Improving students’ chemical literacy levels on thermochemical and thermodynamics concepts through a context-based approach
Ceyhan
Cigdemoglu
*a and
Omer
Geban
b
aAtılım University, Department of Educational Sciences, Ankara, Turkey. E-mail: ceyhan.tas@gmail.com; ceyhan.cigdemoglu@atilim.edu.tr; Fax: +90 312 5868091; Tel: +90 312 5868673
bMiddle East Technical University, Department of Secondary Science & Mathematics Education, Ankara, Turkey. E-mail: geban@metu.edu.tr; Fax: +90 312 2107971; Tel: +90 312 2104049
First published on 19th February 2015
Abstract
The aim of this study was to delve into the effect of context-based approach (CBA) over traditional instruction (TI) on students’ chemical literacy level related to thermochemical and thermodynamics concepts. Four eleventh-grade classes with 118 students in total taught by two teachers from a public high school in 2012 fall semester were enrolled in this particular study. The treatments were randomly assigned to the already formed classes; experimental groups were treated as CBA, the control groups as TI. Each teacher had one experimental and one control group. Open-ended contextual item sets were developed to assess students’ chemical literacy level in thermochemical and thermodynamics concepts. The test was administered to both groups as a post-test at the end of the implementation. Students’ responses to item sets were analyzed based on the rubric prepared as the answer key. Analysis of Covariance (ANCOVA) was used for interpreting the data. The results reveal that CBA is superior to TI on improving students’ chemical literacy levels, implying that CBA, as a discussion platform for concepts through real-life experiences, has a significant role in increasing students’ chemical literacy levels in abstract and difficult concepts regardless of the gender difference.
Introduction
The complex and rapidly changing world requires some basic understanding regarding chemistry (Gilbert and Treagust, 2009) since such an understanding is expected to contribute to scientific literacy, which is widely perceived to be the main goal of science education. Being a well-known – yet open to question – term, scientific literacy embodies scientific ideas, concepts, and practices within and across many scientific disciplines (Shwartz et al., 2006). The attainment of scientific literacy for all students can be facilitated provided that the content and instruction of science courses are professionalized. Shwartz et al. (2005) emphasize the science–technology–society movements as contributing to the social, technological, ethical, and personal aspects through incorporation into science courses for improving science literacy. Regardless of being specialized in science major or not, the ongoing educational initiatives have tried to establish a profound and multidimensional model of students’ scientific literacy, sometimes by offering new courses and sometimes by re-designing the curriculum enriched with science–technology–society (STS) issues.Chemistry education around the world conveys this international trend, since as stated by Osborne and Dillon (2008), students find chemistry concepts difficult to learn and consider it to add little value to their lives and professions. That is, students at the secondary level of many western industrialized countries and other non-industrialized countries do not find chemistry as a popular subject area to study in the future. Such undesirable outcomes are generally attributed to conventional chemistry curricula, which lack in relating theoretical knowledge to real-world students' life, thus inadequate in improving their chemical literacy. The research concerning problems of many chemistry curricula reveal some critical issues associated with varying aspects. Gilbert (2006) has categorized these problems into five distinct groups; overloaded, isolated facts, lack of transfer, lack of relevance, and inadequate emphases. Such undesirable diagnosis has gradually challenged chemistry educators to re-consider the aims of chemistry courses and to make significant shifts through scientific and chemical literacy emphasis (Shwartz et al., 2005).
Substantial research has proposed that chemistry curricula if designed in the framework of context-based approach (CBA) can overcome the problems of conventional chemistry curricula (Bennett et al., 2003, 2007; Westbroek, 2005; Bulte et al., 2006; Gilbert, 2006; Pilot and Bulte, 2006). Westbroek et al. (2000) emphasize the necessity of constructing an A-level chemistry course based on an approach stressing chemistry as a human activity, a pedagogy settled on situated learning, and a framework that improves chemical concepts, skills, and attitudes expected to help students to overcome complex social and scientific problems. According to Shwartz et al. (2005), the most popular approach promising to attain the changes in chemistry courses is the context-based approach. Considerable efforts have been put on reconsiderations in chemistry curricula with many projects; for example, ‘ChemCom’ and ‘Chemistry in Context’ in USA, ‘SALTER’s in England and Wales, and ‘Chemie im Context’ in Germany. All these curriculum projects have been intended to find research-based evidence for the impact of contextualized teaching and learning in chemistry by incorporating real-world contexts to attract students’ interest and to be more beneficial to them. Moreover, in their review, Lubben et al. (2005) stated that courses using context-based and STS approaches have drawn attention all around the world since they are regarded to have a major role in the improvement of students’ scientific literacy.
The concepts related to thermochemistry and thermodynamics are perceived as abstract, difficult to learn, and popular in society (Goedhart and Kaper, 2002). Therefore, these concepts whether subtitled as chemical reactions and energy (De Vos and Verdonk, 1985a; Barker and Millar, 1999, 2000; Goedhart and Kaper, 2002) or as thermochemistry and/or thermodynamics (Goedhart and Kaper, 2002; Greenbowe and Meltzer, 2003; Belt et al., 2005; Sözbilir and Bennett, 2007) have been studied in different ways. Goedhart and Kaper (2002) described the rationale for studying these concepts in a way that energy is a popular societal discussion platform, students as educated citizens are expected to have a certain level of understanding of these concepts. Additionally, they stated that students frequently experience these concepts in classroom or outside the school. Moreover, the comprehension of reaction energy and relevant concepts provides students to predict some parameters regarding the chemical processes. According to Goedhart and Kaper (2002), improving students' understanding of these concepts can contribute to their chemical literacy level on these and related concepts.
Although certain justifications have been provided for studying the concepts of thermochemistry and thermodynamics, a majority of the above-mentioned studies have only focused on overcoming students’ learning difficulties related to these issues. The existing literature is short of investigating students’ chemical literacy in thermochemistry and thermodynamics concepts. Within this perspective, this study purposes to investigate the effectiveness of context-based approach (CBA) over traditional instruction (TI) in improving chemical literacy of eleventh-grade science major students by designing a real-world contextualized instruction enriched with STS issues in thermochemistry and thermodynamics.
Background
What is scientific literacy and chemical literacy?
The construction of conceptualization of scientific literacy (SL) requires returning the description of literacy itself. Miller (2010) portrays literacy as the minimum level of reading and writing skills needed to participate and communicate in the society. Based on these descriptions, SL is commonly defined as the level of understanding of scientific and technological notions required for taking a role as a member of the modern industrial society (Bybee, 1997; Miller, 1983a, 1983b, 1995; Laugksch, 2000; Miller and Pardo, 2000). Shwartz et al. (2005) have described some common dimensions that are generally related to scientific literacy, which are “(a) understanding the nature, norms, and methods of science, as well as the nature of scientific knowledge; (b) understanding the key scientific concepts, principles, and theories (science content knowledge); (c) understanding how science and technology actually work together; (d) appreciating and understanding the impact of science and technology on society; (e) communication competencies in scientific contexts-the ability to read, write, and understand systemized human knowledge; and (f) applying some scientific knowledge and reasoning skills to daily life” (p. 323). Although the depth and the exact content of the domains and desirable balance between knowledge and skills do not form a consensus, these dimensions propose a significant framework in defining SL.Investigating to what extent students are literate in science or other domains necessitates an eligible model of assessment. The Program for International Student Assessment (PISA) by the Organization for Economic Co-operation and Development (OECD), and as well as Trends in Mathematics and Science Studies (TIMSS), are two global thorough survey programs aspiring to assess students’ scientific literacy levels. Shwartz et al. (2006) stated that “PISA tends to focus on ‘practical knowledge in action’, namely recognizing questions as scientific, identifying relevant evidence, critically evaluating conclusions, and communicating scientific ideas” (p. 204), whereas TIMSS mainly concentrates on the rehearsal of the content taught. PISA defines SL as the capacity to utilize scientific knowledge, to analyze questions and to draw conclusions that are evidence-based so as to understand and make decisions related to the world and the modifications made by human activities (OECD, 2000, 2003). PISA definition proposes quite similar scientific processes to the common dimensions that are generally related to scientific literacy described by Shwartz et al. (2005).
Based on the definition of SL, several different initiatives with their theoretical frameworks have developed many research tools that try to assess a definite aspect of SL. The recall of scientific knowledge, that is, the content knowledge, is an important and mostly assessed aspect of SL. Laugksch and Spargo (1996a, 1996b) developed test items to measure students’ content knowledge. Another aspect of SL is the ability to apply scientific principles to other contexts. Some tools have been developed to design authentic non-academic tasks (Champagne and Newell, 1992), such as information on a household detergent and to identify students’ skills in reading such information. Thirdly, some research studies investigated students’ literacy abilities in a scientific context that is to evaluate their ability to read, write, reason, and question the given information (Duschl and Osborne, 2002; Norris and Phillips, 2003). As a fourth aspect of SL, students’ understandings of the nature of science, along with their attitudes toward STS issues have been measured as well. A common instrument to do so is developed by Aikenhead and Ryan (1992).
