Creative exercises (CEs) in the biochemistry domain: an analysis of students' linking of chemical and biochemical concepts†
Abdi-Rizak M.
Warfa
* and
N.
Odowa
Department of Natural Sciences, Metropolitan State University, St. Paul, Minnesota 55106, USA. E-mail: AbdiRizak.Warfa@metrostate.edu
First published on 4th August 2015
Abstract
Creative exercises (CEs), a specific form of open-ended assessment tools, have been shown to promote students' linking of prior and newly learned concepts within a course. In this study, we examined how often students in an upper-division undergraduate biochemistry course linked prior chemical concepts to biochemical ones in response to CE prompts. Thematic analysis of participant responses showed students making in response to the CEs multiple connections between prior chemical concepts and biomolecule structure, thermodynamics and enzyme kinetics. In the case of thermodynamics and enzyme kinetics CEs, most students tended to rely on contexts and concepts focused on specific materials from their current course whereas responses to biomolecule structure CEs mostly invoked foundational concepts in acid–base and organic chemistry, such as pH/pKa, pI, ionization, stereochemistry, and organic functional groups. Invoking the cognitive resources activation framework in discussing the findings, we highlight the utility and relevance of CEs in upper division courses that rely on the application of prior chemical knowledge to explain new ones as well as the implications of the findings for research and teaching.
Introduction
Educational theories on learning often draw contrast between meaningful and rote learning, with the distinction between the two attributed to the learning outcomes associated with each, epistemological beliefs about knowledge acquisition, and the cognitive processes involved in knowledge construction (Ausubel, 2000; Mayer, 2002; Novak, 2002; Taber, 2014). In the most basic form, promoting meaningful learning requires the coordination and active linking of concepts learned in various settings in a coherent and meaningful manner. In chemistry, Taber (2014) specifically argues that instruction should help students view the subject as a set of linked concepts with explanatory and predictive power rather than an assembly of disjointed facts to be memorized. The author wrote, “Chemistry, as a science, is not primarily about isolated facts… “but about concepts that can be used to build extensive theoretical frameworks that offer explanatory value” (Taber, 2014, p. 9). Biochemistry, a subject concerned with the study of chemical processes in living things, illustrates Taber's point well. As an interdisciplinary subject, it requires the application of prior chemical concepts to explain how biological systems work. For example, students will need to apply foundational concepts in acid–base chemistry, thermodynamics and the nature of intermolecular forces as well as insights from organic chemistry to explain a host of biochemical topics such as protein structure and dynamics (Villafane et al., 2011a; Wolfson et al., 2014). That is, students need to use concepts they learned in prerequisite courses as theoretical frameworks that can explain biochemical behavior.In order to help students make links between foundational concepts and biochemical ideas, we need to better understand the contexts that promote making such links. To our knowledge, there is limited research in biochemistry that directly investigated this phenomenon. Much of the previous research in this area focused on uncovering students' incorrect chemical ideas brought to biochemistry (see Grayson et al., 2001; Minderhout and Loertscher, 2007; Villafane et al., 2011a, 2011b) (incorrect ideas or alternate conceptions/misconceptions, as used in this study, refer to ideas/concepts held by students but are inconsistent with scientifically accepted explanations). For example, Villafane et al. (2011a) proposed the use of 21 multiple-choice diagnostic instruments to identify students' incorrect ideas related to prior knowledge required for biochemistry, recommending the use of their instrument as a pre-post assessment tool to inform biochemistry instruction. Several other instruments were specifically designed to uncover student misconceptions/alternate conceptions in biochemistry, e.g., the Molecular Life Science Concept Inventory (MLS-CI; Howitt et al., 2008; Wright and Hamilton, 2008, 2011) and the Enzyme-Substrate Interactions Concept Inventory (ESICI; Bretz and Linenberg, 2012).
Multiple other studies, such as the work of Schönborn and Anderson (2009, 2010) and Linenberger and Bretz (2012), address student struggles with external representations in biochemistry or put their energy into curriculum reform efforts (see Minderhout and Loertscher, 2007; Loertscher and Minderhout, 2010). Loertscher and colleagues have similarly worked to identify threshold concepts in biochemistry (Loertscher et al., 2014). Other studies, such as the work of Wolfson et al. (2014), examine students' learning progressions from general chemistry to biochemistry. Wolfson and colleagues specifically used energy transformations in biological systems as foci for probing student conceptions of energy. In addition to examining the alternate concepts students held when solving energy problems in the context of biochemistry, these authors also examined the information students transferred from introductory courses to solve biochemically situated energy problems.
Our contribution to the existing literature arises from our close examination of the links students make between prior concepts and biochemical ones. Such examination is necessary if we are to view students' conceptual struggles as a failure to understand the interconnectedness of foundational concepts in prerequisite courses and those found in more advanced courses such as biochemistry. We also believe it is important to go beyond simple identification of students' incorrect ideas to understanding the underlying processes in which the incorrect ideas form and the contexts in which they are prompted. Understanding such processes requires close examination of the links students make between prior knowledge and their ability to use it in new contexts.
Because we were interested in understanding students' abilities to link prior chemical concepts and biochemical ones, we utilized Creative Exercises (Trigwell and Sleet, 1990; Lewis et al., 2010) as a mechanism to probe students' abilities to make such links. Creative Exercises (CEs) are open-ended assessment tools containing a single prompt in which students must develop a response. Lewis et al. (2010, 2011) describe the features of CEs as follows:
– students develop as many distinct, correct, and relevant statements to a single prompt
– the instructor decides the allowable maximum number of statements for receiving credit (e.g., in this study, students receive one point for each correct statement in a prompt, with a maximum of five correct statements resulting full credit)
– in order to receive credit, student responses must be relevant to the original prompt and distinct from other statements for which the student has received credit
– the instructor develops a scoring rubric with possible correct responses and a room for add-ons for additional correct answers per student responses
Given the large range of possible answers students can develop in response to a prompt, CEs are considered an open-ended assessment tool (Lewis et al., 2010). Unlike concept maps (Stoddart et al., 2000; Francisco et al., 2002), CEs do not require students to show a network of relationships between their statements but rather to develop a number of relations pertaining to the original prompt. That is, whereas concept maps are concerned with showing how concepts are connected to each other, with a possible central node or nodes in which ideas branch off (Francisco et al., 2002), CEs are mainly concerned with developing a list of concepts related to the original prompt. Consequently CEs have a simpler grading scheme than concept maps. Comparison of CEs with other traditional science assessments as well as examples of CEs from General Chemistry can be found in Lewis et al. (2010, 2011). These authors also examined the validity of CEs as an assessment tool and found them to be comparable to other traditional measures used to assess student achievement such as the American Chemical Society's (ACS) Standardized General Chemistry Exam and concept maps.
