconception: “Decarboxylation is an event where the carboxyl group (–COOH) separates from the carboxylic acid” (see Tables 7 and 10). Apart from this, another misconception regarding “phenol” is demonstrated by the following statement of some students (26.7% in the concept maps and 6.7% in worksheets – see Tables 7 and 10): “Phenol is a cyclical structure of monoalcohols”. That is, they were unable to understand the nature of a phenol compound and confused it with alcohol. A number of students failed to respond to these concepts at all, which could be due to the fact that they first encounter them in organic chemistry, and thus they were unable to understand them after a relatively short time. Students encountering new concepts may not be able to correctly interpret the facts that are required to assimilate them, and consequently, they may be unable to internalize the concepts. Memorizing these concepts may also lead to misconceptions, as with the study of Henderleiter et al. (2001) that found that students were unable to explain certain events and concepts and make interrelations, and thus they tried to learn through memorization alone.Conclusions
Considered in general terms, this study is a pioneer in the field, as there are only a limited number of misconceptions regarding functional groups that have been established in the existing literature. Evaluating all the results, it has been established that undergraduate students following the Organic Chemistry Laboratory Course had low levels of conceptual understanding regarding functional groups, as well as a considerable number of misconceptions, in spite of just having completed a course in organic chemistry. This result could be primarily associated with their deficient and erroneous knowledge of general chemistry, as is indicated in the title of this paper. Since students have been unable to fully learn subjects related to general chemistry such as intramolecular and intermolecular bonds, acidity and basicity, oxidation and reduction and determination of molecular structures, they fail to accurately transfer their knowledge to organic chemistry. Thus, they have difficulties in understanding and explaining concepts regarding functional groups. Indeed, the American Chemical Society (ACS) considers bonds, molecular structure and reactivity, stereochemistry, Lewis and Brønsted acidity and basicity among the basic subjects necessary to understand both functional groups and organic chemistry in general (as cited in Duis, 2011).Likewise, as a result of interviews conducted in her study, Wasacz (2010) contended that the achievements of students in organic chemistry are associated with their achievements in general chemistry. Therefore, she underscores the necessity for instructors to review information related to general chemistry with students before beginning instruction in organic chemistry. This approach, she argues, may enable students to reach the level of relevant comprehension and affect their attitudes towards organic chemistry in a positive manner.
At this point in time, it should be possible to examine the principal subjects being taught in general chemistry at the university in previous years and emerging in the study in detail and support them with different teaching activities. Also, in organic chemistry lessons including the subject of functional groups, it should now be possible to apply assessments – evaluations questioning the extent of knowledge of the principle topics in chemistry – in order to facilitate the learning of organic chemistry. According to the results of these assessments, lesson plans and the learning goals of organic chemistry lessons could be reformulated. This would remove the deficiencies and mistakes by determining students' levels of conceptual understanding in advance. In addition to this, these deficiencies, could be specifically targeted in introductory organic chemistry lessons within the curriculum.
In addition to poor understanding of the concepts of general chemistry, inappropriate teaching strategies used by instructors may also generate misconceptions. To counter this issue, it is suggested that teachers should create an active learning environment that enables students to develop their conceptual understanding. For instance, it is recommended that students be asked to perform various experiments and activities in laboratories in order to explore concepts regarding functional groups, explain these concepts and adapt them into different situations. The inclusion of activities that increase critical thinking, reasoning and spatial thinking abilities may lead to a more effective understanding of the functional groups in organic chemistry. As such, the researchers emphasize the importance of using three-dimensional visual representations such as animation effects and molecular modelling in the representation of organic molecules (as with Fleming et al., 2000; Gaddis, 2001; Bhattacharyya, 2004), as students first need to visualize the particles and manipulate them in their minds in order to understand the dynamics of the processes.
