Grade 12 students' conceptual understanding and mental models of galvanic cells before and after learning by using small-scale experiments in conjunction with a model kit
Saksri
Supasorn

Faculty of Science, Ubon Ratchathani University, Ubon Ratchathani, 34190 Thailand. E-mail: saksri.supasorn@gmail.com
First published on 13th March 2015
Abstract
This study aimed to develop the small-scale experiments involving electrochemistry and the galvanic cell model kit featuring the sub-microscopic level. The small-scale experiments in conjunction with the model kit were implemented based on the 5E inquiry learning approach to enhance students' conceptual understanding of electrochemistry. The research tools consisted of (1) four small-scale experiments involving electrochemistry, which were oxidation and reduction reactions, galvanic cells, cathodic protection of iron nails, and connecting batteries in series, and (2) the galvanic cell model kit with the ability to generate various galvanic cells. The data collecting tools included (1) a conceptual test of electrochemistry and (2) the mental model drawing of a galvanic cell. Thirty-four grade 12 students participated in the series of four 5E learning activities for a total of 10 hours. Paired samples T-test analysis revealed that the mean scores of the post-conceptual test (mean 36.63, SD 7.69) was statistically higher than that of the pre-conceptual test (mean 21.51, SD 6.83) at the significance level of 0.05. In addition, the mean scores of the post-mental models in both the macroscopic (mean 3.56, SD 1.30) and sub-microscopic features (mean 5.98, SD 2.93) were statistically higher than those of the pre-mental models (mean 1.85, SD 1.11 and mean 2.20, SD 2.45) at the significance level of 0.05. Prior to intervention, most students were in the categories of less correct conceptions, Partial Understanding with Specific Misunderstanding (PMU) to No Understanding (NU). However, after the intervention, they moved to the categories of more correct conceptions, Partial Understanding (PU) to Sound Understanding (SU). This indicated that this intervention can enhance students' conceptual understanding of electrochemistry and mental models of galvanic cells.
Introduction and background
Almost all high school students are required to study electrochemistry in both lecture and laboratory settings. Many students revealed that it is one of the difficult chemistry topics since it involves intangible concepts that cannot be accessed with direct perception. In addition, some students may hold alternative conceptions – conceptions that are not consistent with the consensus of the scientific community which may be partially right but incomplete, or just simply wrong (Mulford and Robinson, 2002). Students' misunderstandings, alternative conceptions, or misconceptions, some of which cannot be measured by traditional instruments (Stears and Gopal, 2010), influence their future learning. Therefore, instructors should encourage learning by the use of activities that promote students' conceptual change (Demirbas and Ertugrul, 2014). Requiring students to draw and explain molecular representations of some electrochemistry experiments, such as reactions in galvanic cells, may reveal their understandings and identify some of their alternative conceptions.Alternative conceptions in electrochemistry
Electrochemistry, including its involved concepts, is a complex subject that has considerable importance in many applications (Miller, 2014). It is one of the topics in which students tend to hold alternative conceptions since it is difficult to visualize and relate what occurs at the sub-microscopic level (also called particulate nature or molecular level) to the macroscopic (experiment observation) and symbolic levels (Çalik et al., 2010; Taber, 2013). It involves both qualitative and quantitative aspects which concern the transfer of electrons between different chemical species. The amount of transfer depends on the concentration of the presented species (Miller, 2014).There are many research studies that investigated students' alternative conceptions involving electrochemistry and utilized various intervention tools to improve students' conceptions. It is reported that not only high school students (Garnett and Treagust, 1992; Niaz, 2002; Niaz and Chacón, 2003; Acar and Tarhan, 2007; Osman and Tien Lee, 2014) and college (university) students (Sanger and Greenbowe, 1997a; Hawkins and Phelps, 2013), but also pre-service and in-service science and chemistry teachers (Ahtee et al., 2002; Ökaya, 2002; Aydeniz and Krbulut, 2011; Ekiz et al., 2011; Yakmaci-Guzel, 2013) tended to have alternative conceptions and misconceptions in electrochemistry and its related topics. These studies found that both high school and college students had learning difficulties and misconceptions about galvanic, electrolytic, and concentration cells. The identification of misconceptions is important to help learners understand these topics meaningfully (Sanger and Greenbowe, 1997a).
Sanger and Greenbowe (1997b) summarized common alternative conceptions or misconceptions in electrochemistry. Examples of these common misconceptions are that electrons move through a solution by being attracted from one ion to another, move through a solution by attaching themselves to ions at the cathode and are carried by that ion to the anode, enter a solution from the cathode, travel through a solution and the salt bridge, and emerge at the anode to complete the circuit. Other examples include the idea that electrons can flow through aqueous solutions without assistance from the ions, anions in the salt bridge, and the electrolyte transfer electrons from the cathode to the anode, and cations in the salt bridge and the electrolyte accept electrons and transfer them from the cathode to the anode, only anions constitute a flow of current in the electrolyte and the salt bridge, and the anode is positive because it has lost electrons, while the cathode is negative because it has gained electrons.