Our choosing of CEs in biochemistry was in large part influenced by the work of the Lewis group (Lewis et al., 2010; Ye and Lewis, 2014) on CEs in a General Chemistry classroom. Specifically, their body of work showed students made considerable interconnections between materials learned within the course. Given the prerequisite knowledge of foundational chemical concepts to explain biochemical problems, this work motivated us to utilize CEs as a mechanism to probe biochemistry students' ability to link prior chemical concepts to biochemical ones. It was our hope that by incentivizing students to link the concepts through the use of CEs, they will be able to see the inherent value of chemistry as a set of linked concepts with explanatory and predictive power (Taber, 2014; Ye and Lewis, 2014). Thus, the guiding research questions for the study were: (1) how often do biochemistry students link prior chemical concepts to biochemical ones in response to CE prompts? And, (2) what is the nature of students' linked chemical and biochemical concepts?
Theoretical framework
This study is grounded in the cognitive constructivist theory of learning (Ausubel et al., 1978; Bodner, 1986). In the cognitive constructivist perspective, meaningful learning occurs when students incorporate new knowledge into existing schemas and refine it to make sense of the new information (Bodner, 1986; Mayer, 2002; Ferguson, 2007). That is, students construct their own meaning of new knowledge by matching new information against existing knowledge and making necessary links between the prior and the new, and evaluating and re-evaluating their understanding (Ferguson, 2007). Consistent with this view of knowing and learning, CEs posit that students learn best when they are able to link previously learned concepts with newly presented information. Lewis et al. (2011) summarized CE assessment as the act of assessing students “based on their ability to connect and incorporate new course information with information that was presented previously in the course” (p. 159). Thus, because of the focus of mapping new information against existing knowledge in CEs, cognitive constructivism provided us a useful theoretical framework to probe student use of prior chemical concepts to explain biochemical ones.Study methods
Setting and data collection
This study was conducted at a primarily undergraduate university in the Midwestern United States. During the time of data collection there were roughly 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 attendees at this comprehensive urban university, with female attendees accounting for 56% of the student body and males 44%. Unique among its sister institutions, the university is home to a diverse student body (38% of the student body were students of color). Reflecting this make-up, 58% of the study participants were female and 42% male.
000 attendees at this comprehensive urban university, with female attendees accounting for 56% of the student body and males 44%. Unique among its sister institutions, the university is home to a diverse student body (38% of the student body were students of color). Reflecting this make-up, 58% of the study participants were female and 42% male.
We collected data for the study from upper division biochemistry classrooms (N = 48) that met twice a week for three-hour lecture/lab per day. The course was the first undergraduate biochemistry course the participants took. The required textbook for the course was Lehninger's Principle of Biochemistry (Nelson and Cox, 2013). However, during lecture students worked in small cooperative groups of 3–4 members using POGIL (process-oriented guided inquiry learning) reform materials called BioChemActivities. Using the learning cycle approach, the material was carefully designed to guide students to construct biochemical knowledge through discussion and active participation. For the interested reader, Loertscher and Minderhout's (2010) Foundations of Biochemistry provides equivalent problems in a commercially available workbook. The first of the BioChemActivities provided a review of organic chemistry concepts (e.g., functional groups, polarity) and basic general chemistry concepts (acid–base concepts, solubility, etc.) during the first week of classes.
We used a total of three CEs, listed in Table 1, to collect the data. The first of this, Structure of Amino Acids CE, contained a single prompt showing the structure of glutamic acid. The learning objectives assessed by this CE in the context of biochemistry included knowing the acid/base properties of biochemical compounds; generalizing the concept of a titration to biochemical measurements; identifying the type of intermolecular forces amino acids can potentially form; identifying the organic functional groups present in an amino acid side chain; and, knowing how protonating/deprotonating affects amino acid properties.
| Prompt items | Generalizable chemical concepts to biochemistry assessed |
|---|---|
| a The following instructions accompanied each prompt (see Appendix A): write as many correct, distinct, and relevant facts about [prompt shown]. Five statements will get you full credit for the problem. Recall the information you use should be information you learned in a chemistry course, including our current course. All other outside information, combined, will only count as one distinct fact towards the correct responses. | |
| Structure of amino acids CE |
• Acid–base properties of biochemical compounds
• Polarity, hydrophobicity • Titration • Intermolecular forces • Organic functional groups • Stereochemistry and chirality |
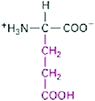
|
|
| Thermodynamics CE |
• Relating thermodynamic parameters (ΔG′°, ΔG, and Keq) to each other
• Understanding the thinking behind coupling energetically favorable and unfavorable reactions together • Identifying reaction types (hydrolysis, group transfer, etc.) |
|
Glucose + Pi → glucose-6-phosphate + H2O ΔG′° = 13.8 kJ mol−1
ATP + H2O → ADP + Pi ΔG′° = –30.5 kJ mol−1 Sum: glucose + ATP → glucose-6-phosphate + ADP |
|
| Enzyme kinetics CE |
• Using the concept of activation energy to describe catalysis
• Suggesting what the relationship might be between activation energy and the relative rates of the reactions depicted in the diagram • Describing the purposes and function of catalysts such as enzymes • Interpreting reaction coordinate diagram of un-catalyzed and enzyme-catalyzed reactions • Knowing how energy diagrams provide information about the overall change in energy |
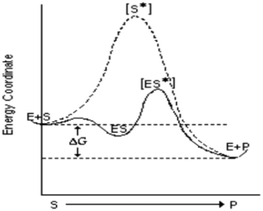
|
|
The second CE contained a thermodynamics topic – the phosphorylation reaction of glucose to glucose-6-phosphate coupled to ATP hydrolysis (Table 1). The glucose reaction is energetically unfavorable while the hydrolysis of ATP is energetically favorable. The main learning objective assessed by this CE, then, was for students to understand how coupling an energetically unfavorable reaction to an energetically favorable one drives product formation forward. In this case, students needed to generalize chemical concepts they learned in introductory courses to biochemical concepts – for instance, using ΔG′° values to predict whether a reaction is product or reactant-favored, relating thermodynamic parameters to each other, identifying reaction types present in the prompt, and the use of other possible foundational concepts.