Furthermore, it is important to encourage different ways of thinking in students in order to enable them to understand concepts regarding functional groups. Thus, the use of symbolic, microscopic and submicroscopic representations, as well as terminological discussions, is recommended. The ‘terminological’ level in chemistry refers to both chemical vocabulary and the uses of common language in the scientific sense. This use of “multi-relational representations” provides a way of scientific thinking and communicating that develops an understanding of functional groups. Mandl and Levin (1989) assert that the recognition of concepts via language-based communication such as words, statements and texts alone places a burden on the mental coding system, whereas pictorial representations are coded as images as well as also enabling verbal coding.
With this in mind, both language-based communication and pictorial representations may enable students to understand the concepts regarding functional groups more effectively within the scope of their interaction. Moreover, examining the results further under the headings of intermolecular bonds, acidity and basicity, reduction and oxidation, stereoisomerism and structural isomerism, it may be further asserted that students are not able to thoroughly learn the inorganic systems that explain the behaviours of atoms, ions and molecules in reality. As McMurry (2000) asserts, “The same principles that explain the simplest inorganic systems also explain the most complex organic ones” (p. 2). Thus, it is very important to also understand the inorganic systems that explain the behaviours of atoms, ions and molecules and the extent to which they are learned in understanding the phenomena of organic chemistry. At this point, emphasis should be placed on some of the topics being taught in Inorganic Chemistry at the university in previous years in order to support future lessons in Organic Chemistry. For instance, we could conduct more effective teaching in valence bond theory, resonance and hybridization in order to enable the students to understand the aromatic compounds; symmetry in molecular structures to understand stereoisomers and structural isomerism; and interparticular attractions to understand the physical properties of functional group compounds.
Rather than improving the levels of conceptual understanding of undergraduate students regarding functional groups and removing the misconceptions, this case study is aimed at revealing the present condition of this knowledge. It also approaches all the functional groups. Future studies could thus be conducted on how to improve students' conceptual understanding by using the results found here.
Appendix
Fig. 2 and 3.References
- Abraham M. R., Williamson V. M. and Westbrook S. L., (1994), A cross-age study of the understanding of five concepts, J. Res. Sci. Teach., 31(2), 147–165.
- Abraham M., Varghese V. and Tang H., (2010), Using molecular representations to aid student understanding of stereochemical concepts, J. Chem. Educ., 87(12), 1425–1429.
- Anderson J. P., (2009), Learning the language of organic chemistry: how do students develop reaction mechanism problem-solving skills? Doctoral dissertation, Purdue University, West Lafayette, Indiana.
- Anselme, J.-P., (1997), Understanding oxidation–reduction in organic chemistry, J. Chem. Educ., 74(1), 69–72.
- Ausubel D. P., (1968), Educational psychology: a cognitive view, New York: Holt, Rinehart and Winston.
- Bauer R. C., (1998), Teaching innovation in organic chemistry: an inquiry into what happens when the lecturer stops lecturing, Doctoral dissertation, Science Education, Purdue University, West Lafayette, Indiana.
- Bhattacharyya G., (2004), A recovering organic chemist's attempts at self-realization: how students learn to solve organic synthesis problems, Doctoral dissertation, Purdue University, West Lafayette, Indiana.
- Bhattacharyya G. and Bodner G. M., (2005), It gets me to the product: how students propose organic mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Birk J. and Kurtz M., (1999), Effect of experience on retention and elimination of misconception about molecular structure, J. Chem. Educ., 76(1), 124–128.
- Bodner G. M., (1991), A view from chemistry, in Smith M. U. (ed.), Toward a unified theory of problem solving: views from the content domains, Hillsdale, NJ: Lawrence Erlbaum Associates, pp. 21–34.
- Brandriet A. J. and Bretz S. L., (2014), The development of the redox concept inventory as a measure of students' symbolic and particulate redox understandings and confidence, J. Chem. Educ., 91(8), 1132–1144.
- Bryan L. C. H., (2007), Identifying students' misconceptions in ‘a-level’ organic chemistry, http://conference.crpp.nie.edu.sg/2007/paper/papers/SCI352.pdf, accessed 12 August 2014.
- Cartrette D. P. and Mayo P. M., (2011), Students' understanding of acids/bases in organic chemistry contexts, Chem. Educ. Res. Pract., 12(1), 29–39.