Karsli and Çalik (2012) reviewed many articles and summarized major alternative conceptions encountered in electrochemistry as follows: (1) the cathode is a negative electrode, an oxidation half-cell that loses electrons, and decreases mass over time, (2) the anode is a positive electrode, a reduction half-cell that gains electrons, and increases mass over time, (3) the salt bridge allows electrons to travel from the anode to the cathode, supplies the ions which are necessary to move from the cathode to the anode, allows the cations to migrate toward the anode electrode, whereas the anions migrate towards the cathode electrode, and (4) a lack of reporting the cell reaction correctly.
Cullen and Pentecost (2011) reported evidence that chemistry textbooks and instructors are responsible for many students' alternative conceptions involving electrochemistry. Instructors tend to use everyday language that can lead to misinterpretation by their students. They then designed a paper model to teach about galvanic (voltaic) cells to address students' alternative conceptions by adaptation of an inexpensive, portable, and flexible teaching model by Huddle et al. (2000). This paper model was used in conjunction with electrochemistry laboratory activities and allows students to visualize that no electrons pass into the solutions and how mass of electrodes is gained and lost. The researchers also commented that as students demonstrated and discussed the model within their groups, they were constructing their own understanding of galvanic cells.
Three levels of representations in chemistry
Previous studies reported that many alternative conceptions in some intangible concepts arose from the fact that students have difficulty in understanding the relationship among representations in chemistry (Çalik et al., 2010; Cullen and Pentecost, 2011). Representations in chemistry, also called chemical representations, refer to various types of formula, structures, and symbols used to represent chemical processes and conceptual entities, such as molecules and atoms. They can be viewed as metaphors, models, and theoretical constructs of chemists' interpretations of nature and reality (Hoffmann and Laszlo, 1991). Previous research highlighted three levels of representations in chemistry (Johnstone, 1993; Chandrasegaran et al., 2007; Taber, 2013):(1) Macroscopic representation. This describes bulk properties of tangible and visible phenomena in the everyday experiences of learners when observing changes in the properties of matter, such as colour changes, formation of gases, and precipitates in chemical reactions.
(2) Sub-microscopic representation. This is also called molecular representation, and provides explanations at the particulate level in which matter is composed of atoms, molecules and ions.
(3) Symbolic representation. This involves the use of chemical symbols, formula, and equations, as well as molecular structure drawings, diagrams, and models to symbolize matter. It can provide information for both macroscopic (relative amounts or moles of involved substances) and molecular levels (numbers of formula unit of involved substances).
Çalik et al. (2010) considered studies of students' alternative conceptions in such topics as electrochemistry, acids and bases, chemical equilibrium, and rates of reactions. They concluded that some alternative conceptions arose because many students find it difficult to visualize chemical phenomena and/or processes at the sub-microscopic level and to link the macroscopic, sub-microscopic, and symbolic levels to each other. They also explored Turkish grade 11 students' conceptions of chemical reaction rates. Their intervention consisted of nine classes of 45 minutes using three guide sheets and 11 computer animations. The students were first given a student guide sheet containing scientific questions to promote curiosity and draw out their prior knowledge. They then were asked to interact with the animations followed by group discussions. Next, they were introduced to the concepts and processes of rates of reactions to enhance their conceptual understanding. Finally, they attempted to extend their understandings of the concept which were assessed by means of questions at the bottom of each guide sheet. The researchers reported that this teaching intervention could help students correct their alternative conceptions but may not completely eliminate them. Therefore, instructors should utilize more than one intervention model to overcome students' alternative conceptions.
Roles of mental models in learning chemistry
Students' conceptual understanding, especially intangible concepts in phenomena/processes/systems, involves the ability to relate to the three representations in chemistry. The term ‘mental model’ was introduced to describe how students construct a model of understanding of a specific process by the incorporation of new received information into their existing knowledge (Johnstone, 1993). Mental models are representations of objects, ideas, thinking, or processes which individuals intrinsically construct during cognitive functioning (Ibrahim and Rebello, 2013; Liu et al., 2014). People use these models to reason, describe, explain, and/or predict scientific phenomena, processes, or systems. Mental models can be generated in various formats to communicate ideas to other people or to solve problems (Ibrahim and Rebello, 2013; Liu et al., 2014), and can represent either physical entities via verbal descriptions, diagrams, simulations, and concrete models, or conceptual understanding, such as models of ideas, thinking, or intangible concepts (Coll and Treagust, 2003; Chandrasegaran et al., 2011). If their mental models fail to assimilate new experiences, students may modify their existing models or generate alternative models (Glynn and Duit, 1995). Mental models are considered as an important part of learners' conceptual frameworks (Glynn and Duit, 1995) and they play a potential role in learning chemistry at the molecular level because much of the chemistry involved at this level cannot be accessed by direct perception (Briggs and Bodner, 2005). Full understanding of chemical processes involves the ability to connect events at a macroscopic level with events at the molecular level (Johnstone, 1993). Therefore, students need to transform these invisible events or phenomena into equivalent mental or conceptual models or representations, which is difficult for many students (Doymus et al., 2010; Dixon and Johnson, 2011; Duis, 2011).Based on the information above, the term ‘mental models’ in this study context are the models of understanding (in form of drawings) that students use to relate and describe their understanding of how a galvanic cell functions at macroscopic, symbolic, and sub-microscopic levels.