The theoretical perspectives addressing the aspects of SL pose certain levels and expressions. For example, ‘functional literacy’ is usually perceived as the lowest level of SL which refers to the ability needed to function normally in daily life (Shwartz et al., 2006). ‘Civic literacy’ is a higher level compared to functional, and meaning participating wisely in a social debate related to science and technology issues (Miller, 2004). Shwartz et al. (2006) describe ‘cultural literacy’ (or ideal literacy) as a situation in which scientific endeavor is appreciated and perceived as a major intellectual activity. The theoretical framework suggested by Bybee (1997) is more suitable for assessment of SL because of its transferable order to instructional purposes. The levels of SL are scientific illiteracy, nominal SL, functional SL, conceptual SL, and multidimensional SL. According to Bybee (1997) the latter is more difficult to attain at all and a student may have high level of literacy on a specific subject matter, but a low level in other subjects. It is also stressed that the attainment of SL should be considered as a lifelong process, which means, assessing SL during a specific school year may not be the final level of SL attained. Shwartz et al. (2006) stated that assessing SL at school years demonstrates whether the seeds of literacy are found in students or not.
Building on this conceptualization of SL, Shwartz et al. (2006) made a comprehensive description for chemical literacy established on the framework of Bybee (1997) and Shwartz et al. (2005). The definition of chemical literacy was developed by obtaining a consensus among chemists, educators, and high school teachers. As a result, the definition of chemical literacy is developed and is given in Table 1. As seen from Table 1, there are four domains of chemical literacy (CL). According to Shwartz et al. (2006), the definition of CL proposes that each high-school graduate is expected to know the key ideas and have the capacity to do with that knowledge.
| 1. Chemical content knowledge |
| A chemically literate person understands the following: |
| A. General chemical ideas |
| • As an experimental discipline, chemists carry out scientific investigations, generalize findings, and propose theories to explain to the world. |
| • Serves knowledge to other fields in order to explain phenomena |
| B. Characteristics (key ideas) of chemistry |
| • Explain the macroscopic level by means of the molecular structure of matter |
| • Seek the dynamics of processes and reactions |
| • Investigate the energy changes accompanied in a reaction |
| • Understand/explain life by chemical processes and structures of living systems |
| • Appreciate the contribution of scientific language to this discipline |
| 2. Chemistry in context |
| Chemically literate students are able to: |
| • Understand the knowledge of chemistry in explaining everyday situations |
| • Understanding of daily life chemistry, such as being user of new products/technology, a decision process, and involving in social argumentation on chemistry-related issues. |
| • See the relatedness of innovations in chemistry and sociology. |
| 3. Higher-order learning skills |
| Students who are chemically literate ask questions, investigate relevant information when required. Additionally, he/she can evaluate pros/cons of debates. |
| 4. Affective aspects |
| Students who are literate have a fair and rationale perspective of chemistry and its applications. Furthermore, literate students show interest in issues of chemistry, specifically in a non-formal environment like mass media. |
How to assess CL?
Related to the assessment of CL, Witte and Beers (2003) explained that in chemistry exams they would assess chemical literacy when they assessed students’ ability of using and dealing with given information in a chemistry problem and students’ ability of using chemistry knowledge and skills in order to comprehend information regarding an everyday problem. These skills are understanding given information, capacity to select needed information from the text, capacity to alter given information to another form, and capacity of assessing information from acceptability or plausibility aspects. Additionally they state that the capacity to manage valid arguments with pro and con ideas and having a standpoint during the arguments are required skills for high CL. Based on such a description, if students have skills in using and dealing with given information and skills in argumentation (valid arguments with pro and con ideas) that means they have high chemical literacy levels.Having contexts with real-life situations give students the possibility of showing their literacy skills. The chemistry examinations assessing these skills may be presented in the form of a newspaper article, information on a product or drug, print from web, a comic book chapter, story or advertisement (Witte and Beers, 2003). A context in these forms can be an industrial process, an environmental issue, an everyday life problem from school or science. PISA questions constructed by OECD are generally in this form and these questions are developed to assess students’ science knowledge, science skills, and several other skills. The questions are generally context-based and they mainly try to assess students’ science knowledge and skills (75%) and other skills (25%).
How CBA is expected to contribute to CL?
Westbroek (2005) stated that the aim of chemistry education should be qualifying students in a way that they could use scientific knowledge, solve problems, show evidence-based results, and evaluate the changes in nature made through human activities. Curriculum studies incorporating context-based approach (CBA) have such expressions and propose to increase students’ chemical literacy skills too. Whitelegg and Parry (1999) stated that context-based learning possesses similar aims since it presents students real-world controversial and social issues in order to make argumentations and integrates social or civic lessons to increase social awareness. An instructional design expected to contribute to literacy skills must enhance the learning processes in class in a way that the social and cognitive development of competencies are supported. In such a design, teachers must create effective learning environments in which students are given opportunities to ask relevant and scientifically sound questions (Penick et al., 1996) in order to develop scientific literacy among students. When such a design is framed, assessment of students’ chemistry knowledge, chemical literacy skills and ability of using scientific terms in their explanations or argumentations could be carried out through contextual questions, problems or situations from students’ everyday lives (OECD, 2004; Witte and Beers, 2003). As Taber (2003) stated standardized examinations with multiple-choice and open-ended questions are altered to more structured questions that are embedded in real-life contexts. Considerable effort has been put into the integration of such real-life contexts into assessment tools since they are perceived as relevant for students (Kelly, 2007) and measure the literacy skills.The gender issues in science related fields have been a concern of research studies for a long time. A difference may exist in the cognitive or affective domain across the gender. Taasoobshirazi and Carr (2008) stated that a large gender difference is found in students’ achievement level, specifically in physics education. According to Koballa and Glynn (2007) physiological and sociological functions have a direct effect on the reason why the attitude toward science is low among females when compared to males. Scantlebury and Baker (2007) stated that according to TIMSS data, gender differences start at the fourth-grade level and go on through the final years of secondary school in favour of males, and this gap widens at each level in the European countries. Related to contextual learning, Taasoobshirazi (2007) stated that gender differences in achievement as well as the motivation to learn physics may be minimized by context-based instruction through making the lessons more relevant to students. De Jong (2006) stated that a context is required to be well-known and relevant for both boys and girls in order to avoid situations or contexts which will favor males or females. That is, the selection of the context in the context-based approach also becomes critical in order to avoid causing superiority for males or females. According to Gilbert (2006), a context should be designed in such a way to engage all students, and the collection of such contexts should be in such a way that it makes chemistry more relevant to all students. Therefore, this study finds it necessary to explore the effect of treatments on the gender as well.
To sum up, the ongoing reform in science education sets the attainment of “scientific literacy for all” as its main goal, and therefore, teaching high school chemistry should address this aim too. As one of the promising ways to help students bridge their gap between science, applications of science and technology and their critical evaluation can be brought about by designing chemistry lessons to include societal issues and discussions involving science and technology (Albe, 2008). One of the most popular approaches promising to attain the changes in chemistry courses, the context-based approach (CBA), is utilized in designing lessons that have the possibility to improve eleventh-grade science major students’ chemical literacy level in thermochemistry and thermodynamics. By designing a real-world contextualized instruction enriched with science–technology–society issues that cover the concepts; this study investigates the effectiveness of CBA over traditional instruction (TI). Based on the definition of chemical literacy (CL) developed by Shwartz et al. (2006), and items developed to assess CL by Witte and Beers (2003) and PISA Science Literacy questions, the study developed its own framework to assess CL of students on a definite topic. Specifically, the study investigates (1) the effect of CBA on improving the chemical literacy of eleventh grade science major students on thermochemical and thermodynamics (TT) concepts, (2) the effect of interaction between the gender and treatment with respect to students’ mean scores obtained from chemical literacy items.