The third and final CE showed a reaction coordinate diagram depicting enzyme-catalyzed and un-catalyzed reactions (see Table 1, enzyme kinetics CE). The learning objectives for this CE included using the concept of activation energy in the context of enzyme-catalyzed reactions, interpreting a reaction coordinate diagram and its elements, including any depictions of transition state species, and explaining the function and purposes of catalysts such as enzymes.
The underlying concepts in each CE were chemical ideas students learned in prerequisite foundational courses but generalized to biochemical problems – this particular biochemistry course required two semesters of general chemistry and one semester of organic chemistry as a prerequisite. For this reason we chose the specific CEs shown in Table 1 based on the extent in which they required the application of prior chemical concepts covered in the prerequisite introductory courses and not present in fresh unique biochemistry concepts. To become familiar with CE expectations, students completed before data collection ensued practice CEs based on a separate concept to those for which data was collected. Only in-class CEs were used as part of the data collection as their validity is established (Lewis et al., 2011). The first CE was administered as part of the first midterm exam and the second and third CEs as part of an optional 4th course exam. The lower participation number in CEs 2 and 3 reflects the number of students who chose not to take the exam or did not provide responses to the prompts. Before data collection ensued, the participants were informed about the purposes of the study and its voluntary nature. The human subjects' committee in our university has approved the study and consent forms were obtained from the participants.
Data analysis
To analyze the data, we first used the method of Lewis et al. (2011) and Ye and Lewis (2014) by developing a scoring rubric for each CE as shown in the ESI† for the structure of amino acids CE. Once there was an agreement on the scoring rubric, we did a practice scoring session as recommended by Lewis et al. (2011), grading independently a set of four student responses to the structure of amino acids CE. Following the dry-run session, and upon negotiation of any differences that arose, we each graded independently a set of twenty-two student responses to the structure of amino acids CE for 100% overlap on grading and without the knowledge of the scores assigned by the other grader (see Lewis et al., 2011, p. 161). Since the inter-rater reliability based on this grading was high (Cohen's kappa, κ = 0.86) and disagreements were easily negotiated, one author (AW) scored all the remaining CEs.Once all responses were graded, we carried out thematic analysis by pooling the responses around common themes found in chemistry and biochemistry textbooks (Ye and Lewis, 2014). For example, in response to the structure of amino acids CE, statements referring to “it [glutamic acid] will accept a proton; it will donate a proton” and “when in this form the pH is equal to its pI” were themed together under the common chemistry topic of “acid–base concepts.” Statements directly referring to titrations or buffer regions were more specifically organized under the common topic of “titration.” Similarly, statements pointing out functional groups in the prompt structure or invoked organic chemistry concepts such as “there is stereochemistry” or “the molecule is chiral” were put under the common topic of “organic chemistry concepts.” We created the theme “Structure–Activity–Relationship” for responses that made direct correlation between the structure shown in the prompt and its function – for example, student statements such as “when deprotonated, its side chain can participate in ionic interactions as well as hydrogen bonding” or “addition of another amino acids would form a peptide bond under the right conditions.” Similar thematic analysis was used for the other CEs. Responses in each CE were further coded for correctness (i.e., response is correct, incorrect, or irrelevant) as described by Ye and Lewis (2014) and using our scoring rubrics as guideline. Thus, tabulated data in the results' section list emerging themes by topic and the frequency of correctness for each theme.
Results
Participant responses, organized in terms of the most to least common, as well as the frequency of correct and incorrect statements in percentage are shown in Tables 2–4. Overall, our research findings indicate that students made considerable connections between foundational concepts in chemistry and biochemical ideas. With respect to the structure of amino acids and enzyme kinetics, students used various foundational concepts in response to the prompt questions whereas responses to the thermodynamics CEs were confined to traditional energy topics with limited interconnections with other chemical concepts. Responses to the enzyme kinetics CE revealed multiple student struggles with free energy diagrams and confusions about the relationship between energy, equilibrium, and kinetics. Specific findings in response to each CE are detailed in the following sub-sections.| Theme | Sample responses | Number selecting response (%) | Percent correct (%) | Percent incorrect (%) |
|---|---|---|---|---|
| a Given the small number of responses in these case, the frequencies of correct and incorrect statements were not tabulated. | ||||
| Acid–base concepts |
(i) “When in this form the pH is equal to its pI”
(ii) “It can lose two more protons (H+); it can gain a proton on the C-terminus end” (iii) “During ionization, COOH will deprotonate first as its pKa will always be smaller than NH3+” |
39 (81.3%) | 97.4 | 2.6 |
| Compound classification |
(i) “It is a polar molecule”
(ii) “This is classified as acidic due to the COOH group in its side chain” (iii) “Polar, acidic amino acid” |
36 (75.0%) | 91.7 | 8.3 |
| Structure identification |
(i) “This is glutamic acid”
(ii) “It is an amino acid” |
31 (64.6%) | 96.8 | 3.2 |
| Organic chemistry concepts |