5E inquiry learning activities
Inquiry learning activities were considered to be effective in teaching chemistry and have been highly advocated in the last few decades (Sanger, 2009). These types of activities possess more advantages over traditional approaches. These advantages include the encouragement of students to practice using learning resources and working in groups to enhance their conceptual understandings, and the opportunities for teachers to play roles as facilitators who motivate and challenge students to carry out the activities through a science inquiry process (Deters, 2005). The 5E learning cycle has been proven to be one of the most effective inquiry learning in chemistry (Bybee et al., 2006). It involves students through the following steps: (1) students are engaged in inquiry questions, (2) students explore answers to the questions by planning, designing, and carrying out their experiment, and recording the experiment data, (3) students make explanations from the experimental data to answer the questions, (4) students elaborate, extend, or apply their findings in a new context, and (5) students evaluate their experimental processes and results in a variety of ways. This learning cycle is effective to support students to notice and correct their alternative conceptions (Balci et al., 2006; Bybee et al., 2006).The literature review above suggests that the implementation of corresponding experiments through the 5E inquiry learning approach is effective to enhance students' conceptual understanding of the corresponding concepts. The use of inquiry experiments in conjunction with a corresponding model featuring the sub-microscopic level could be more effective to enhance students' conceptual understanding and mental models of the corresponding concepts. As a result, the combination of 5E inquiry experiments and a galvanic cell model kit featuring the sub-microscopic level was used as the intervention tools in this study to minimize students' difficulty in visualizing and relating what occurs at the sub-microscopic level to the macroscopic and symbolic levels of galvanic cells.
Research questions
Based on results from a pilot study, the implementation of small-scale experiments via the 5E inquiry learning approach is effective to enhance students' conceptual understanding at the macroscopic and symbolic levels, but not at the sub-microscopic level (Supasorn et al., 2014). This view arose from the fact that the implemented experiments contained insufficient information regarding sub-microscopic features. As a result, the galvanic cell model kit featuring macroscopic, sub-microscopic, and symbolic levels was developed to help students relate these representations to each other. The main purpose of this study was to investigate students' conceptual understanding of electrochemistry and mental model of a galvanic cell prior to and after the performance of corresponding experiments and the model kit based on 5E inquiry learning activities.These research questions were posed when the small-scale experiment activities in conjunction with the use of the model kit of galvanic cells were implemented:
(1) How do students' scores on the conceptual test of electrochemistry and on the mental model drawing of galvanic cells change before and after they performed the experiments in conjunction with the model kit of galvanic cells?
(2) How do the percentages of students in each conceptual understanding category in the conceptual test of electrochemistry and in the mental model of galvanic cells change before and after they performed the experiments in conjunction with the model kit of galvanic cells?
Research methodology
This one group pre-test/post-test study used a quantitative method in its research paradigm. However, some qualitative data obtained from informal interview regarding students' mental models of galvanic cells were applied to fulfill the quantitative part.Treatment tools
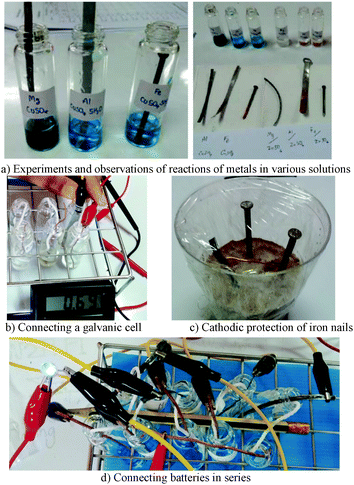
Two types of treatment tools were developed in this study, small-scale experiments and the galvanic cell model (zinc metal chart). The small-scale experiments consisted of (1) oxidation and reduction reactions (Fig. 1a), (2) galvanic cells (Fig. 1b), (3) cathodic protection of iron nails (Fig. 1c), and (4) connecting batteries (Fig. 1d).The experiments were designed with regard to some ‘green’ chemistry principles, such as reducing the amounts of chemicals used, toxic chemicals, and generated wastes (Poliakoff and Licence, 2007). The concentration and volume of solutions used in this study were 2.50 mL and 0.01 M. The terms ‘small-scale’ and ‘low-cost’ were applied since these experiments reduced the scale of the normal experiments by at least 1000 or 2000 times and used inexpensive equipment, chemicals, and substances. A cotton thread (with the length of 15–20 cm) pre-treated in 5.00 mL of 0.01 M of saturated potassium nitrate solution was used as a salt bridge (Khattiyavong et al., 2014). Such experiments can minimize costs and amounts of chemicals, laboratory glassware and equipment requirements, waste production, high expense for waste disposal, and time-consuming activities, while maintaining the concepts of the experiments and necessary laboratory techniques and skills (Martin and Gilbert, 2011). The experiments were tried out with grade 12 students studying in Satrisiriket School in Srisaket Province of Thailand (Supasorn et al., 2014). Comments and suggestions from the students were used to improve the effectiveness of the experiments.
The galvanic cell model kit featuring the sub-microscopic level, also called the model kit or a model kit of galvanic cells, was adapted from the inquiry model approach to the electrochemical cell by Cullen and Pentecost (2011). The model kit consisted of two parts (Fig. 2).