Methodology
Experimental design of the study
The quasi-experimental design as a type of experimental research was utilized for the study. In order to compare the effect of CBA over TI on improving students’ chemical literacy in thermochemical and thermodynamics concepts, the treatments (CBA and TI) were randomly assigned to the intact classes. There were two teachers; (Teacher X and Teacher Y), Teacher X had class 11-A and class 11-B (‘11’ is the grade level, the letter is the class name), Teacher Y had class 11-C and class 11-D. We placed the names of the treatments (CBA and TI) in a closed box, and mixed the names of the treatments thoroughly. Then, we asked Teacher X to draw out a treatment for his class 11-A. The other treatment which remained in the box was assigned to class 11-B. The same procedure was carried out for the classes taught by Teacher Y. Each teacher’s one intact class was the experimental group and the other was the control group. Table 2 briefly describes the design of the study.| Groups | Pre-test | Treatments | Post-test |
|---|---|---|---|
| a Note: EG: experimental group, CG: control croup, TTCT: thermochemical and thermodynamics concept test, CBA: context based approach, TI: traditional instruction, CLIs: chemical literacy items. | |||
| EG | TTCT | CBA | CLIs |
| CG | TTCT | TI | CLIs |
Sample
Eleventh-grade science major students in a public high school were conveniently selected as participants of the study. Four intact classes of two different teachers from the same school were selected for implementation. These two teachers were volunteers for participating in the study. The subjects consisted of 118 eleventh grade science major students. Of these, 69 were females and 49 were males. 58 (26 male and 32 female) of the participants were in CGs and 60 (23 male and 37 female) of them were in EGs. The age range of the students was between 16 and 18 years.Procedure
Prior to the treatment, the necessary permissions were obtained from the ethical boards, and the school administration was informed about the research. Also, the families of the students were informed about the treatment and all of the students accepted to participate in the study after their family signed the consent form. Later, the materials, lesson designs, and instruments were introduced to the teachers in a small workshop, during which, they were briefed on the main requirements of CBA for meaningful chemistry instruction that is expected to contribute to chemical literacy and the phases of each framework designed for groups. These requirements are described by Westbroek (2005) as, (a) context, (b) need-to-know, and (c) attention for students input, and the authors were inspired by these requirements while developing the frameworks for EGs and CGs. Additionally, teachers were informed on the spider web metaphor (Fig. 1) for teaching TT concepts in their EGs. Based on the frameworks developed, the volunteer teachers were informed on the how to teach TT concepts through CBA in their EGs and how to teach TT concepts through TI in their CGs. Fig. 3 and 4 show these frameworks. Just before the treatment period started, the pre-test, TTCT, was administered to the students of both EGs and CGs in order to reveal if any significant differences across the groups exist. The implementation took almost two months.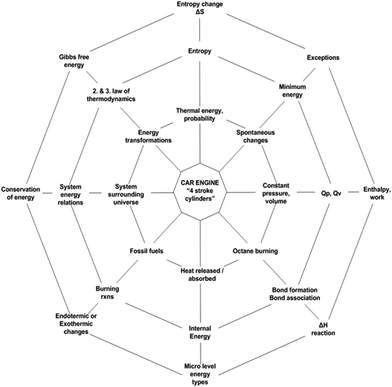 | ||
| Fig. 1 The spider web metaphor for learning thermochemical-thermodynamics concepts. (Adapted from Schwartz, 2006). | ||
Treatment (CBA) in experimental groups, a sample module for “Systems and Energy”
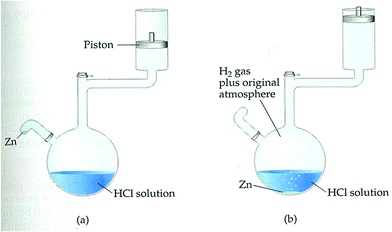
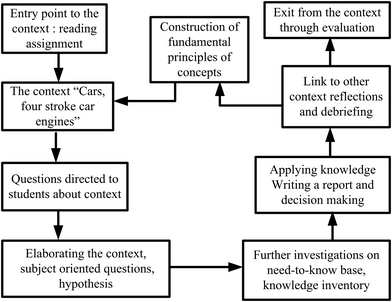
The developed framework was utilized throughout all lesson plans designed for EGs (see Fig. 3). For example, for the first lesson plan, the entry point was a reading assignment (historical development of car engines) related to the context of ‘cars’. This step was the contact phase, in which the students’ prior knowledge and ideas about the context were evoked. During the class, their curiosity was stimulated by asking questions related to the reading assignment. Some of which included: How do steam machines work? How heat is converted to work? What about the car engines, how do they work? What kind of a system is a four-stroke cylinder? Then, a simulation video of “how car engines work” was displayed and students were expected to write their own notes to answer the questions. In this way the need-to-know phase was established over the context for new knowledge construction. After the video display, the teacher further inquired by asking whether the changes in a four-stroke cylinder are endothermic or exothermic? What kinds of energy transformations occur? The teacher expected students to write their observations after watching the video. The students wrote their ideas on endothermic–exothermic, matter–energy transformations, heat-mechanical and energy-internal energy changes occurred in car cylinders with justifications. They debriefed with friends and teacher to elaborate subject-oriented and context-oriented questions. The teacher explained that a system contains internal energy and gave the definition of it.Next, the teacher further investigated how energy is exchanged through a system with its surroundings; how the internal energy of a system can be calculated, when a change is observed (Zn is added in Fig. 2), in what forms the internal energy of the system will change. Then, an experimental setup as shown in Fig. 2 was constructed, and the students were asked to write their hypothesis on what will be observed when the change is observed.
Zinc was added to HCl solution, and the students observed the reaction. The teacher again asked what changes occurred in the system. A new need-to-know base was established for knowledge inventory, and the teacher later asked the students to calculate the work. Students worked with their peers and discussed their notes and observations, and then wrote a brief report on how to calculate the work. One of the parameters, the area of cross-section for the piston, was given by the teacher and the height difference of the motion was measured by the students. They wrote the work as “force × height changed” and pressure as “force/cross-sectional area” when F is replaced by P × A the formula becomes P × A Δh. As, A × Δh means volume (ΔV), the formula is condensed to P × ΔV for work. The students were expected to conclude that H2(g) produced from the reaction causes the piston to move up, and therefore a work which is just the pressure multiplied by the change in the volume (constant pressure) is produced.
The teacher constantly guided, posed additional questions to students so as to receive their ideas. While anticipating the responses, the teacher provided additional explanations whenever needed as well as guiding students’ ideas and observation related to work done on the system and work done by the system. Then, the formula related to internal energy and parameters that contribute to internal energy were also explored. Later, the teacher asked the students to write a brief report on the work done by/on the system in a single cylinder of a four-stroke engine using thermodynamic terms to explain the phenomena. The students discussed with friends and most of them wrote that during the power stroke (gas expands on the piston to move it outwards and thus transfers the energy outside the system to the other cylinders) the work is done by the system. Also they wrote that during the exhaust stroke (force is supplied by the power stroke of a different cylinder) the work is done on the system. Related to explaining the mechanism, the students wrote that the heat transfer to the gas in a cylinder increases the internal energy of the gas, thus creating higher pressure and temperature. Then, as the gas expands, a force is exerted on the movable piston, so the system (cylinder) does work. Discussions and talks occurred among students, eventually directing them to agree on the idea that gas pressure and temperature decrease when it expands, indicating that its internal energy is decreased by doing work. They continued with their reports, stating that heat transfer to the environment reduces pressure in the gas, so that the piston can more easily return to its starting position.
In order to take students’ reflections, they watched another video (a closed system) and the teacher asked even more questions for the purpose of elaborateness. The teacher elicited more examples from the daily life so as to further explain the types of systems, such as open, closed, isolated, and isothermal along with energy transformations and the heat–work–energy relationship in systems. At this stage the teacher guided debriefing among students to reach the knowledge construction through the context of ‘car engines’. For the next lesson, students were assigned to write a brief report on their ideas about how more efficient cars can be produced. They were expected to make decision on efficient car engines and efficient fuels. The next lesson started with a summary and a small discussion on students’ decision reports on efficient cars and fuels. The whole unit was instructed through similar phases. In CBA classes students were not only made familiar with the context, they also learned to question the chemistry behind it through inquiry-based activities and decision making processes. Through the CBA framework, the attributes of an effective context were addressed as described in Table 3. Gilbert (2006) designed such a table for any context, and the table is adapted to the context of car engines for this study.
| Attribute | Focal event: car engines | |
|---|---|---|
| a | Where, when, and how is the focal event situated? | Historical development of cars, from steam to 4 stroke engines |
| b | What do people do in the situation, and what actions do they take? | Production of more efficient cars, more efficient fuels, bio-fuels, high octane ratings, reduction of environmental hazards |
| c | In what language do people speak about their actions? | Related to the efficiency of car engines, 1.4, – 1.6 or 2.0 capacity; different energy types; energy related discussion in the society |
| d | What is the background knowledge of those who act? | Chemical reactions, energy terms in car engines: heat, work, system, surrounding, internal energy |
Treatment in control groups; traditional instruction
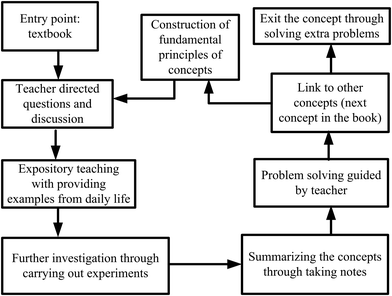
A traditional framework which was utilized in all lesson plans designed for control groups (see Fig. 4) was followed in CGs. For the first lesson plan, the entry point was the textbook. The teacher asked whether the students had read the related part of the book, then directed questions about the content. The basic framework for traditional instruction was set as teacher-centered. The teacher asked what is a system? What is a surrounding? What kinds of transitions occur between systems and surroundings? What are the types of systems? After taking students’ responses, the teacher briefly introduced (as done in their routine class hours) basic concepts of thermochemistry and thermodynamics. For example, the definition of systems and surroundings were given. A bottle of water was given as an example of a system. Some more water was added into the bottle, and the teacher asked what kinds of transition occur between the systems and their surroundings? Through expository teaching, the teacher explained open, closed, and isolated systems. To deepen students’ understanding, they were expected to provide examples to open, closed, and isolated systems.Then, the teacher asked to the students to categorize the types of systems in a kettle used in the kitchen, an iron machine, a car engine, and a coffee cup. The instruction continued with explaining possible energy transformations such as matter–energy transformations, heat-mechanical and energy-internal energy changes in the given examples. The expository teaching about these concepts was connected to the heat, work, and internal energy and continued with explanations related to internal energy. The same experimental set-up (Fig. 2) was constructed for CGs. The teacher asked the students how to calculate the work. The parameters, the area of cross-section of the piston and the height difference of the motion were given. The teacher wrote on a blackboard the work as “force × height changed” and pressure as “force/cross-sectional area” and when F is replaced by P × A the formula becomes P × A × Δh. As, A × Δh means volume (ΔV), the formula is condensed to P × ΔV for work. The teacher concluded that H2(g) produced from the reaction caused the piston to move up and therefore work which is just the pressure multiplied by the change in volume (constant pressure) is produced.