- Case J. M. and Fraser D. M., (1999), An investigation into chemical engineering students' understanding of mole and the use of concrete activities to promote conceptual change, Int. J. Sci. Educ., 21(12), 1237–1249.
- Childs P. E. and Sheehan M., (2009), What's difficult about chemistry? An Irish perspective, Chem. Educ. Res. Pract., 10, 204–218.
- Collins M. J. and Easdon J., (2001), Demonstrating chirality: using a mirror with physical models to show non-superimposability of chiral molecules with their mirror images, J. Chem. Educ., 78(11), 1484–1485.
- Coştu B., Ayas A. and Niaz M., (2010), Promoting conceptual change in first year students' understanding of evaporation, Chem. Educ. Res. Pract., 11(1), 5–16.
- Cruz-Ramirez de Arellano D. and Towns M. H., (2014), Students' understanding of alkyl halide reactions in undergraduate organic chemistry, Chem. Educ. Res. Pract., 15, 501–515.
- Çalık M., (2005), A cross-age study of different perspectives in solution chemistry from junior to senior high school, Int. J. Sci. Math. Educ., 3, 671–696.
- Deci E. L., Vallerand R. J., Pelletier L. G. and Ryan R. M., (1991), Motivation and education: the self-determination perspective, J. Educ. Psychol., 26(3), 325–346.
- Domin D. S., Al-Masum M. and Mensah J., (2008), Students' categorizations of organic compounds, Chem. Educ. Res. Pract., 9(2), 114–121.
- Dori Y. J. and Barak M., (2001), Virtual and physical molecular modeling: fostering model perception and spatial understanding, J. Educ. Tech. Soc., 4(1), 61–74.
- Driver R. and Easley J., (1978), Pupils and paradigms: a review of the literature related to concept development in adolescent science students, Stud. Sci. Educ., 5, 61–84.
- Driver R. and Erickson G., (1983), Theories-in-action: some theoretical and empirical issues in the study of students' conceptual frameworks in science, Stud. Sci. Educ., 10, 37–60.
- Duis M. J., (2011), Organic chemistry educators' perspectives on fundamental concepts and misconceptions: an exploratory study, J. Chem. Educ., 88(3), 346–350.
- Duffy A. M., (2006), Students' ways of understanding aromaticity and electrophilic aromatic substitution reactions, Doctoral dissertation, Mathematics and Science Education, University of California, San Diego.
- Ealy J. and Hermanson J., (2006), Molecular images in organic chemistry, J. Sci. Educ. Technol., 15(1). 59–68.
- Evans M. J., (2013), Development and analysis of educational technologies for a blended organic chemistry course, Doctoral dissertation, University of Illinois, Urbana-Champaign.
- Ferguson R. L., (2003), Investigating chemistry students' understanding of arrow-pushing formalism, Doctoral dissertation, Purdue University, West Lafayette, Indiana.
- Ferguson R. and Bodner G. M., (2008), Making sense of arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9, 102–113.
- Fishback V., (2010), Pedagogical content knowledge of organic chemistry mechanisms, Doctoral dissertation, University of Northern Colorado, Greeley, Colorado.
- Fleiss J. L., Levin B. and Paik M. C., (2003), Statistical methods for rates and proportions, 3rd edn, New York: John Wiley and Sons.
- Fleming S. A., Hart G. R. and Savage P. B., (2000), Molecular orbital animations for organic chemistry, J. Chem. Educ., 77(6), 790–793.
- Francisco, J. S., Nakhleh, M. B., Nurrenbern, S. C., and Miller, M. L., (2002), Assessing student understanding of general chemistry with concept mapping, J. Chem. Educ., 79(2), 248–257.
- Gaddis B., (2001), Conceptual change in an organic chemistry laboratory: a comparison of computer simulations and traditional laboratory experiments, Doctoral dissertation, University of Colarodo, Denver.
- Galley W. C., (2004), Exothermic bond breaking: a persistent misconception, J. Chem. Educ., 81, 523.