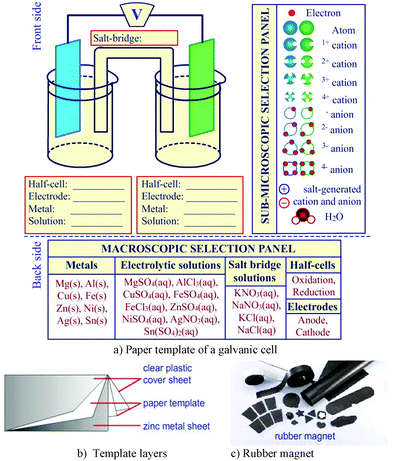 | ||
| Fig. 2 The galvanic cell model kit salt-generated ions and H2O molecules could be dismissed to focus on particles involving oxidation and reduction reactions). | ||
The first part was a galvanic cell template containing three layers, in which a sheet of zinc metal is inserted as the core layer followed by a paper template of the galvanic cell, and then covered with a clear plastic sheet. The back side of this template contained the instructions related to the use of the model. This part was printed on A3-size paper. The second part was the package containing sets of various metal electrodes, metal cations, electrons, and salt-generated anions and cations. All templates in the second part were glued with rubber magnets (cutting by using scissors or cutters) to make it possible for students to magnetize them into the zinc metal core of the model kit. If students found it difficult to illustrate the water (H2O) molecules and/or salt-generated ions (i.e., K+, Na+, Cl−, NO3− ions) in the model kit, these particles were able be omitted to allow students to concentrate on only the particles involving oxidation and reduction in galvanic cells as suggested by Cullen and Pentecost (2011). In addition, covering one out of two half-cells in the model kit can demonstrate how the oxidation or reduction reactions of metals occur in each half-cell (containing the metal ion solution).
Data collection tools
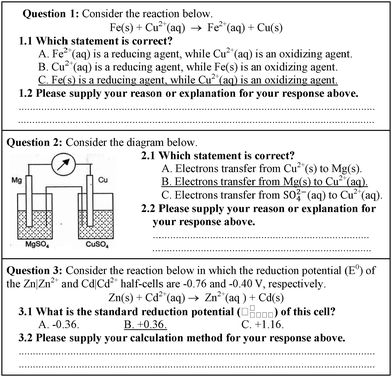
There were two types of data collection tools in this study. The first one was the conceptual test of electrochemistry containing 24 items in a two-tier three-choice test. There were 9 and 15 items regarding the concepts of oxidation–reduction reactions and galvanic cells, respectively. The concepts of batteries (3 out of 15 items) and cathodic protection (3 out of 15 items) were considered as sub-concepts of galvanic cells so they were included in the concepts of galvanic cells in this study. Students were required to make their choices of answers in the first tier and then provide their explanations for those choices in the second tier (examples of the test are shown in Fig. 3) (Treagust, 1988; Chandrasegaran et al., 2007). The test was content-validated by two senior chemistry lecturers and one chemistry education professor. There were four main concepts in the test including oxidation–reduction reactions, galvanic cells, batteries, and cathodic protection.The test items were analyzed by the use of a software called Simple Item Analysis (SIA) which is commonly used in Thailand. The difficulty index (P) for each item was in the range of 0.20–0.70, in which the percentages of items with P in the ranges of 0.20–0.39 (difficult), 0.40–0.59 (medium), and 0.60–0.80 (easy) were 20.00, 30.00, 40.00, and 10.00 respectively. The discrimination index (r) for each item was in the range of 0.30–0.90, in which the percentages of items with r in the ranges of 0.20–0.39 (fair), 0.40–0.59 (medium), 0.60–0.79 (good), and 0.80–1.00 (excellent) were 12.50, 20.83, 41.67, and 25.00 respectively. In addition, the reliability based on Kuder–Richardson Formula 20 or KR20 for the entire test was 0.87.
The second data collection tool was a mental model drawing of a galvanic cell. Students were asked to draw their understandings of what happens at the molecular level in a galvanic cell from two half-cells randomly provided Zn|Zn2+, Cu|Cu2+, and Ni|Ni2+ half-cells (see Fig. 4). For example, if students were asked to draw a mental model of a galvanic cell from Cu|Cu2+ and Ni|Ni2+ half-cells, they had to consider which one was an oxidation or a reduction half-cell, and to provide how ions and atoms in each half-cell (both in solutions and electrodes) changed regarding the progress of the reaction.
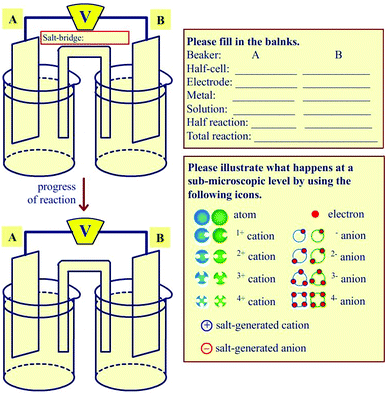 | ||
| Fig. 4 The task for a mental model drawing of a randomly given galvanic cell (i.e., Mg|Mg2+, Zn|Zn2+, Fe|Fe2+, Cu|Cu2+ and Ni|Ni2+ half-cells). | ||
Participants
With prior permission from the school principal and the instructor of the chemistry course during the first semester of the academic year 2014, 34 students out of 41 students (one classroom) who attended all activities throughout the study were purposively selected as the participants of this study. They were studying grade 12 at Srimuang Wittayakhan School, a regular, large high school, in Ubon Ratchathani province of Thailand. The participants were asked for permission to use their conceptual test information and to reproduce their drawings for the study report and publication.Notice that all research tools both treatment tools (lesson plans) and data collecting tools (conceptual tests and mental model drawings) were in Thai language. The class was taught in Thai language and all examples included in this article involved translation into English. In addition, these students had a chance to experience a two-tier conceptual test in which they were asked to explain their choices in the second tier in the previous semester. This could support students to be able to provide fruitful information in their explanation.