Then, the teacher solved some simple exemplary problems, gradually increasing the difficulty level of questions and expecting students to solve them. Through these activities, concepts of the topic were clarified in detail. Then, the teacher read certain parts from the textbook and the students followed on to take notes for themselves. The definitions and the formulas related to heat, work, and internal energy were directly presented by the teacher, while the students took notes of all explanations and problem solvings. The entire concepts covered in the EGs were also covered in CGs, but not over the context, and rather through expository teaching. The questions and problems solved in CGs were also designed by the researchers and contextual items were particularly utilized to prevent favoring EGs.
The same videos and simulations displayed in EGs were also showed to CGs after introducing the concepts. In essence, the teachers were doing whatever they were doing in their routine class hours and the methods were mainly included in lecturing and questioning, with discussions around the context designed for the topic. Here, the difference was that real-life contexts were introduced at the end of concepts, the discussions were not carried out through the context, but the teacher generally utilized contextual questions while problem solving. In CGs, the John Henry effect was tried to reduce by movies and simulation displays, the same as those used in the EGs. The traditional framework utilized in CGs is shown in Fig. 4.
To verify the treatments, the researchers carried out systematic classroom observations both in the experimental and control groups throughout the implementations. After each class hour, the teacher and the researchers evaluated the progress of implementations, and the same procedure was followed with other teachers. The researchers supported the teachers at any given time together with additional feedback and suggestions in order to make the intervention more in line with the purpose of the study. Additionally, the teachers were informed to teach in their control groups in the way that they were accustomed to. In all, the implementations took six weeks, in each of which, students had three chemistry lecture hours, that is, there were 18 hours (45 minutes each). For EGs the concepts of thermochemistry and thermodynamics were summarized around the context, (see Fig. 1; the spider web metaphor). In CGs, movies and simulation displays were the same as those used in the experimental groups; here they served as an example to application of concepts. Additionally, to avoid favoring EGs about the familiarity with contextual items, the teachers used the same contextual exercises in their CGs too.
Instruments
One of the instruments used in this study, thermochemistry and thermodynamics concept test (TTCT), was developed based on the main concepts required to understand thermochemistry and thermodynamics (TT). The instrument was originally constructed by Yeo and Zadnick (2001) and translated to Turkish by Ceylan (2004). In its original form, there were 20 multiple-choice items, thirteen items of them were taken directly from the translated version and 7 new items were developed by authors. The validity of the test was established by two faculties in chemistry education and a chemistry teacher. Also, feedback by one Turkish language expert and another chemistry teacher was used for both understandability and face validity of the instrument. The test was piloted before treatment and resulted in some revisions. Based on the pilot study scores, the item difficulties and item discrimination indexes were checked using ITEMAN, item analysis program, and they were within the range of 0.159–0.756 and 0.219–0.539 respectively. The Cronbach alpha reliability coefficient of the scores was found to be 0.72 for TTCT. The questions were classified into categories such as heat-temperature and energy, energy release/absorption of bond dissociation and bond formation, endothermic and exothermic changes, heat of reactions, enthalpy, spontaneous changes, systems and energy, entropy changes, and Gibbs free energy. After revisions, the instrument was administered to the groups before the implementation to reveal whether the groups have similar prior knowledge to understand the thermochemistry and thermodynamics concepts.The other instrument of the study was Chemical Literacy Items (CLIs) which was developed in an open-ended format. CLIs were constructed based on the sample questions of PISA measuring students’ scientific literacy skills and the questions developed by Witte and Beers (2003) to reveal students’ chemical literacy skills. Chemistry related news covered in the media along with the tasks embodying scientific facts were prepared from students’ daily lives since they are perceived to attract students’ attention. The everyday contexts used as contextual item sets not only investigate students’ content knowledge, but also their abilities and interpretations on a task or a given data. That is, the items were measuring students’ cognitive variables such as ability in using and dealing with given information in a chemistry problem and students’ ability in using chemistry knowledge/skills in order to comprehend information regarding everyday context. Bybee et al. (2009) described this framework for PISA items as having the components of scientific contexts, the scientific competencies - which means identifying scientific issues, explaining phenomena scientifically, and using scientific evidences -, the domains of scientific knowledge, and student attitudes toward science. The study constructed contextual items because Bennett and Holman (2002) claimed that if the scope of a context-based lesson design is to develop conceptual understanding, then students could be assessed in de-contextualized ways about their understanding of chemical ideas. On the other hand, they state that if the lessons aim to develop scientific literacy and ideas about science, assessment should be contextualized. Additionally, Ratcliffe and Millar (2009) stated that contextual problems require a student to do certain things competently: to identify what the issue is, to come to some understanding of the underlying concepts, and to weigh up the evidence that bears on the viewpoints expressed.
There were four different contextual item sets with sub-questions to measure students’ chemical literacy level on thermochemical and thermodynamics concepts in CLIs. The first item set was related to the combi-boiler which is a technological household appliance possessed by almost each student in his/her house. A sample item set is provided in Appendix A. The second item set was about energy concepts in biological systems. To avoid covering technological issues at all, the authors needed to construct questions concerning biological issues as well. The energy required for daily routines of a teenager was provided for students as a task. The third item was about the elastic polymer bands having widespread usage in daily life, and students were expected to connect their pure conceptual knowledge of order of molecules to the entropy of the system and the entropy of surroundings. The last item was related to the volcanoes. Types of systems and changes, the sign of basic quantities such as ΔG, ΔH and ΔS, and the laws of thermodynamics were investigated.
Table 4 briefly summarizes the contextual item sets and investigated TT concepts. The maximum and minimum scores obtained from the overall CLIs were 45 and 0, respectively, and the distribution of maximum score to each item set is provided in Table 5.