(i) “It is chiral [molecule]”
(ii) “It has carboxylic acid functional group” |
26 (54.2%) | 100 | 0.0 |
| Structure activity relationship |
(i) “[it] can H-bond, giving it stronger force”
(ii) “When deprotonated, it's side chain can participate in ionic interactions…” (iii) “It can peptide bond to form a protein” |
19 (39.6%) | 78.9 | 10.5 |
| Titrationa |
(i) “It's titration curve has 3 buffer regions”
(ii) “There are 3 ‘bumps’ on the titration curve for this amino acid” |
6 (12.5%) | — | — |
| Structure-specific informationa | (i) “The N-terminus is NH3+; the C-terminus is COO−” | 4 (8.3%) | — | — |
| Theme | Sample responses | Number selecting response (%) | Percent correct (%) | Percent incorrect (%) |
|---|---|---|---|---|
| a Given the small number of responses in this case, the frequencies of correct and incorrect statements were not tabulated. | ||||
| Free energy |
(i) “ΔG° for coupled reaction is −16.7 kJ mol−1”
(ii) “ΔG° = −RT ln Keq; “ΔG° = −16.7; Keq = 845.27” (iii) “This is reaction is exergonic” (iv) “It uses exergonic reaction to drive an endergonic reaction” (v) “ΔG is Gibbs free energy” |
26 (92.9%) | 96.0 | 4.0 |
| Spontaneity and equilibrium |
(i) “Since ΔG° is negative, the reaction will be spontaneous”
(ii) “The hydrolysis of ATP is spontaneous” (iii) The coupled reaction proceeds forward and favors the products” (iv) “Keq for the ATP (reaction) and the coupled reaction is >1” |
22 (78.6%) | 95.0 | 5.0 |
| Reaction types |
(i) “The first reaction is a group transfer; the second reaction is hydrolysis”
(ii) “This is group transfer” |
17 (60.7%) | 76.5 | 23.5 |
| Glycolysis |
(i) “First step of glycolysis”
(ii) “This is the rate-limiting step in glycolysis” |
14 (50.0%) | 100 | 0.0 |
| Entropya | (i) “An increase in entropy occurs as a result of this reaction” | 1 (3.6%) | — | — |
| Theme | Sample responses | Number selecting response (%) | Percent correct (%) | Percent incorrect (%) |
|---|---|---|---|---|
| a Given the small number of responses in these cases, the frequencies of correct and incorrect statements were not tabulated. | ||||
| Activation energy |
(i) “The activation energy is lower with enzyme”
(ii) “The non-catalyzed reaction is exergonic but has high activation energy” (iii) “The enzyme lowers the activation energy required to form the product” |
20 (80.0%) | 100.0 | 0.0 |
| Free energy |
(i) “This reaction has a negative ΔG and releases energy”
(ii) “This reaction is exothermic; energy is released” (iii) “ΔG is the same for both reactions” (iv) “ΔG is negative since E + S starts at a higher energy than E + P end up” |
19 (76.0%) | 100.0 | 0.0 |
| Catalysis |
(i) “The enzyme is the catalyst”
(ii) “Reaction progresses much easier with enzyme present” (iii) “Substrate can react w/ or w/o enzyme” |
12 (48.0%) | 100.0 | 0.0 |
| Visual representation |
(i) “The solid line is a reaction that is much more favorable considering the ΔG that is presented between the dashed horizontal lines”
(ii) “This appears to show noncompetitive inhibition” (iii) “This reaction is a competitive inhibition.” |
12 (48.0%) | 50.0 | 50.0 |
| Kinetics |
(i) “With an enzyme, this reaction will proceed much faster”
(ii) “The enzyme increases the rate of the reaction” (iii) “Reaction is slower without enzyme” (iv) “The rate-limiting step of the catalyzed reaction is the formation of [ESa]” |
10 (40.01%) | 90.0 | 10.0 |
| Chemicala equilibrium |
(i) “This reaction proceeds forward and favors products”
(ii) “Product is favored in the reaction” |
3 (12.0%) | — | — |
| Entropya | (i) “The products are more disordered than the reactions” | 1 (3.6%) | — | — |
The structure of amino acids CE
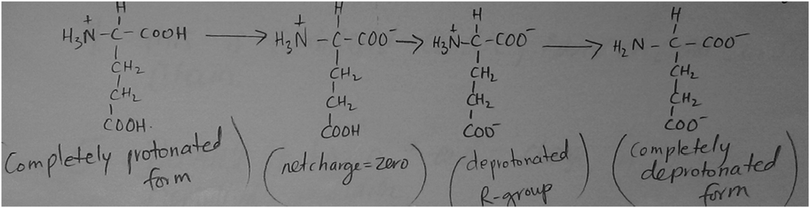
A total of 48 students completed the structure of amino acids CE. Their responses fell into one of seven broad themes based on the chemical concepts present in the responses. The themes and the frequency in which they occurred, summarized in Table 2, were (i) use of acid–base concepts (N = 39), (ii) compound classification (N = 36), (iii) structure identification (N = 31), (iv) use of organic chemistry concepts (N = 26), (v) structure activity relationship (N = 19), (vi) titration (N = 6), or (vii) use of structure specific information (N = 4). Findings within each theme are further described below.The most common student response in this CE, mentioned by 39 students, used foundational concepts in acid–base chemistry to describe the structure of glutamic acid. Specific references included statements indicating that the prompt structure can lose or accept proton(s) or that the pH of the shown structure will be equal to its pI (isoelectric point) in the form shown (see Table 2 for actual student responses). Some students drew the ionization steps of the compound, as shown in Fig. 1, or mathematically calculated the pI value of the structure. Others indicated that the molecule is in zwitterion form, with only one student erroneously indicating that the structure has an overall charge of +2. That is, in the responses included in the acid–base theme, students touched upon the concepts of pH/pKa, pI, and used various terms associated with acid–base chemistry such as ionization, protonation and deprotonation. This indicates that, when incentivized to do so, students use foundational concepts in chemistry to describe biochemical molecules. Unsurprisingly, student responses tended to rely on current context and concepts, often commenting on the pH–charge relationships of the shown structure. Nevertheless, students referred continuously to foundational acid–base concepts such as the ability of the molecule to accept or donate a proton or the relationship between the pH of the structure and its pKa. Moreover, as can be seen in Table 2, six students specifically described the titration behavior of the prompt structure. Students made comments such as “there will be 3 bumps on the titration curve of this amino acid” or “It's titration curve has three buffer regions,” This suggests students were using foundational concepts in chemistry to describe biomolecule structure and chemical behavior.