Implementation
Prior to the series of four 5E inquiry learning activities, the participants spent one hour to complete the conceptual test of electrochemistry and mental model drawing of a galvanic cell (pre-test and pre-mental model). The students were then divided into groups of four or five students and requested to participate in four 5E inquiry learning activities as shown in Table 1. They then started the inquiry activities in order of oxidation and reduction of metals in metal ion solutions, generation of galvanic cells, connection of series and parallel batteries, and cathodic protection of iron nails by using Zn and Mg metals. In each 5E learning activity, the students were requested to participate in the following process.| Plan (hours) | Key activities |
|---|---|
| 1. Pre-test (1.0) | – Pre-conceptual test and pre-mental model drawing. |
| 2. 5E learning (10) | |
| 2.1 Oxidation and reduction (3.0) |
Main question: How do metals react with metal ion solutions?
– Observing oxidation and reduction reactions of metals (Mg, Zn, Fe, Al, Cu) in metal ion solutions (Mg2+, Zn2+, Fe2+, Cu2+). – Demonstrating oxidation and reduction reaction by using the galvanic cell model kit. |
| 2.2 Galvanic cells (3.0) |
Main question: How can galvanic cells be generated from variety of half-cells? How do they react?
– Constructing galvanic cells from various half-cells (Mg|Mg2+, Zn|Zn2+, Fe|Fe2+, Cu|Cu2+) and measuring their cell voltages. – Generating galvanic cells by interacting with the model. |
| 2.3 Batteries (2.0) |
Main question: How can series and parallel batteries be generated and how do their reactions occur?
– Connecting batteries in series and in parallel using highest voltage galvanic cell obtained from previous experiment. – Demonstrating what occurs at sub-microscopic level of batteries by using the model kit. |
| 2.4 Cathodic protection (2.0) |
Main question: How can Mg and Zn metal protect iron nails from rusting?
– Observing cathodic protection of iron nails by using Mg and Zn as anode. – Demonstrating how cathodic protection works by using the model kit. |
| 3. Post-test (1.0) | – Post-conceptual test and post-mental model drawing |
| 4. Informal interview | – Unstructured interview regarding students, explanations in conceptual tests and in mental model drawings. |
(1) Engagement: they were engaged in a scientifically-oriented question in regard to electrochemistry (one main question in each experiment, see Table 1).
(2) Exploration: they explored and gathered data to answer the question by planning and carrying out an experiment.
(3) Explanation: they formulated explanations based on their summarized data and scientific knowledge to answer the question.
(4) Elaboration: they elaborated, extended, related, or applied their macroscopic and symbolic findings from the experiment to the sub-microscopic level by interacting with the model kit in regard to the question “Based on your experiment results at a macroscopic level and the equation at a symbolic level, how does the reaction occur at a sub-microscopic level?”
(5) Evaluation: they were evaluated regarding their understanding by means of class and group discussions together with demonstration of the model kit regarding the experiment concepts.
The oxidation and reduction topic was raised as an example of 5E inquiry learning activities in this study. The students were firstly engaged with the inquiry question “How does each metal (Mg, Fe, Al, Zn, and Cu) react with various metal ion solutions?” The instructor then summarized and wrote students' responses on the whiteboard. After that the instructor divided them into small groups and allowed them to plan and conduct an experiment to answer the engaged question by using the provided metals, solutions, and equipments. The instructors and two teaching assistants acted as facilitators during this step. After they completed their experiments, they were asked to summarize their experimental data and then formulate explanations to answer the engaged question. This step involved both macroscopic and symbolic representations. Next, they were asked to interact with the model kit to explain what happens at the sub-microscopic level based on their experiment results about how each metal (Mg, Fe, Al, Zn, and Cu) reacts with various metal ion solutions in front of the class. This step allowed students to elaborate what they experienced at the macroscopic and symbolic levels to the sub-microscopic level. Finally, they were evaluated regarding their conceptual understanding by requesting them to generate oxidation and reaction reactions for other metals (i.e., Sn, Ni and Ag) by using a model kit together with writing chemical equations. Please note that group and class discussions were encouraged during the explanation and elaboration steps.
After the completion of the four learning activities for a total of 10 hours, the students were asked to complete the conceptual test of electrochemistry (the same test with rearrangement of choice and item orders) and make changes to their mental model drawings or draw a new one (post-test and post-mental model). Finally, participants in each of “Sound Understanding: SU”, “Partial Understanding: PU”, “Partial Understanding with Specific Misunderstanding: PMU”, “Specific Misunderstanding: MU”, and “No Understanding: NU” categories were purposively selected for informal unstructured interview regarding their supplied reasons in the explanation tiers of the conceptual test and in their mental model drawings.