| Chemical literacy items on thermochemical and thermodynamics concepts | |||
|---|---|---|---|
| Item 1: chemistry of combi-boiler (max. 15 points) | Item 2: energy concepts in biological systems (max. 10 points) | Item 3: elastics polymer bands and entropy (max. 10 points) | Item 4: volcanoes and related chemical terms (max. 10 points) |
| Investigated concepts | |||
| Systems, surroundings, exothermic and endothermic changes, heat, enthalpy, | Energy transformations, calorimeters, bond formation/dissociation, Gibbs free energy | Order/disorder, entropy, isothermal process, ΔSsystem, qsystem | Systems, energy transformations, internal-energy, entropy, spontaneous/nonspontaneous changes, laws of thermodynamics |
| Item sets | The sub-categories of chemical literacy definition |
|---|---|
| a Note: numbers in parentheses show the maximum score that students can obtain from that choice. CK: content knowledge, CC: chemistry in context. | |
| 1.a | CK/key ideas/dynamics of processes and reactions |
| (2) | Higher-order learning skills/investigate relevant information |
| 1.b | CK/key ideas/dynamics of processes and reactions |
| (3) | CC/understanding daily-life chemistry, decision process |
| 1.c | CK/general chemical ideas/generalize findings |
| (3) | CK/key ideas/explaining macroscopic level with molecular structures |
| CK/key ideas/dynamics of processes and reactions | |
| Higher-order learning skills/investigate relevant information | |
| 1.d | CK/key-ideas/explaining macroscopic level with molecular structures |
| (3) | CK/key ideas/dynamics of processes and reactions |
| CK/key ideas/energy changes accompanied in a reaction | |
| CC/understanding of daily-life chemistry, decision process | |
| Higher-order learning skills/investigate relevant information | |
| 1.e | CK/key ideas/explain life and structures of living systems |
| (4) | CC/knowledge of chemistry in explaining everyday situations |
| CC/understanding of daily-life chemistry, decision process | |
| 2.a | CK/general chemical ideas/knowledge to explain phenomena in other fields |
| (2) | CK/key ideas/energy changes accompanied in a reaction |
| CK/key ideas/explain life and structures of living systems | |
| 2.b | CK/general chemical ideas/knowledge to explain phenomena in other fields |
| (2) | CK/key ideas/explain life and structures of living systems |
| 2.c | CK/general chemical ideas/knowledge to explain phenomena in other fields |
| (3) | CK/key ideas/energy changes accompanied in a reaction |
| CC/knowledge of chemistry in explaining everyday situations | |
| CC/understanding of daily-life chemistry, decision process | |
| Higher-order learning skills/evaluate pro-con of and debates | |
| 2.d | CK/key ideas/explaining macroscopic level with molecular structures |
| (3) | CK/key ideas/explain life by chemical processes |
| CC/understanding of daily-life chemistry, decision process | |
| Higher-order learning skills/evaluate pro-con of and debates | |
| 3.a | CK/general chemical ideas/knowledge to explain phenomena in other fields |
| (5) | CK/key ideas/explaining macroscopic level with molecular structures |
| CK/key ideas/dynamics of processes and reactions | |
| CC/knowledge of chemistry in explaining everyday situations | |
| CC/understanding of daily-life chemistry, decision process | |
| Higher-order learning skills/evaluate pro-con of and debates | |
| 3.b | CK/key ideas/explaining macroscopic level with molecular structures |
| (5) | CK/key ideas/dynamics of processes and reactions |
| CC/knowledge of chemistry in explaining everyday situations | |
| CC/understanding of daily-life chemistry, decision process | |
| Higher-order learning skills/evaluate pro-con of and debates | |
| 4. | CK/general chemical ideas/generalize findings |
| (10) | CK/general chemical ideas/knowledge to explain phenomena in other fields |
| CK/key ideas/explaining macroscopic level with molecular structures | |
| CK/key ideas/dynamics of processes and reactions | |
| CK/key ideas/explain life by chemical processes | |
| CC/knowledge of chemistry in explaining everyday situations | |
| CC/understanding of daily-life chemistry, decision process | |
| Higher-order learning skills/investigate relevant information when required | |
For content validity, first, the instrument was sent to one chemistry professor majoring in physical-chemistry and one professor from chemistry education. They reviewed each item of the CLIs for accuracy of content and appropriateness to measure the investigated concepts. They also categorized the concepts of thermochemistry and thermodynamics which are investigated through the instrument (see Table 4) and confirmed the validity of the content. Next, chemical literacy items were examined based on the definition of chemical literacy (Table 1) framed by Shwartz et al. (2006). The researchers specified each alternative of the item sets according to Table 1. Three faculty members of chemistry education also examined these items in order to categorize them based on chemical literacy definition used in this study. Chemistry educators matched each sub-item of the instrument with sub-categories of definition of chemical literacy, that is, what aspect of chemical literacy is supposed to be measured by these sub-items partially emerged. For example, the fourth alternative of item set 1 (1d) includes chemical content knowledge, chemistry in context, and higher order learning skills. After that, the researchers came together for consensus, and Table 5 was constructed with 0.90 agreements. Additionally, the professors and two researchers from the field examined the rubric which is prepared for the evaluation of CLIs. The confirmation of the accuracy and appropriateness of the content was established upon expert agreement. Each researcher evaluated all of the students’ responses according to the rubric. The inter-rater reliability of the scores was established as 0.89.
Analysis of the data
Students’ responses to the thermochemistry and thermodynamics concept test (TTCT) and chemical literacy items (CLIs) were entered to SPSS (Statistical Package for the Social Sciences). Total scores of students for each test were calculated. The gender and the type of the treatment were the independent variables of the study. Students’ CLI scores were the dependent variables of the study. TTCT scores served as a covariate in the main analysis. The descriptive and inferential statistics of the analysis are provided in the next section.Results and discussion
Statistical analysis of TTCT scores
Prior to implementation, an independent sample t-test was used to determine if statistically significant mean differences exist between EGs and CGs with respect to thermochemistry and thermodynamics concept test (TTCT) scores. The descriptive statistics for the TTCT scores is given in Table 6.| Test | N | Mean | Std. dev. | |||
|---|---|---|---|---|---|---|
| CG | EG | CG | EG | CG | EG | |
| TTCT | 58 | 60 | 12.22 | 12.20 | 2.18 | 2.45 |
Before this computation, the assumptions of the t-test; normality, independence of observations along with the equality of variances were checked. The distribution was normal, the assumptions were not violated.
As seen from Tables 6 and 7, the mean difference of EGs (M = 12.20, SD = 2.45) and CGs (M = 12.22, SD = 2.18) was not statistically significant with respect to TTCT scores, t(116) = 0.046, p > 0.05. Such a result indicated that no difference between groups in their pre-conception related to TT concept scores prior to implementation existed. Although no significant difference is observed, by subtracting its effect out we remove more extraneous variability from the post-test scores. That means if a significant mean difference could be observed in CLI scores of groups, it could be totally attributed to the effect of the treatment types.
| t | df | p | |
|---|---|---|---|
| TTCT | 0.046 | 116 | 0.963 |
Statistical Analysis of Chemical Literacy Items (CLIs)
The research questions stated previously were tested using ANCOVA (Analysis of covariance) with the CLI score which was used as a dependent variable, and TTCT as a covariate. The treatment and gender were independent variables with two categories. The analyses were conducted at a 0.05 significance level using SPSS. The descriptive statistics of CLI scores is given for both EGs/CGs and males/females. The number of participants was 58 for CGs, 60 for EGs for CLIs. Table 8 gives related descriptive statistics.| Mean | Std. dev. | |||
|---|---|---|---|---|
| CG | EG | CG | EG | |
| CLIs | 26.79 | 32.97 | 7.18 | 5.47 |
The mean score of CLIs for students in EGs was higher than the mean of the CGs (32.97 and 26.79 respectively).
The mean score of CLIs for females was higher than the mean of the males (30.80 and 28.71 respectively, Table 9).
| Mean | Std. dev. | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| CLIs | 28.71 | 30.80 | 5.76 | 7.78 |
In addition to means reported in Table 10, the standard deviation for males of CGs was 5.65, females of CGs was 8.29, males of EGs was 4.64, and females of EGs was 5.79. Later the assumptions of ANCOVA were controlled and no violation was observed.
| Mean | ||||
|---|---|---|---|---|
| CG | EG | |||
| Male (26) | Female (32) | Male (23) | Female (37) | |
| CLIs | 26.31 | 27.19 | 31.43 | 33.92 |
Based on the results given in Table 11 research questions were answered, the findings indicated a significant mean difference (p = 0.000) between the chemical literacy level of the groups exposed to CBA and groups exposed to traditionally designed instruction in favor of EGs. Whether there was a difference between mean scores of males and females was also investigated. The results revealed that the difference was not statistically significant (p = 0.151) across the gender with respect to CLI scores. The results showed that the gender and treatment did not interact for CLI scores, that is, the treatment did not significantly favor females or males in improving their chemical literacy level.
| Source | df | Mean square | F | Sig. | Partial eta squared | Noncent. parameter | Observed power |
|---|---|---|---|---|---|---|---|
| Corrected Model | 4 | 307.681 | 7.550 | 0.000 | 0.211 | 30.202 | 0.996 |
| Intercept | 1 | 3914.254 | 96.056 | 0.000 | 0.459 | 96.056 | 1.000 |
| Treat | 1 | 1000.921 | 24.563 | 0.000 | 0.179 | 24.563 | 0.998 |
| Gender | 1 | 85.058 | 2.087 | 0.151 | 0.018 | 2.087 | 0.299 |
| Treat* Gender | 1 | 18.307 | 0.449 | 0.504 | 0.004 | 0.449 | 0.102 |
In general, EG students had higher correct responses than control groups for all items (see Table 12), implying better comprehension of chemical content knowledge, chemistry in context issues, and higher-order learning skills. The first item-set which was about the Combi-boiler (STS issue, see Appendix A) was the most answered item set with the smallest percentage of no response (CGs has 10.34; EGs has 8.2). Probably, such a contextual item attracted students’ attention more than other items since the news used was posted widespread and the society had speculated on it extensively. Another reason may be the nature of this item which mainly tries to measure chemical content knowledge. Both contextual and traditional frameworks are designed to support students’ content knowledge with a slight difference in terms of supporting abilities.