- Garnett P. J. and Treagust D. F., (1992), Conceptual difficulties experienced by senior high school students of electrochemistry: electric circuits and oxidation–reduction equations, J. Res. Sci. Teach., 29(2), 121–142.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: how do students deal with the invisible? Chem. Educ. Res. Pract., 16, 9–21.
- Grove N. P., Hershberger J. W. and Bretz S. L., (2008), Impact of a spiral organic curriculum on student attrition and learning, Chem. Educ. Res. Pract., 9, 157–162.
- Harrison A. G. and Treagust D. F., (1996), Secondary students' mental models of atoms and molecules: implications for teaching chemistry, Sci. Educ., 80(5), 509–534.
- Harrison A. G. and Treagust D. F., (2000), Learning about atoms, molecules, and chemical bonds: a case study of multiple-model use in grade 11 chemistry, Sci. Educ., 84(3), 352–381.
- Hassan A., Hill R. and Reid N., (2004), Ideas underpinning success in an introductory course in organic chemistry, Univ. Chem. Educ., 8(2), 40–51.
- Henderleiter J., Smart R., Anderson J. and Elian O., (2001), How do organic chemistry students understand and apply hydrogen bonding? J. Chem. Educ., 78(8), 1126–1130.
- Jennings D., (2012), Concept maps for assessment. UCD Teaching and Learning. http://www.ucd.ie/t4cms/UCDTLA0040.pdf, accessed 24 September 2014.
- Klein D. R., (1999), Symmetry, photolithography, and curriculum development: a fresh perspective, Doctoral dissertation, University of California, Los Angeles.
- Kozma R. B., Chin E., Russell J. and Marx N., (2000), The role of representations and tools in the chemistry laboratory and their implications for chemistry learning, J. Learn. Sci., 9(3), 105–144.
- Köseoğlu F. and Tümay H., (2013), Bilim eğitiminde yapılandırıcı paradigma (Teoriden öğretim uygulamalarına), [Constructivist paradigm in science education (From theory to teaching practices)], Ankara: Pegem A Publishing.
- Kuwajima S., (1984), Valence bond theory of aromaticity, J. Am. Chem. Soc., 106, 6496–6502.
- Lin H.-S., Yang T., Chiu H.-L. and Chou C.-Y., (2002), Students' difficulties in learning electrochemistry, Proc. Natl. Sci. Coun., 12(3), 100–105.
- Liu R. S. H., (2005), “You're repulsive!” – Teaching VSEPR in a not-so-elegant way, J. Chem. Educ., 82(4), 558–560.
- Lujan-Upton H., (2001), Introducing stereochemistry to non-science majors, J. Chem. Educ., 78, 475–477.
- Mandl H. and Levin J. R., (ed.), (1989), Knowledge acquisition from text and pictures, Amsterdam: Elsevier.
- McClary L. M., (2010), Of mental models, assumptions and heuristics: the case of acids and acid strength, Doctoral dissertation, Department of Chemistry and Biochemistry, The University of Arizona.
- McClure R. J. and Bell P. E., (1990), Effects of an environmental education related STS approach instruction on cognitive structures of pre-service science teachers, Pennsylvania State University, University Park, PA (ERIC Document Reproduction Service No. ED 341 582).
- McClure, J. R., Sonak, B. and Suen, H. K., (1999), Concept map assessment of classroom learning: reliability, validity and logistical practicality, J. Res. Sci. Teach., 36(4), 475–192.
- McMurry J., (2000), Organic Chemistry, 5th edn, Pacific Grove, CA, New York: Brooks/Cole Thomson Learning.
- Menzek A., (2002), A new approach to understanding oxidation–reduction of compounds in organic chemistry, J. Chem. Educ., 79(6), 700–702.
- Meyer P. G., (2005), A study of how precursor key concepts for organic chemistry success are understood by general chemistry students, Doctoral dissertation, Western Michigan University, Kalamazoo, Michigan.
- Miles M. B. and Huberman A. M., (1994), Qualitative data analysis, Thousand Oaks, CA: Sage.