For the model kit part, the participants were asked to generate a specific galvanic cell out of various galvanic cells by interaction with the model of galvanic cells. For the macroscopic feature, they had to select the anode and cathode electrodes, electrolytic solution, and salt bridge solution and then put them on the oxidation and reduction half-cells of the model. For the sub-microscopic feature, they were required to choose the metal ions (with the right oxidation number), neutral atoms, electrons, and salt-generated cations and anions, and then put them into the oxidation and reduction half-cells of the model (see Fig. 5). Finally, they had to move these particles into the correct positions as the oxidation and reaction reactions progressed over time. The students also had to consider the numbers of neutral atoms, cations and anions in each half-cell, and electrons. These details were used as the criteria for grouping students into conceptual understanding categories.
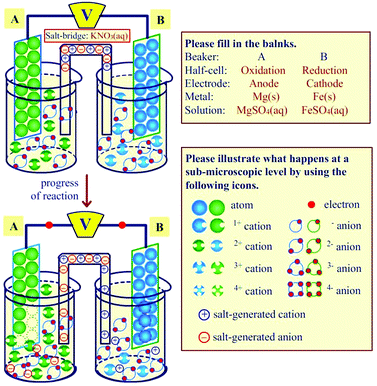 | ||
| Fig. 5 Example of a student generated Mg–Fe galvanic cell in the model kit, randomly given among Mg|Mg2+, Zn|Zn2+, Fe|Fe2+, Cu|Cu2+ and Ni|Ni2+. | ||
Data analysis
The data collected in this study were analyzed as follows:(1) The pre- and post-conceptual test answers were awarded 1.00 and 0.00 point for each correct and incorrect choice, respectively, in the first tier. Please note that the first tier may not be enough to identify if students accommodate misconceptions, while the explanation tier contains more relevant information about students' conception. Each explanation provided in the second tier was awarded 0.00, 0.25, 0.50, 0.75, or 1.00 point regarding the completeness of their explanation. The total possible score for each item was 2.00 points. Considering the explanation score, the total possible score in this part was 24 points. Students were categorized into five categories according to their explanation scores. Students whose percentages of explanation scores fell in the ranges of 0–19, 20–39, 40–59, 60–79, and 80–100 were classified as ‘very poor’, ‘poor’, ‘fair’, ‘good’, and ‘excellent’ categories, respectively.
(2) The pre- and post-mental model drawings were categorized into five groups according to the information expressed in their drawing both macroscopic (including symbolic) and sub-microscopic (molecular) levels. The macroscopic and symbolic levels were combined in this study as MacSym criteria as sometimes they were difficult to separate symbolic information from macroscopic information in students' mental drawings. There were three criteria (5 points available) for the macroscopic and symbolic features (MacSym A1, A2, and A3) and the other three criteria (10 points available) for the sub-microscopic features (Mol B1, B2, and B3). Therefore, the total available score was 15 points. Four main scientific concepts were considered in each criterion (see Table 2). Drawings with information corresponding to none, one, two, three, and four out of four scientific concepts in each criterion were classified as “Sound Understanding: SU”, “Partial Understanding: PU”, “Partial Understanding with Specific Misunderstanding: PMU”, “Specific Misunderstanding: MU”, and “No Understanding: NU” categories, respectively. These drawings were, respectively, awarded 100%, 75%, 50%, 25%, and 0% of the possible score in each criterion.
| Criteria | Scientific concepts |
|---|---|
| 1. Macroscopic and symbolic | The following concepts are considered. |
| 1.1 MacSym A1 (2 points) |
Electrodes, solutions and salt bridges.
1. Provide correct cathode (Mac). 2. Provide correct anode (Mac). 3. Provide correct salt-bridge (Mac). 4. Provide correct solution in each half-cell (Mac). |
| 1.2 MacSym A2 (2 points) |
Atoms, ions and electrons (particles).
1. Show right Ox. No. for metal cations in each half-cell (Sym). 2. Show right Ox. No. for electrolytic anions in each half-cell (Sym). 3. Define particles in electrodes as neutral atoms (Sym). 4. Show free electrons (e−) only on wire (Sym). |
| 1.3 MacSym A3 (1 point) |
Oxidation and reduction half-cells.
1. Identify correct oxidation and reaction half-cells (Mac). 2. Provide right oxidation reaction for oxidation half-cell (Sym). 3. Provide right reduction reaction for reaction half-cell (Sym). 4. Provide right total oxidation-reaction reaction (Sym). |
| 2. Molecular | The following concepts are considered. Please note that sizes of particles are not included in these criteria. |
| 2.1 Mol B1 (4 points) |
Position of particles and oxidation number.
1. All neutral atoms appear only on electrodes. 2. All ions appear only in solutions or in salt-bridge. 3. Free electrons appear only on a wire. 4. Ox. No. of metal ions in each half-cell is correct. |
| 2.2 Mol B2 (4 points) |
Numbers of particles in solutions and electrodes.