| Item-sets | Choices | Control groups | Experimental groups | ||||
|---|---|---|---|---|---|---|---|
| Mean | Mean per item | % NR | Mean | Mean per item | % NR | ||
| a Note: % NR: percentage of students with no response; numbers in parentheses show the maximum scores that can be obtained from an item or a choice. | |||||||
| 1 (15) | a (2) | 1.14 | 10.82 | 10.34 | 1.17 | 11.41 | 8.2 |
| b (3) | 2.08 | 2.14 | |||||
| c (3) | 2.29 | 2.31 | |||||
| d (3) | 2.13 | 2.16 | |||||
| e (4) | 3.18 | 3.63 | |||||
| 2 (10) | a (2) | 1.22 | 5.19 | 18.97 | 1.47 | 7.49 | 11.67 |
| b (2) | 1.35 | 1.56 | |||||
| c (3) | 1.33 | 2.87 | |||||
| d (3) | 1.29 | 1.59 | |||||
| 3 (10) | a (5) | 1.64 | 3.76 | 20.69 | 1.88 | 5.62 | 13.33 |
| b (5) | 2.12 | 3.74 | |||||
| 4 (10) | Fill in the blanks (10) | 7.02 | 7.02 | 12.07 | 8.45 | 8.45 | 8.33 |
| Overall | 45 | 26.79 | 32.97 | ||||
As seen from the data reported in Table 12, the mean scores of the groups are slightly different for the items assessing students’ chemical content knowledge; however, because of a striking difference in the mean scores of related sub-items, it can be stated that EGs were better in utilizing their chemistry knowledge in explaining everyday phenomena (see Table 12 for the results of the sub-items such as 1e). According to PISA literacy description, we can report that EGs used more scientific terms in explaining life by chemical processes and structures of living systems. One of the students from the experimental groups wrote about the 5th sub-item of the combi-boiler which was about scientifically explaining the death of young people as follows:
“Possibly, there was a problem with the combi-boiler system in that house, so the products of burning reactions were entering the house instead of going out. This means the concentration of oxygen in the house was decreasing, so both people and burning reaction has affected from. The product of reaction becomes carbon monoxide instead of carbon dioxide, which is seriously harmful to our body. Since this gas leaked into the house, the people had died due to poisoning”.
Another student from the same group explained the reason for their death in this way:
“CO2 molecules cannot combine with the complex molecule of hemoglobin. When the concentration of gases – changed in the room, the CO concentration increased and CO molecules combined with hemoglobin in a coordinated covalent bond, thus contaminating the nature of blood and its pH level, and result in dead”.
A student from CGs explained the phenomena as given below:
“The combi-boiler used in that house was problematic; the burning of CH4 produced more carbon monoxide. This carbon monoxide (CO) is quite harmful to human health, so the people in that house died because of this dangerous gas and because the amount of oxygen decreased in that house”.
Compared to the responses of CG students, the researchers detected more explanations about life by utilizing chemical processes and the structures of living systems. Although both groups mostly responded to the items on the combi-boiler, EG students used more scientific terms in their answers.
In addition, one can attribute higher mean scores to better content knowledge and abilities; we think that such an outcome can also be attributed to high or less interest of students. In our designs, the percentages of no response (NR) for EGs were lower than CGs, which can be evaluated as more interest in explaining the issues. Based on the Shwartz et al. (2006) chemical literacy definition, literate students show high interest in issues of chemistry, specifically in media news, so especially about item-set 1 and item-set 4; we can say that affective aspects are supported because of low NR percentages. Besides, as Fensham (2009) reported, the difficulty or ease that students have in explaining a contextual set of item may be due to the more/less familiarity, more/less interesting, or more/less relevance to individuals.
The contextual item set on ‘energy concepts in biological systems’ had revealed a striking difference in terms of correct responses between content knowledge-based items (2a, 2b) and chemistry in context decision-process required items (2c, 2d) (see Table 12). The sub-item (2c) of this set required students to connect their chemistry knowledge to biology knowledge (multidimensional literacy). The CBA framework for EGs was enriched with reading and writing activities that could serve as tools for students to analyze, interpret, and declare scientific ideas. In our design, as Glyn and Muth (1994) stated, such kinds of activities could help students’ minds to engage in complex reasoning and problem-solving processes. Additionally, although such a kind of item enables a more holistic and balanced approach to the underlying science (Fensham, 2009), a majority of the students had difficulty in making this connection for control groups. A possible reason for such a result is explained by Bybee (1997) as this level of literacy is more difficult to be fully attained and a student may have a high level of literacy on a specific subject matter, but a low level in others.
Some common difficulties of students related to concepts of thermochemistry and thermodynamics were also addressed within sub-items of CLIs. For example, in the third sub-item of the second item set (2c), the students were expected to classify correctly ATP-ADP and ADP-ATP conversions to the concepts of bond formation, bond dissociation, energy absorption, or energy release. Possibly, due to more minds-on activities, such as reading, writing, discussions, and decision-making processes on real-world issues in experimental groups, these students provided better explanations about that during ATP-ADP conversion both bond dissociation and bond formation occur. They were generally better in providing valid pro and con ideas related to this knowledge. Boo and Watson (2001) revealed that students categorize bond-formation as energy requiring reactions and bond-dissociation as energy releasing reactions. They claimed that this was about students’ ideas from the macroscopic world in which energy is usually required to do something. Additionally, they stated that such a kind of conceptualization might be related to students’ everyday lives or biology lessons in which they learn that degradation of food is the energy source for living. Barker and Millar (2000) reported that some students aging 16–18 have the idea that energy was released from fossil fuels. Students consider that when bonds are broken energy is released. Goedhart and Kaper (2002) stated that students’ conceptualization may be aroused from their biology knowledge since in this lesson they learn that ATP contains energy-rich bonds.
The third contextual item set concerning order–disorder was the least answered item by students, it might be perceived as the most difficult item in CLIs and students have the smallest mean score related to it. Besides, percentage of NR for this item was higher than for other items. Few students from experimental and control groups could get the maximum correct response score (5) for one sub-item of this set (3b), which is related to isothermal stretching of an elastic polymer band. Control group (CG) students lacked more in explaining the macroscopic level with molecular structures when compared to experimental groups (EGs). Similarly, EG students had also problems in utilizing knowledge of chemistry in explaining everyday situations and effectively decision making processes for understanding daily life chemistry. Possibly the reason is that they had difficulty in transferring the knowledge of ΔSsystem = qsystem/T to an isothermal process. The common misconception that ‘Entropy of the whole system decreases or does not change when a spontaneous change occurs in an isolated system’ (Sözbilir and Bennett, 2007) may be another reason for the lack of knowledge and knowledge transfer in that item set. The unfamiliarity of the context to the students’ everyday lives can be also regarded as a cause for lower mean scores.
Volcanoes and related chemical terms was a contextual item set which was answered by majority of students correctly. Experimental group students had high scores compared to control group students on transferring their thermochemistry and thermodynamics knowledge to a real-life context about volcanic eruption. This item set was developed from inspirations taken from the eruption occurred in Iceland in 2010. Probably because of the acquaintance of the news covered in the media, students engaged in this item-set more easily and they could transfer their knowledge about systems, energy transformations, internal energy, entropy, spontaneous/nonspontaneous changes, and laws of thermodynamics to a text about volcanic eruption. Such implementation utilized in this study contributed to students’ higher order thinking skills in developing their chemical literacy level in TT concepts by providing them to transfer their knowledge to other real-life contexts. Additionally, higher mean scores of EG students can be interpreted as this design stimulated the affective domain of students’ more positively than the traditional one since the item is answered by EGs more than CGs.
The framework of CBA in EGs offered better learning environments for students. As Fensham (2009) stated that context-based teaching led to engagement of more students in science concepts and therefore, students of CBA groups have better learning gains specifically on developing literacy skills on the specific concepts. Similarly, referring to the PISA description of literacy, students in EGs showed better skills in argumentation by providing pro and con ideas; they used more scientific terms in explaining the problems. According to Kortland (2007) relating science to everyday situations has potential to make teaching more interesting and this will make large proportion of students to be more motivated to learn so that students will better understand concepts.
Conclusion
The present study revealed that the design used as a context-based approach (CBA) is better to improve students’ chemical literacy level on thermochemical and thermodynamics (TT) concepts when compared to the traditional instruction (TI). A framework in which students’ literacy skills are promoted through contextual instruction was implemented in experimental groups, and a traditional design was framed for control group students. The contextual item sets were developed based on the PISA 2006 items proposing to measure students’ scientific literacy and the questions developed by Witte and Beers (2003) for the assessment of chemical literacy. The components of chemical literacy defined by Shwartz et al. (2006) were utilized during the development and assessment of chemical literacy items.The whole unit of thermochemistry and thermodynamics was instructed through a specific design of contextual approach for experimental groups. In this design the context was used as an entry point, a reading assignment on historical development of cars and car engines established the contact phase for students. Watching the videos on four-stroke car engines served to attract students’ curiosity for the next steps. Teacher-driven activities, such as questions directed to elaborate the context and the subject established a need-to-know base for concept construction. The design enabled the teacher to drive questions and connect the answers to the context used and other relevant contexts. As Parchmann et al. (2006) stated the use of context creates learning environments to stimulate students’ personal mental activities to enable progression of learning successfully. In our design, the next phases continued with hypothesis-testing and knowledge inventory in which well-established activities, such as carrying out experiments, were integrated into context-based lessons as necessary means to comprehend the topic. In later phases, the students wrote a report by applying their knowledge and discussed their reports with peers. Such activities supported social embeddedness through participation in discussions and decision-making processes. In these discussions and reflection processes students asked relevant and scientifically sound questions which is perceived as critical in learning environments to develop scientific literacy (Penick et al., 1996).