- Mintzes J. J., Wandesee J. H. and Novak J. D., (1997), Teaching science for understanding, San Diego, CA: Academic Press.
- Nakhleh M. B., (1992), Why some students don't learn chemistry, J. Chem. Educ., 69(3). 191–196.
- Nakhleh M. B., (1993), Are our students conceptual thinkers or algorithmic problem solvers? Identifying conceptual students in general chemistry, J. Chem. Educ., 70(1), 52.
- Nakiboğlu C. and Ertem H., (2010), Comparison of the structural, relational and proposition accuracy scoring results of concept maps about atom, J. Turk. Sci. Educ., 7(3), 60–77.
- Nandagopal K., (2008), An expert performance approach to examining factors contributing to academic success in organic chemistry, Doctoral dissertation, Department of Pyschology, Florida State University, Tallahassee, Florida.
- Nicoll G., (2001), A report of undergraduates' bonding misconceptions, Int. J. Sci. Educ., 23(7), 707–730.
- Nieves E. L. O., Barreto R. and Medina Z., (2012), JCE classroom activity #111: redox reactions in three representations, J. Chem. Educ., 89(5), 643–645.
- O'Dwyer A. and Childs P. E., (2011), Second-level Irish pupils' and teachers' view of difficulties in organic chemistry, http://lsg.ucy.ac.cy/esera/e_book/base/ebook/strand10/ebook-esera2011_ODWYER-10.pdf, accessed 28 October 2014.
- Osborne M. and Freyberg P., (1985), Learning in science: implications of children's knowledge, Auckland, New Zealand: Heinemann.
- Othman J., Treagust D. F. and Chandrasegaran A. L., (2008), An investigation into the relationship between students' conceptions of the particulate nature of matter and their understanding of chemical bonding, Int. J. Sci. Educ., 30(11), 1531–1550.
- Pakhira D., (2012), Analysis of the effect of sequencing lecture and laboratory instruction on student learning and motivation towards learning chemistry in an organic chemistry lecture course, Doctoral dissertation, Chemistry and the Graduate Faculty of the University of Kansas.
- Palmer D. H., (1998), Measuring Contextual error in the diagnosis of alternative conceptions in science, Issues. Educ. Res., 8(1), 65–76.
- Palmer D., (2001), Students' alternative conceptions and scientifically acceptable conceptions about gravity, Int. J. Sci. Educ., 23(7), 691–706.
- Peterson R. and Treagust D., (1989), Grade – 12 students' misconceptions of covalent bonding and structure, J. Chem. Educ., 66(6), 459–460.
- Peterson R. F., Treagust D. F. and Garnett P., (1989), Development and application of a diagnostic instrument to evaluate grade-11 and -12 students' concepts of covalent bonding and structure following a course of instruction, J. Res. Sci. Teach., 26(4), 301–314.
- Piaget J., (1964), Development and learning, in Ripple R. and Rockcastle V. (ed.), Piaget rediscovered, Ithaca, NY: Cornell University Press, pp. 7–20.
- Pungente M. D. and Badger R. A., (2003), Teaching introductory organic chemistry: ‘Blooming’ beyond a simple taxonomy, J. Chem. Educ., 80(7), 779–784.
- Raker J. R., (2011), Connecting scientific research and classroom instruction: developing authentic problem sets for the undergraduate organic chemistry curriculum, Doctoral dissertation, Purdue University, West Lafayette, Indiana.
- Reid N. and Yang, M-J., (2002), The solving of problems in chemistry: the more open-ended problems, Res. Sci. Tech. Educ., 20(1), 83–96.
- Ross B. and Munby H., (1991), Concept mapping and misconceptions: a study of high-school students' understandings of acids and bases, Int. J. Sci. Educ., 13, 11–23.
- Ruiz-Primo M. A., (2004), Examining concept maps as an assessment tool, concept Maps: Theory, Methodology, http://cmc.ihmc.us/papers/cmc2004-036.pdf, accessed 25 November 2014.