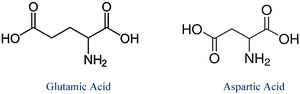
In the classification theme, 36 students (N = 36, Table 2) stated the molecule is polar or “this is acidic amino acid.” Some justified their claim by further stating that the structure shown is acidic due to the carboxyl group present in the side chain. We note this reflects material coverage in the course – students engaged early in the semester activities that tasked them to classify the naturally occurring twenty amino acids into polar, nonpolar, acidic or basic depending on the chemical properties of their side chains. Thus, the focus on “this is polar” or “this is acidic amino acid” is contextualized response. Three students incorrectly classified the structure as nonpolar, basic, and hydrophobic respectively. When not classifying the amino acid as polar or acidic, students simply identified the shown structure as that of glutamic acid or simply stated “this is an amino acid.” Responses from thirty-one students fit into the structure identification theme (see Table 2). Only one student misidentified the shown structure as that of aspartic acid (2.1%, Table 2), a reasonable mistake given that glutamic acid and aspartic acid differ only by one-methylene group (see Fig. 2).
Twenty-six students used organic chemistry insights in their response. These responses invoked stereochemistry, chirality and presence of carboxylic acid and amino functional groups to describe the prompt structure. Some commented on how the pKa's of the carboxyl moieties would be lower than that of the amino group; others noted the resonance features of the carboxyl functional groups. There was uniformity in students' use of organic chemistry insights, with most noting the different functional groups present in glutamic acid and few using chirality and other organic concepts in their response. The students were therefore, in our view, linking concepts covered in prerequisite courses to the prompt question or at least able to mention such concepts when prompted to do so.
Interestingly 19 students (Table 2) made direct correlation between the structure of glutamic acid and its function. We pooled such responses under the broad theme of Structure–Activity-Relationship, exemplified by the sample responses shown in Table 2. In one such response, a student wrote “when deprotonated, its [glutamic acid] side chain can participate in ionic interactions as well as hydrogen bonding.” Others mentioned that the structure would be water soluble, making connection between the shown structure and its chemical behavior in aqueous solutions. Another student wrote that “if this amino acid were to be put into a pH that is bigger than its pKa, it would deprotonate; if this amino acid was placed into a solution with pH less than its pKa(acidic solution), it would be unaffected.” This particular student is attempting to describe how the net charge of molecules with ionizable functional groups is dependent on pH conditions, with different net molecule charges expected at high pH vs. low pH. This response illustrates how students were attempting to make a generalization about when a structure is protonated, deprotonated, or remains unaffected by changes in the pH. The other responses illustrate student attempts to make connection between the structure and its ability to form hydrogen bonds, peptide bonds, or participate in electrostatic interactions. Two students incorrectly stated the structure is not soluble in an aqueous environment or that it cannot form a hydrogen bond.
Finally, in response to the structure of amino acids CE, a smaller number of students (N = 4, Table 2) provided structure-specific information that either labeled the N-terminus and the C-terminus of the structure or stated so – for example by writing the H3N+ would be the N-terminus and the COOH the C-terminus. While three of the four students invoked N-terminus and C-terminus language, the remaining student in this group simply wrote “covalent bond.” With respect to this theme, we caution making any claims based on the shown responses given the small number of students who invoked these ideas (4 and 6).
Thermodynamics CE
Responses (N = 28) to the thermodynamics CE fell into one of five themes as summarized in Table 3. Three of the five represented traditional thermodynamic concepts of free energy, entropy and spontaneity and equilibrium. The exceptions were contextualized responses that described reaction types or mentioned how the reactions were part of the glycolysis pathway. There was no evidence in the responses on attempts to link the thermodynamic concepts to other foundational concepts in chemistry, as was the case with the structure of amino acids CE.Not surprisingly the most common response, mentioned by 26 students, featured free energy concepts (Table 3). All calculated correctly the ΔG° for the coupled reaction to be −16.7 kJ mol−1 except one student who incorrectly calculated it to be +16.7 kJ mol−1 and subsequently concluded “the reaction is nonspontaneous.” This suggests most students correctly understand the conventional signs of ΔG° and how it is used to predict reaction spontaneity. Similarly, students related the ΔG° value to other thermodynamic values, e.g., by calculating the value of Keq, and made statements such as “it uses exergonic reaction to drive endergonic reaction” or simply “this reaction is exergonic.” Only one student mentioned entropy as part of their response, stating “an increase in entropy occurs as a result of this reaction.”
Twenty-two students invoked spontaneity and equilibrium principles. Within this theme, most indicated that the overall reaction will be spontaneous or that the coupled reaction proceeds forward and favors product formation. One student wrote “+ΔG° value of glucose + Pi → glucose-6-phosphate + H2O indicates the product is favored; when both reactions are coupled together, we get a −ΔG° value (reactants favored),” correctly noting the conventional signs in ΔG° but incorrectly using them to describe reaction equilibrium and directionality. Most used equilibrium in conjunction with spontaneity by, for example, mentioning in their responses that the reaction is spontaneous and subsequently stating the coupled reaction will proceed forward and favor product formation (see Table 3 for sample student responses).
The remaining student responses to the thermodynamic CEs were contextualized to the particulates of their current course. Seventeen students identified the reaction types involved in the coupled reaction. Of these, 13 students correctly identified the phosphorylation of glucose to glucose-6-phosphate as a group transfer reaction and the second reaction as that of ATP hydrolysis, reflecting the emphasis of many biochemistry textbooks and instructors on the transfer of the phosphate group from ATP to glucose with little attention paid to the ester synthesis mechanism of the coupled reaction. Four students thought the coupled reaction involved oxidation–reduction, most likely misapplying memorized algorithms (Nyachwaya et al., 2014). Half of the student respondents (N = 28) noted the reaction of glucose to glucose-6-phosphate constitutes the first step of the glycolysis pathway, with some mentioning that this is a rate-determining step for this pathway. From our perspective, the responses on reaction types and responses mentioning glycolysis reflect localized information recall and are not significant to the students' conceptual understanding or linking of chemical concepts to biochemical ones.