In the traditional framework implemented in the control groups, the entry point was the textbook instead of a context. The expository teaching with questioning and discussions were parallel to the flow of the teaching in experimental groups (EGs). The same experiments were carried out in the control groups (CGs), but without hypothesis forming and connecting the concepts to a specific context. In EGs, the unit was covered within the context through inquiry-based learning environment since, as reaffirmed by National Science Education Standards (National Research Council, 1996), inquiry is central to the achievement of scientific literacy. In both EGs and CGs, inquiry as content understanding, in which students have opportunities to construct concepts, patterns and to create meaning about an idea in order to explain what they experience was satisfied. According to Bybee (2000) inquiry is more than the content understanding and also refers to abilities. Different from CGs, in EGs, about the abilities or skills, questions were identified, hypotheses were formed, scientific investigations were designed and conducted, scientific explanations were formulated and revised, and scientific arguments were communicated and defended.
We conclude that our specific design was an effective way of instruction for better chemical literacy level on TT concepts regardless of the gender difference. As De Jong (2006) pointed out the contexts used should be well-known and relevant for girls and boys. Obeying such an outcome, our contextual design possibly made the lessons more relevant to students and the context was well-known for them, thus did not cause a difference between the groups. Furthermore, the selection of context in context-based approaches is critical in order to avoid causing superiority for males or females; we can state that the instruction used in this study favored neither males nor females. The recommendation given by Taasoobshirazi (2007) was that gender differences in achievement as well as the motivation to learn physics may be minimized by context-based instruction. Our findings support this due to non significant difference between males and females.
Related to the limitations of the study, our findings are narrowed to the concepts of TT. Additionally, students’ literacy skills are not in a steady state; rather they progress through the educational process; so that, not only experimental groups, but also control groups’ literacy level may improve at the end of the implementations. However, as the results indicated, the improvement is higher in EGs. A different research design (pre-post test design) would compare the gain of the groups. Also, the conclusions drawn from the study are limited to the instant chemical literacy level of eleventh-grade science major students exposed to contextual teaching and traditional teaching. Besides, CLIs may seem to have little about affective concerns, which is the fourth component of chemical literacy definition of Shwartz et al. (2006). When comparing both groups’ responses, regardless of being correct, we observed that the EGs had more things to say. This may be evaluated as the contribution of the design to affective issues in EGs. Besides, the use of a contextual approach itself is perceived as a way of evoking students’ affective aspects.
Further studies may utilize an instrument measuring students’ interests or attitudes as pre- and post-test to strengthen their results. Other educational approaches may have a similar effect on students’ literacy skills as well. CBA with such a framework has the possibility of improving students’ chemical literacy skills in abstract and difficult concepts. Also, further studies may utilize such a design in other chemistry concepts, too. In order to develop higher-order cognitive skills, that is, in communication, reflection and evaluating controversial issues within the STS-framework further studies may also create their own contextual designs and items to develop literacy skills.
In our opinion, one promising way to help students to close the gap between school chemistry, its applications in the development of science and technology, and its critical evaluation can be handled by designing chemistry lessons including real-world contextual issues and discussions involving science and technology. Students’ literacy skills may improve provided that a design is oriented towards their interests, the relevance of chemistry to their lives is ensured, and that inquiry-based content understanding along with inquiry as supporting the abilities is utilized. Although Bennett et al. (2007) recommend that CBA implementations enhance literacy skills, the literature lacks intervention studies pointing out this. The contribution of the present study to chemistry education is that, as an intervention study, it designs its own framework for improving literacy skills through constructing original items to measure these skills.
Appendix A: the chemistry of combi-bolier
Above, on 3 January 2009, a high circulation newspaper headline “Turkey Awoke to Big Pain” is given. The deaths of seven young on the New Year's Eve in Ankara were announced. The cause of the death was the combi-boiler.
Based on the working principle, a combi-boiler is divided into three categories, these are, a combi with chimney, hermetic combi, and combi with a condensing mechanism. Below, the difference between a combi with chimney and a hermetic combi is given.
There is no fan in a combi-boiler with chimney. Waste gas expands due to temperature increase, and is thrown to the outside through the chimney due to a natural chimney draft. Therefore, a combi-boiler with chimney must be connected to a building in accordance with standards. Otherwise, the leaking waste gas contacts and poisons the people in the environment. The hermetic combi has a fan. Waste gas is thrown out through the inner pipe with the help of the fan to the external environment and, the outer pipe draws the air required for combustion in the combustion chamber. So a hermetic combi does not need a building with chimney. It can be mounted on any space with a connection to an open external environment. This type of combi does not consume inner oxygen and pollute the air in the room.
Combi-boilers use the heat evolved from the oxidation reaction of natural gas. Natural gas consists of approximately 95% of methane (CH4), the rest is hydrocarbons such as ethane (C2H6), propane (C3H8), butane (C4H10).
Please answer the following questions:
(a) One of the reactions proceeding in the heat room of the combi-boiler is given below. Complete the reaction and determine the sign of heat of the reaction.
CH4(g) + ⋯ → ⋯ + 2H2O(g) ΔH = ⋯
(b) What kind of systems is a hermetic combi-boiler? (Open, closed, isolated, isothermal…) Explain with your reasons.
(c) When 1 mole of methane (CH4(g)) is burned in the heat room of the combi-boiler, 212 kcal heat is evolved. What is the amount of heat when 256 g of oxygen is consumed? Decide whether the reaction is exothermic or endothermic? (MW of O2 = 32 g mol−1)
(d) It is known that the use of methane, CH4(g), is more advantageous compared to other hydrocarbons. What kind of advantages do you think they are?
(e) When the amount of oxygen is not enough, the oxidation reaction cannot completely proceed and one of the major products is CO(g) instead of CO2(g). When the concentration of CO(g) exceeds its normal value, it poisons people.
In the light of given information, explain the reason of death more scientifically. (There was a combi-boiler with chimney in the house of seven young people).
Notes and references
- Aikenhead G. and Ryan A., (1992), The development of a new instrument: 'Views on Science-Technology-Society' (VOSTS), Sci. Educ., 76(5), 477–492.
- Albe V., (2008), When scientific knowledge, daily life experience, epistemological and social considerations intersect: students’ argumentation in group discussions on a socioscientific issue, Res. Sci. Educ., 38(1), 67–90.
- Barker V. and Millar R., (1999), Students' reasoning about chemical reactions: what changes occur during a context-based post-16 chemistry course? Int. J. Sci. Educ., 21(6), 645–665.
- Barker V. and Millar R., (2000), Students' reasoning about basic chemical thermodynamics and chemical bonding: what changes occur during a context-based post-16 chemistry course? Int. J. Sci. Educ., 22(11), 1171–1200.
- Belt S. T., Leisvik M. J., Hyde A. J. and Overton T. L., (2005), Using a context-based approach to undergraduate chemistry teaching, Chem. Educ. Res. Pract., 6(3), 166–179.
- Bennett J. and Holman J., (2002), Context-based approaches to the teaching of chemistry: what are they and what are their effects? in Gilbert J. K., De Jong O., Justi R., Treagust D. F. and Van Driel J. H. (ed.), Chemical education: towards research-based practice, Dordrecht, the Netherlands: Kluwer Academic Publishers, pp. 165–184.
- Bennett J., Hogarth S. and Lubben F., (2003), A systematic review of the effects of context-based and Science–Technology–Society STS approaches in the teaching of secondary science, in Research Evidence in Education Library, London: EPPI-Centre.
- Bennett J., Campbell B., Hogarth S. and Lubben F., (2007), A systematic review of the effects on high school students of context-based and science–technology (STS) approaches to the teaching of science, York, UK: Department of Educational Studies, the University of York, retrieved June 12, 2007, from http://www.york.ac.uk/depts/educ/projs/EPPI/bennettsaarmste.pdf.
- Boo H. K. and Watson J. R., (2001), Progression in high school students’ (aged 16–18) conceptualizations about chemical reactions in solution, Sci. Educ., 85, 568–585.
- Bulte A. M. W., Westbroek H. B., De Jong O. and Pilot A., (2006), A research approach to designing chemistry education using authentic practices as contexts, Int. J. Sci. Educ., 28(9), 1063–1086.
- Bybee R. W., (1997), Achieving Scientific Literacy: From Purposes to Practice, Portsmouth, NH: Heinemann.
- Bybee R. W., (2000), Teaching science as inquiry, in Inquiring into Teaching Inquiry Learning and Teaching in Science, Washington, DC: American Association for the Advancement of Science.