- Rushton G. T., Hardy R. C., Gwaltney K. P. and Lewis S. E., (2008), Alternative conceptions of organic chemistry topics among fourth year chemistry students, Chem. Educ. Res. Pract., 9, 122–130.
- Sanger M. J., (2005), Evaluating students' conceptual understanding of balanced equations and stoichiometric ratios using a particulate drawing, J. Chem. Educ., 82, 131–134.
- Sanger M. J. and Greenbowe T. J., (1997), Common student misconceptions in electrochemistry: galvanic, electrolytic, and concentration cells, J. Res. Sci. Teach., 34(4), 377–398.
- Schmidt H. J., (1992), Conceptual difficulties with isomerism, J. Res. Sci. Teach., 29, 995–1003.
- Schroeder J. D., (2008), Implementing the science writing heuristic laboratory report format in the undergraduate organic chemistry laboratory, Doctoral dissertation, Iowa State University, Ames, Iowa.
- Schwartz L. D., (1993), The construction and analogical transfer of symbolic visualizations, J. Res. Sci. Teach., 30(10), 1309–1325.
- Shatila A., (2007), Assessing the impact of integrating POGIL in elementary organic chemistry, Doctoral dissertation, The University of Southern Mississippi.
- Shibley I. A., Amaral K. E., Aurentz D. and Mccaully R. J., (2010), Oxidation and reduction reactions in organic chemistry, J. Chem. Educ., 87(12), 1351–1354.
- Silverstein T. P., (2011), Oxidation and reduction: too many definitions? J. Chem. Educ., 88, 279–281.
- Strange de Soria L. E., (2001), The methods that college students use to answer questions about stereochemistry involving spatial ability, Doctoral dissertation, The Department of Middle-secondary Education and Instructional Technology, Georgia State University.
- Taagepera M. and Noori S., (2000), Mapping students' thinking patterns in learning organic chemistry by the use of knowledge space theory, J. Chem. Educ., 77(9), 1224–1229.
- Taagepera M., Arasasingham R., Potter F., Soroudi A. and Lam G., (2002), Following the development of the bonding concept using knowledge space theory, J. Chem. Educ., 79, 756–762.
- Taber K. S., (2001), Building the structural concepts of chemistry: some considerations from educational research, Chem. Educ.: Res. Pract. Eur., 2(2), 123–158.
- Taber K. S., (2014), Ethical considerations of chemistry education research involving ‘human subjects', Chem. Educ. Res. Pract., 15, 109–113.
- Taber, K. S. and Coll R. K., (2002), Chemical bonding, in Gilbert J. K., de Jong O., Justi R., Treagust D. F. and Van Driel J. H. (ed.), Chemical Education: Research-based Practice, Dordrecht: Kluwer Academic Publishers BV, pp. 213–234.
- Topal G., Oral B. and Özden M., (2007), University and secondary school students misconceptions about the concept of “aromaticity” in organic chemistry, Int. J. Environ. Sci. Educ., 2(4), 135–143.
- Ülgen G., (2004), Kavram geliştirme: Kuramlar ve uygulamalar [Concept development: Theories and practices], 4th edn, Ankara: Nobel Publishing.
- Ünal S., Coştu B. and Ayas A., (2010), Secondary school students' misconceptions of covalent bonding, J. Turk. Sci. Educ., 7(2), 3–29.
- Vosniadou S. and Ioannides C., (1998), From conceptual development to science education: a psychological point of view, Int. J. Sci. Educ., 20, 1213–1230.
- Wasacz J. T., (2010), Organic chemistry preconceptions and their correlation to student success, Doctoral Dissertation, University of Northern Colorado.
- Yin Y., Vanides J., Ruiz-Primo M. A., Ayala C. C. and Shavelson R. J., (2005), Comparison of two concept mapping techniques: implication for scoring, interpretation and use, J. Res. Sci. Teach., 42(2), 166–184.
- Yong W., (1994), STS education and social organic chemistry, J. Chem. Educ., 71(6), 509–510.
| This journal is © The Royal Society of Chemistry 2016 |

![[X with combining overline]](https://www.rsc.org/images/entities/i_char_0058_0305.gif)