Enzyme kinetics CE
The enzyme kinetics CE resulted in the most variation among the responses. Of 25 students who completed the CE, 20 invoked activation energy in their response. Students made statements such as “the activation energy is lower with enzyme,” and “the non-catalyzed reaction is exergonic but has higher activation energy” (see Table 4). We infer from the high percentage of students who invoked activation energy in their response as evidence that students understand the ability of enzyme catalysts to enhance reaction rates by lowering the activation energy. Given that students did not mention transition state theory in their response and there was no opportunity for follow-up questions, it was not possible to ascertain if students had a deeper level understanding of the mechanism by which the energy of transition state is lowered via enzyme catalysis.Of ten students who invoked kinetics in their response, 9 correctly made a link between reaction rates and the function of enzymes (see Table 4). Students made statements such as “reaction is slower without enzyme” or “the enzyme increases the rate of the reaction.” Moreover, almost half of those who completed this CE (N = 12, Table 4) noted the enzyme was the catalyst, making statements such as “substrate can react with or without the enzyme” or “the enzyme is the catalyst.” A smaller number (3 students) invoked chemical equilibrium principles by stating that the reaction will proceed forward and favor product formation. One student mistook kinetics for equilibrium, stating “it will take less time for the reaction with the enzyme to hit equilibrium.”
Similar to the thermodynamic CE, most students (N = 19, Table 4) made links to free energy concepts. For example, students made statements such as “this reaction is exothermic; energy is released” or “this reaction has a negative ΔG and releases energy.” Others noted that ΔG is the same for the enzyme-catalyzed and non-catalyzed reactions. However, the most problematic responses to the enzyme kinetics CE were student struggles when directly attempting to describe the free-energy diagram. We grouped 12 student responses under the theme of visual representations based on student attempts to interpret information contained in the free energy diagram (see Table 4), with six of the twelve incorrectly interpreting the diagram. Two thought the diagram represented enzyme inhibition plots, stating “this appears to show noncompetitive inhibition” and “this reaction is a competitive inhibition.” This finding seems to suggest some students rely on memorized shapes of graphical data rather than localized concepts in the diagrams. That is, from our perspective, the data do not seem to suggest students struggled with interpreting the graph but rather used memorized graphical sketches to interpret the given data, hence the misapplication of enzyme inhibition plots for a free energy diagram. Further exploration of student reasoning and the processes they used to interpret the graphical data will require in-depth data based on interviews or other sources.
Discussion
The main purpose of this study was to examine how often students in an upper-division undergraduate biochemistry course linked prior chemical concepts to biochemical ones in response to CE prompts. Tables 2–4 provide the number of students who made these links as well as sample of their responses. The data provided in these tables indicate that students were successful in most part in linking foundational concepts to biochemical problems. For example, the students made links between the structure of glutamic acid (the prompt structure) and the acid–base concepts of pH/pKa, pI, titration, ionization steps and pH–charge relationships. Likewise, participants listed concepts of stereochemistry, chirality, and organic functional groups as relevant facts for the structure of glutamic acid. The thermodynamics CE similarly promoted students to link thermodynamic-related topics to each other though unsuccessful in facilitating links to other foundational concepts in chemistry. We observed similar findings with respect to the enzyme kinetics CE – students invoked free-energy, catalysis, kinetics, and equilibrium principles as relevant facts for the enzyme kinetics prompt. In our view, these findings suggest students are able to make links to foundational concepts when incentivized to do so and appropriate contexts for linking the ideas are presented to them.The wide range of prior chemical concepts students listed as relevant facts for the biochemically situated problems suggests CEs are appropriate facilitative tools to promote students' linking of relevant concepts within or across courses. Furthermore, we agree with the Lewis group that student efforts to link concepts in response to the open-ended prompt questions in the CEs suggest the “connections displayed are of the students' choosing and not an artificial contrivance to address a particular, targeted question” (Ye and Lewis, 2014, p. 581). Moreover, given students in this study previously completed a year of general college chemistry and at least one semester of introductory organic chemistry, and thus were utilizing prior knowledge, we make the claim that CEs can facilitate students' conceptions of chemical ideas as interrelated and with explanatory power (Taber, 2014). Admittedly, for this claim to materialize, much will depend on how instructors utilize CEs as a facilitative learning tool to help students make the necessary links.
We note that the results from our thermodynamics CE differs from the findings reported by Ye and Lewis (2014). In their study, participants were able to link the topics of stoichiometry, solution chemistry, compound classification, gas laws, atomic structure and thermodynamic-related topics with a thermodynamics prompt. Here, our students did not form such links. The differential outcome in the two studies is attributable to the nature of information provided in each prompt. While both CEs provided students with chemical reaction prompts (the dissociation of solid iron(II) chloride, FeCl2, in water in the Ye-Lewis study vs. the ATP-driven phosphorylation of glucose to glucose-6-phosphate in this study), different information was provided to the students in each CE – we provided only ΔG0 values in our case whereas amounts of reactant and water, initial water temperature, and heats of formation (Hfs) values were provided in the Ye-Lewis thermodynamics prompt (Ye and Lewis, 2014). Thus, the information provided in each CE differentially prompted students to make certain links with prior concepts or concepts learned within the course. That is, to use the language advanced by Hammer et al. (2005), the nature of the CE prompts activated certain knowledge resources in which each student population used to make links. In their study, Hammer et al. (2005) speak of context-sensitive activation of knowledge resources, e.g. prior conceptions students hold, in which learner's understanding of the phenomenon under study emerges. This suggests we need to pay close attention to contexts within the CEs that can promote students' link-making processes and ways to activate students' use of desired knowledge resources. This view is consistent with the notion of learners drawing upon their cognitive structures and combining the knowledge resources available to them in new ways so as to make sense of the information presented to them (Taber and García-Franco, 2010).
One of the findings in our study suggests some students confused free-energy diagrams with enzyme inhibition plots (see Table 4). Student struggles with visual representation in biochemistry are well documented in the literature (see Schönborn and Anderson, 2009, 2010; Linenberger and Bretz, 2012; Loertscher et al., 2014; Wolfson et al., 2014). However, findings in this study lead us to believe the faulty linking of enzyme inhibition plots with free-energy diagrams suggests there are underlying reasoning processes students utilize that cannot be explained by the inability to interpret visual data. In the chemical education literature, it is known that students rely on memorized processes and ideas when balancing and describing chemical reaction equations, often incorrectly using the memorized algorithms (Nyachwaya et al., 2014). We contend similar reasoning processes are at play here. Ascertaining whether our explanation is valid will require a follow-up study in which students are interviewed in-depth about their reasoning or the use of other sources to probe their underlying reasoning processes for the observed behavior.