- Bybee R., McCrae B. and Laurie R., (2009), An assessment of scientific literacy, J. Res. Sci. Teach., 46(8), 865–883.
- Ceylan E., (2004), Effects of instruction using conceptual change strategies on students' conceptions of chemical reactions and energy. (Unpublished master's thesis), Middle East Technical University, Ankara, Turkey.
- Champagne A. B. and Newell S. T., (1992), Directions for research and development: alternative methods of assessing scientific literacy, J. Res. Sci. Teach., 29, 841–860.
- De Jong O., (2006), Context-based chemical education, how to improve it? retrieved May 29, 2014 from http://old.iupac.org/publications/cei/vol8/0801xDeJong.pdf.
- De Vos W. and Verdonk A. H., (1985a), A new road to reactions, J. Chem. Educ., 62, 238–240.
- Duschl R. A. and Osborne J., (2002), Supporting and promoting argumentation discourse in science education, Studies in Science Education, 38, 39–72.
- Fensham P. J., (2009), Real world context in PISA science: implications for context-based science education, J. Res. Sci. Teach., 46(8), 884–896.
- Gilbert J. K., (2006), On the nature of “context” in chemical education, Int. J. Sci. Educ., 28(9), 957–976.
- Gilbert J. K. and Treagust D., (2009), Introduction: macro, submicro and symbolic representations and the relationship between them: key models in chemical education, in Gilbert J. K. and Treagust D. (ed.), Multiple representations in chemical education, The Netherlands: Springer, pp. 1–8.
- Glyn S. M. and Muth K. D., (1994), Reading and Writing to Learn Science: Achieving Scientific Literacy, J. Res. Sci. Teach., 31(9), 1057–1073.
- Goedhart M. J. and Kaper W., (2002), From Chemical Energetics to Chemical Thermodynamics, in Gilbert J. K. et al., (ed.). Chemical Education: Towards Research-Based Practice, Wouters-Kluwer: Dordrecht, the Netherlands, 339–362.
- Greenbowe T. J. and Meltzer D. E., (2003), Student learning of thermochemical concepts in the context of solution calorimetry, Int. J. Sci. Educ., 25(7), 779–800.
- Kelly V., L., (2007), Alternative assessment strategies within a context-based science teaching and learning approach in secondary schools in Swaziland, unpublished doctoral dissertation, University of the Western Cape, Cape Town, South Africa.
- Koballa T. R. and Glynn S. M., (2007), Attitudinal and motivational constructs in science learning, in Abell S. K. and Lederman N. G., (ed.), Handbook of research in science education, Mahwah, NJ: Lawrence Erlbaum Associates, pp. 75–102.
- Kortland J., (2007), Context-based science curricula: Exploring the didactical friction between context and science content, paper presented at ESERA Conference 2007, Malmo, Sweden.
- Laugksch R. C., (2000), Scientific literacy: a conceptual overview, Sci. Educ., 84(1), 71–94.
- Laugksch R. C. and Spargo P. E., (1996a), Development of a pool of scientific literacy test-items based on selected AAAS literacy goals, Sci. Educ., 80, 121–143.
- Laugksch R.C. and Spargo P. E., (1996b), Scientific literacy test items, Cape Town, SA: University of Cape Town.
- Lubben F., Bennett J., Hogarth S. and Robinson A., (2005), A systematic review of the effects of context-based and Science-Technology-Society (STS) approaches in the teaching of secondary science on boys and girls, and on lower-ability pupils, in Research Evidence in Education Library, London: EPPI-Centre, Social Science Research Unit, Institute of Education, University of London.
- Miller J. D., (1983a), The American People and Science Policy, New York: Pergamon Press.
- Miller J. D., (1983b), Scientific literacy: a conceptual and empirical review, Daedalus, 112(2), 29–48.
- Miller J. D., (1995), Scientific literacy for effective citizenship, in R. E. Yager (ed.) Science/Technology/Society as Reform in Science Education, New York: State University Press of New York.
- Miller J. D., (2004), Public understanding of, and attitudes toward scientific research: what we know and what we need to know, Public Understanding of Science, 13, 273–294.
- Miller J. D., (2010), The Conceptualization and Measurement of Civic Scientific Literacy for the Twenty-First Century, in Meinwald J. and Hildebrand J. G. (ed.), Science and the Educated American: A Core Component of Liberal Education, American Academy of Arts and Sciences, pp. 1–20.
- Miller J. D. and Pardo R., (2000), The development of civic scientific literacy in the United States, in Kumar D. D. and Chubin D. (ed.) Science, Technology, and Society: A Sourcebook on Research and Practice, New York: Plenum Press, pp. 21–47.
- National Research Council., (1996), National science education standards, Washington, DC: National Academy Press.
- Norris S. P. and Phillips L. M., (2003), How literacy in its fundamental sense is central to scientific literacy, Sci. Educ., 87(2), 224–240.
- OECD, (2000), retrieved from http://www.oecd-ilibrary.org/economics/oecd-annual-report-2000_annrep-2000-en, last accessed on 12 May, 2014.
- OECD, (2003), Retrieved from http://www.oecd-ilibrary.org/economics/oecd-annual-report-2003_annrep-2003-en;jsessionid=2p82ij0g1bm6c.x-oecd-live-0, last accessed on 12 May, 2014.
- OECD, (2004), The PISA 2003 Assessment Framework: Mathematics, Reading, Science and Problem Solving Knowledge and Skills, Paris: PISA, OECD Publishing.
- OECD, (2006), Assessing scientific, reading and mathematical literacy: A framework for PISA 2006, Paris: OECD.
- Osborne J. and Dillon J., (2008), Science education in Europe: critical reflections, London: King’s College.
- Parchmann I., Gräsel C., Baer A., Nentwig P., Demut R., Ralle B. and The ChiK Project Group, (2006), “Chemie im Kontext”: a symbiotic implementation of a context-based teaching and learning approach, Int. J. Sci. Educ., 28(9), 1041–1062.
- Penick J. E., Crow L. W. and Bonnsteter R. J., (1996), Questions are the answers, The Science Teacher, 63, 26–29.
- Pilot A. and Bulte A. M. W., (2006), Why do you “need to know”? context-based education, Int. J. Sci. Educ., 28(9), 953–956.
- Ratcliffe M. and Millar R., (2009), Teaching for understanding of science in context: evidence from the pilot trials of the Twenty First Century Science courses, J. Res. Sci. Teach., 46(8), 945–959.
- Scantlebury K. and Baker D., (2007), Gender issues in science education research: remembering where the difference lies, in Abell S. and Lederman N. (ed.), Handbook of research on science education, Mawhah, New Jersey: Lawrence Erlbaum, pp. 257–286.
- Schwartz A. T., (2006), Contextualized chemistry education: the American experience, Int. J. Sci. Educ., 28(9), 977–998.
- Shwartz Y., Ben-Zvi R. and Hofstein A., (2005), The importance of involving high-school chemistry teachers in the process of defining the operational meaning of ‘chemical literacy’, Int. J. Sci. Teaching, 27, 323–344.
- Shwartz Y., Bez-Zvi R. and Hofstein A., (2006), The use of scientific literacy taxonomy for assessing the development of chemical literacy among high-school students, Chem. Educ. Res. Pract., 2006, 7(4), 203–225.
- Sözbilir M., Bennett M., J., (2007), A study of Turkish chemistry undergraduates’ understandings of entropy, J. Chem. Educ., 84(7), 1204–1208.
- Taasoobshirazi G., (2007), Gender differences in physics: a focus on motivation, Journal of Physics Teacher Education Online, 4(3), 7–12.
- Taasoobshirazi G. and Carr M., (2008), A review and critique of context-based physics instruction and assessment, Educ. Res. Rev., 3(2), 155–167.
- Taber K. S., (2003), Examining structure and context – questioning the nature and purpose of summative assessment, School Science Review, 85(311), 35–41.
- Westbroek H. B., (2005), Characteristics of meaningful chemistry education, the case of water quality, Unpublished doctoral dissertation, The Netherlands: Utrecht University, Utrecht.
- Westbroek H. B., Bulte A. M. W. and Pilot A., (2000), Development of a prototype module: an example of a new vision on an A-level chemistry curriculum, in De Jong O., Savelsbergh E. R. and Alblas A. (ed.), Teaching for scientific literacy: context, competency, curriculum, Utrecht: Cdβ-Press.
- Witte D. and Beers K., (2003), Testing of chemical literacy (chemistry in context in the Dutch national examination), Chem. Educ. Int., 4(1), 1–3.
- Whitelegg E. and Parry M., (1999), Real-life contexts for learning physics: meanings, issues, and practice, Phys. Educ., 34(2), 68–72.
- Yeo S. and Zadnick N., (2001), Introductory thermal concept evaluation: Assessing students' understanding, The Physics Teacher, 39, 495–505.
| This journal is © The Royal Society of Chemistry 2015 |