1. Numbers of neutral atoms increases in cathode, while decreases in anode. 2. Numbers of metal cations increases in oxidation half-cell, while decreases in reduction half-cell. 3. Number of metal ions relate to the gain and loss of electron in each half-cell (mole concept). 4. Total number of metal atoms plus metal ions in each half-cell remains constant (conservation of mass). |
| 2.3 Mol B3 (2 points) |
Transfer (movement) of particles in solution and salt bridge.
1. Salt-generated cations transfer to reduction half-cell. 2. Salt-generated anions transfer to oxidation half-cell. 3. Electrons transfer from anode to cathode via a wire. 4. Electrolytic anions transfer from one to the other half-cell via salt-bridge. |
(3) Students' scores from the pre- and post-conceptual tests and mental model drawings were analyzed by the use of paired-samples T-test to identify the mean differences between the pre- and post-intervention scores at the significance level of 0.05.
(4) Class normalized learning gains or 〈g〉 of students' scores from pre- and post-conceptual tests and mental model drawings were applied to minimize the floor and ceiling effects calculated using the equation:
| 〈g〉 = [(% post-test) − (% pre-test)]/[(100%) − (% pre-test)] |
The floor and ceiling effects are the effects that students who begin with low pre-test scores may have more chance to have large percentage gains, while students who begin with large pre-test scores may gain only small percentage scores. In other words, it is common for students with higher pre-test scores to have results of smaller absolute gains (post-test scores minus pre-test scores). The floor and ceiling effects can be minimized by using normalized gain 〈g〉 analysis. The topics with 〈g〉 ≤ 0.30, 0.30 < 〈g〉 > 0.70, and 〈g〉 ≥ 0.70 were classified into low-, medium-, and high gain categories, respectively (Hake, 1998).
Results and discussion
There were four sections of results in this study: (1) students' scores in the conceptual tests of electrochemistry, (2) student conceptual categories in the conceptual tests of electrochemistry, (3) students' scores in the mental model drawings of a galvanic cell, and (4) students' conceptual categories in the mental models of a galvanic cell.Students' scores in the conceptual tests of electrochemistry
Students' conceptual test scores were divided into two categories, oxidation–reduction reactions and galvanic cells. The mean pre-test scores for the first and second tiers and the totals were 4.41 (SD 1.62), 4.22 (SD 2.21), and 8.63 (SD 2.90), respectively, for the topic of oxidation–reduction reactions, and 8.09 (SD 2.75), 4.79 (SD 3.54), and 12.88 (SD 5.21), respectively, for the topic of galvanic cells, as shown in Table 3. After the completion of the four small-scale experiments, the mean post-test scores for the first and second tiers and the totals were 7.67 (SD 2.19), 6.88 (SD 1.38), and 14.32 (SD 3.32), respectively, for the topic of oxidation–reduction reactions, and 11.67 (SD 3.43), 10.32 (SD 2.80), and 22.26 (SD 5.30), respectively, for the topic of galvanic cells. The normalized learning gains or 〈g〉 for the first and second tiers and the totals were 0.71, 0.55, and 0.61, respectively, for the topic of oxidation–reduction reactions, and 0.52, 0.53, and 0.55, respectively, for the topic of galvanic cells. The 〈g〉 was in the medium gain range of 0.30 and 0.70 in all cases except the choice tier of the topic of oxidation–reduction reactions (〈g〉 = 0.72, high gain). This arose because the oxidation–reduction reactions topic involves just one half-cell (one vial or beaker observation), while the galvanic cells topic involves two half-cells (two vials or beakers) which is more difficult to observe and understand. Therefore, the students provided clearer explanations in the oxidation–reduction section than in the galvanic cells section which involves and assumes knowledge of oxidation–reduction.| Tiers | Available | Pre-test | Post-test | Gain | T | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | % | Mean | SD | % | % | 〈g〉 | |||
| a Statistically different at the significance level of 0.05. | ||||||||||
| Ox.–Red. | 18 | 8.63 | 2.90 | 47.96 | 14.32 | 3.32 | 79.58 | 31.62 | 0.61 | 10.10 |
| Choice | 9 | 4.41 | 1.62 | 49.02 | 7.67 | 2.19 | 85.22 | 36.20 | 0.71 | 8.50a |
| Explanation | 9 | 4.22 | 2.21 | 46.90 | 6.88 | 1.38 | 76.47 | 29.57 | 0.55 | 5.99a |
| Galvanic cells | 30 | 12.88 | 5.21 | 42.94 | 22.26 | 5.30 | 74.19 | 31.25 | 0.55 | 6.27 |
| Choice | 15 | 8.09 | 2.75 | 53.92 | 11.67 | 3.43 | 77.78 | 23.86 | 0.52 | 3.95a |
| Explanation | 15 | 4.79 | 3.54 | 31.96 | 10.32 | 2.80 | 68.19 | 36.23 | 0.53 | 7.94a |
| Total | 48 | 21.51 | 6.83 | 44.82 | 36.63 | 7.69 | 76.39 | 31.57 | 0.57 | 7.58 |
The paired-samples T-test analysis indicated that the differences between the mean scores of the pre- and post-conceptual tests were statistically significant in all cases. In the galvanic cells topic, students obtained much higher percentages of scores in the choice tier than the explanation tier for both the pre- (53.92 and 31.96) and post-conceptual tests (77.78 and 68.19). This situation arose because sometimes the students knew the answers without a complete scientific conceptual explanation of galvanic cells. As a result, they may provide partial understandings, alternative understandings, or misunderstandings in their answers (Sözbilir et al., 2010). The improvements in the percentages of the post-test scores indicated that the corresponding small-scale experiments of electrochemistry in conjunction with the model of galvanic cells were effective in the enhancement of students' conceptual understandings of electrochemistry.