Implications for research and teaching
In chemistry and biochemistry, much effort has gone into developing instructional materials that help students overcome incorrect ideas or alternate conceptions/misconception – defined as those ideas/concepts that students held but are inconsistence with scientifically accepted explanations (Villafane et al. 2011a, 2011b; Wolfson et al., 2014). For instance, Villafane et al. (2011a) developed twenty-one multiple-choice diagnostic instruments to identify students' incorrect ideas prior to biochemistry instruction, with the goal of aiding faculty make targeted changes in instruction to address problematic areas. In addition to addressing students' pre-existing alternate ideas, there is also a need to focus on their emerging alternative ideas during biochemistry instruction as was the case in this study when students invoked enzyme inhibition plots to describe a free-energy diagram (see Table 4). Given the ubiquitousness of free-energy diagrams in introductory chemistry courses, it is reasonable to assume students who had a year and half of such courses would recognize these diagrams at will, yet the data revealed students erroneously thought the diagrams represented competitive or non-competitive enzyme inhibition plots. Wolfson et al. (2014) similarly reported that some of their students confused free-energy diagrams with electron transport or titration curves. We suspect the student mix-up of enzyme inhibition plots with free-energy diagrams is due to their over-activation of the knowledge resources available to them (multiple graphical sketches which when activated some misapply) (Hammer et al., 2005). Ascertaining this hypothesis will require more extensive data collection in the future.The implication of the aforementioned discussion for biochemistry instruction is quite evident – there is a need to focus attention on misconceptions/alternate conceptions that emerge during biochemistry instruction. It is unlikely that students came across enzyme inhibition plots or electron transport before receiving biochemistry instruction. The misapplication of these plots suggests the need for a model that accounts for the emergence of alternate conceptions/misconception in real time. Consequently, knowledge learned in prerequisite courses such as free-energy concepts should facilitate the (re)learning of that concept or related concepts in new contexts such as biochemistry. We thus recommend the use of CE-like prompts as facilitative tools in instruction to assist in the relearning of concepts in new contexts and dealing with incorrect ideas as they emerge during instruction rather than confronting them as unitary concepts brought from previous courses.
On similar grounds, we note that chemical educators often assume student difficulties in upper division courses such as biochemistry stem from the inability to transfer previously learned knowledge into new contexts (Loertscher, et al., 2014; Wolfson et al., 2014). The findings reported in this paper suggest participants were able to provide a varied list of foundational concepts in chemistry as relevant facts for biochemically situated problems. Ye and Lewis (2014) similarly reported students' linking of various chemical concepts presented in a General College Chemistry classroom to each other. In our interpretation, this seems to suggest students can link prior and newly learned concepts when primed to do so. Thus the interaction of the mind and context appear to be an essential component of the knowledge construction process (Hammer et al., 2005; Taber and García-Franco, 2010). We therefore believe that the focus on transfer inability of prior concepts to new contexts may be misplaced. Rather, we argue for the need for research that examines how concepts learned in one context, such as thermodynamics in introductory courses, can facilitate the (re)learning of that concept in a different context such as in biochemistry.
To be clear, we do not claim students' listing of relevant ideas/facts in response to CE prompts constitutes evidence for deep or meaningful learning. Reaching such a conclusion would require further probing of individual students via in-depth clinical or think-aloud interviews to ascertain their understanding of the underlying principles of the concepts that the students are learning, e.g. thermodynamics. We note, for example, that in focus group interviews as part of threshold concepts study in biochemistry, Loertscher and colleagues reported some of their students “had superficial, memorized, or incorrect understanding of the physical basis of noncovalent interactions such as hydrogen bonds, dipole–dipole interactions, and van der Waals interactions” (Loertscher et al., 2014, p. 522). That is, the students could name the interaction but failed during the interviews to provide the electrostatic basis of such interactions. Thus, the mere linking of ideas to each other or listing of related facts to a given prompt, while evidence for students' ability to link such ideas, does not of itself constitute evidence for meaningful learning.
However, as an alternative to the transfer inability reasoning for noted student difficulties, we find the views of Hammer et al. (2005) and their construct of “resource activation” as a more appealing framework to theorize why certain students fail to utilize chemical knowledge they already possess in biochemical contexts. Because individual learner's conceptual knowledge organization is influenced greatly by the context in which the pieces of knowledge they held is activated, as noted by Maeyer and Talanquer (2010) and Taber and García-Franco (2010), it is likely that the instructional approaches fail subsequent reactivation of existing resources. We suggest CE-like prompts can be used as a useful tool to activate certain knowledge resources and research the effects of (re)learning prior chemical concepts in the biochemical context.
Study limitations and future work
The findings reported in this paper came from a single institution and involved a smaller number of study participants and might not necessarily be representative of all biochemistry students. As was pointed out to us by a reviewer, a “representative sample” would ideally involve different institutions or trajectories to make durable claims about the prevalence of ideas. Thus, in the absence of such data, one should be cautious with generalizations. As part of our ongoing research, we plan to solicit data from different institutions and students to further examine our hypothesis in a representative sample of all biochemistry students. A second limitation of our study is that students were instructed to use ideas from chemistry courses and this narrow focus was in line with our specific goal of examining the links students make between chemical and biochemical ideas. However, as an interdisciplinary subject, biochemistry draws on science content beyond what is taught in general and organic chemistry. Thirdly, during data analysis, only one author (AW) scored the second and the third CEs following high inter-rater agreement on the first CE. This might represent a methodological limitation. A fourth limitation is related to student participation in exam four. Because this exam was optional (students had the opportunity to drop the lowest exam score), there is a greater likelihood of low-performing students oversampled. While we note that non-participants included both students with comparatively low exam scores and students with high exam scores, nevertheless the likelihood of oversampling low-performing students affects data interpretation with respect to CEs two and three. Finally, our study design does not permit us to make claims about the durability of the links student make in response to the CE prompts. We aim to address this limitation in our future work.Acknowledgements
We thank faculty members at the department of natural sciences at Metropolitan State University who provided feedback on earlier iterations and expert responses on the structure of glutamic acid CE. We also thank the students who participated in this study and made it possible. We are also very thankful for reviewer comments and their helpful suggestions which greatly improved the paper.References
- Ausubel D. P., (2000), The acquisition and retention of knowledge, Dordrecht: Kluwer.
- Ausubel D. P., Novak J. D. and Hanesian H., (1978), Educational Psychology: A Cognitive View, 2nd edn, New York: Holt, Rinehart, Winston.
- Bodner G. M., (1986), Constructivism: a theory of knowledge, J. Chem. Educ., 63, 873–878.
- Bretz S. L. and Linenberg K. J., (2012), Development of the enzyme-substrate interactions concept inventory, Biochem. Mol. Biol. Educ., 40, 229–233.
- Ferguson R. L., (2007), Constructivism as a research lens, in Bodner G. M. and Orgill M. (ed.), Theoretical Frameworks for Research in Chemistry/Science Education, Upper Saddle River, NJ: Pearson/Prentice Hall, pp. 27–47.
- Francisco J. S., Nakhleh M. B., Nurrenbern S. C. and Miller M. L., (2002), Assessing student understanding of general chemistry with concept mapping, J. Chem. Educ., 79, 248–257.
- Grayson D. J., Anderson T. R. and Crossley L. G., (2001), A four-level framework for identifying and classifying student conceptual and reasoning difficulties, Int. J. Sci. Educ., 23, 611–622.
- Hammer D., Elby A., Scherr R. E. and Redish E. F., (2005), Resources, framing, and transfer, in Mestre J. (ed.), Transfer of Learning from a Modern Multidisciplinary Perspective, Greenwich, CT: Information Age Publishing, pp. 89–120.
- Howitt S. anderson T., Costa M., Hamilton S. and Wright T., (2008), A concept inventory for molecular life sciences: how will it help your teaching practice? Aust. Biochemist, 39, 14–17.
- Lewis S. E., Shaw J. L. and Freeman K. A., (2010), Creative exercises in General Chemistry: a student-centered assessment, J. Coll. Sci. Teach., 40, 18–23.
- Lewis S. E., Shaw J. L. and Freeman K. A., (2011), Establishing open-ended assessments: investigating the validity of creative exercises, Chem. Educ. Res. Pract., 12, 158–166.
- Linenberger K. J. and Bretz S. L., (2012), Generating cognitive dissonance in student interviews through multiple representations, Chem. Educ. Res. Pract., 13, 172–178.
- Loertscher J. and Minderhout V., (2010), Foundations of biochemistry, 2nd edn, Lisle, IL: Pacific Crest.
- Loertscher J., Green D., Lewis J. E., Lin, S and Minderhout V., (2014), Identification of threshold concepts for biochemistry, CBE—Life Sciences Education, 13, 516–528.
- Maeyer J. and Talanquer V., (2010), The role of heuristics in students thinking: ranking of chemical substances, Sci. Educ., 94, 963–984.
- Mayer R. E., (2002), Rote versus meaningful learning, Theory Pract., 41, 226–232.
- Minderhout V. and Loertscher J., (2007), Lecture-free biochemistry, Biochem. Mol. Biol. Educ., 35, 172–180.
- Nelson D. L and Cox M. M., (2013), Lehninger's Principles of Biochemistry, 6th edn, New York, NY: W. H. Freeman & Company.
- Novak J. D., (2002), Meaningful Learning: The Essential Factor for Conceptual Change in Limited or Inappropriate Propositional Hierarchies Leading to Empowerment of Learners, Sci. Educ., 86, 548–571.
- Nyachwaya J. M., Warfa A. M., Roehrig G. and Schneider J. L., (2014), College chemistry students' use of memorized algorithms in chemical reactions, Chem. Educ. Res. Pract., 15, 81–93.
- Schönborn K. J. and Anderson T. R., (2009), A model of factors determining students' ability to interpret external representations in biochemistry, Int. J. Sci. Educ., 31, 193–232.
- Schönborn K. J. and Anderson T. R., (2010), Bridging the educational research-teaching practice gap: foundations for assessing and developing biochemistry students' visual literacy, Biochem. Mol. Biol. Educ., 38, 347–354.
- Stoddart T., Abrams R., Gasper E. and Canaday D., (2000), Concept Maps as assessment in science inquiry learning – a report of methodology, Int. J. Sci. Educ., 22, 1221–1246.
- Taber K. S., (2014), Constructing active learning in chemistry: Concepts, cognition and conceptions, in Devetak I. and Glazar S. A. (ed.), Learning with Understanding in the Chemistry Classroom, Dordrecht, Netherlands: Springer.
- Taber K. S. and García-Franco A., (2010), Learning processes in chemistry: drawing upon cognitive resources to learn about the particulate structure of matter, J. Learn. Sci., 19(1), 99–142.
- Trigwell K. and Sleet R., (1990), Improving the relationship between assessment results and student understanding, Assess. Eval. High. Educ., 15, 190–197.
- Villafañe S., Bailey C. P., Loertscher J., Minderhout V. and Lewis J. E., (2011a), Development and analysis of an instrument to assess student understanding of foundational concepts prior to biochemistry coursework, Biochem. Mol. Biol. Educ., 39, 102–109.
- Villafañe S., Loertscher J., Minderhout V. and Lewis J. E., (2011b), Uncovering students' incorrect ideas about foundational concepts for biochemistry, Chem. Educ. Res. Pract., 12, 210–218.
- Wolfson A. J., Rowland S. L., Lawrie G. A. and Wright A. H., (2014), Student conceptions about energy transformations: progressions from general chemistry to biochemistry, Chem. Educ. Res. Pract., 15, 168–183.
- Wright T. and Hamilton S., (2008), Assessing student understanding in the molecular life sciences using a concept inventory, ATN Assess., 8, 216–224.
- Wright T. and Hamilton S., (2011), Diagnostic assessment for biological sciences – development of a concept inventory (Final Report), Canberra, Australia: Australian Office of Learning and Teaching.
- Ye L. and Lewis S. E., (2014), Looking for links: examining student responses in creative exercises for evidence of linking chemistry concepts, Chem. Educ. Res. Pract., 12, 158–166.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5rp00110b |
| This journal is © The Royal Society of Chemistry 2015 |