Levels of students' understanding in the explanation tier of conceptual tests of electrochemistry
The students were categorized into five levels of understanding regarding their explanations in the conceptual tests. Prior to the involvement of 5E inquiry experiments and the model kit of electrochemistry, the percentages of students in the very poor, poor, fair, good, and excellent categories were 48.37, 21.24, 20.26, 8.50, and 1.63, respectively, for the oxidation–reduction reactions topic, and 65.29, 13.73, 13.53, 6.67, and 0.78, respectively, for the galvanic cell topic (Table 4). After the intervention, the percentages of students in the very poor, poor, fair, good, and excellent categories were 6.54, 7.52, 14.38, 16.67, and 54.90, respectively, for the oxidation–reduction reactions topic, and 11.96, 11.57, 13.53, 15.29, and 47.65, respectively, for the galvanic cell topic. Notice that the percentages of students decreased in the less understanding categories but increased in the more correct categories.| Conceptual test (no. of items) | Percentage of students (%) | ||||
|---|---|---|---|---|---|
| Very poor | Poor | Fair | Good | Excellent | |
| Pre-test (24) | 58.95 | 16.54 | 16.05 | 7.35 | 1.10 |
| Ox.–Red. (9) | 48.37 | 21.24 | 20.26 | 8.50 | 1.63 |
| Galvanic (15) | 65.29 | 13.73 | 13.53 | 6.67 | 0.78 |
| Post-test (24) | 9.93 | 10.05 | 13.84 | 15.81 | 50.34 |
| Ox.–Red. (9) | 26.54 | 7.52 | 14.38 | 16.67 | 54.90 |
| Galvanic (15) | 11.96 | 11.57 | 13.53 | 15.29 | 47.65 |
| Change (24) | −49.02 | −6.50 | −2.20 | 8.46 | 49.26 |
| Ox.–Red. (9) | −41.83 | −13.72 | −13.72 | 8.17 | 53.27 |
| Galvanic (15) | −53.33 | −2.16 | 0.00 | 8.62 | 46.87 |
Examples of students' responses in conceptual test
Consider the students' responses in the explanation tier for Question 1 in the conceptual test of electrochemistry (see also Fig. 3). Please note that if students did not supply any response in the explanation tier, they were awarded 0.00 point automatically. Some students chose the correct choice (C) but supplied incorrect explanation such as ‘Fe(s) is a reducing agent because it gained electrons, while Cu2+(aq) is an oxidizing agent because it lost electrons’. This case was awarded 0.25 point in the explanation tier because it was considered as misunderstood. Some students chose incorrect choice (A) and provided almost correct explanation such as ‘Fe2+(aq) is a reducing agent because its oxidation number increased from 0 to +2, while Cu2+(aq) is an oxidizing agent because its' oxidation number decreased from +2 to 0’. This case was awarded 0.75 point in the explanation tier. Although the explanation about decreasing and increasing oxidation numbers was correct, the consideration of oxidation number of Fe(s) and Fe2+(aq) was switched from the right to left hand-side of the chemical equation (incorrect). Some students chose incorrect choice (B) but provided correct explanation such as ‘Cu2+(aq) is a reducing agent because it gained electrons and became Cu(s), while Fe(s) is an oxidizing agent because it lost electrons and became Fe2+(aq)’. This case was awarded 1.00 point in the explanation tier because the explanation about gaining and losing electrons of reducing and oxidizing agents was correct.Students' alternative conceptions and misconceptions in the explanation tier of the conceptual tests were consistent with the summarized alternative conceptions in electrochemistry by Karsli and Çalik (2012). The alternative conceptions included: (1) the cathode electrode is negatively charged, which allows an oxidation reaction to occur, (2) the anode electrode is positively charged, which allows a reduction to occur, and (3) there was a lack of ability to write the correct cell reactions. The misconceptions were also consistent with the common misconceptions summarized by Sanger and Greenbowe (1997b), such as the anode is positively charged and getting smaller because it lost electrons, while the cathode is negatively charged and getting larger because it gained electrons.
The improvement of students' conceptual understanding and the conceptual changes to the more correct scientific conception categories are consistent with the studies by Cullen and Pentecost (2011) and by Huddle et al. (2000) who found that the use of a paper model of a galvanic cell in conjunction with electrochemistry laboratory activities allowed students to visualize what happens at the sub-microscopic level of a galvanic cell. As a result, students gained more conceptual understanding of galvanic cells.
Students' scores in the mental models of a galvanic cell
Prior to the intervention, students' mean scores for the pre-mental models in the macroscopic and symbolic (MacSym) and sub-microscopic (Mol) features were 1.85, 2.35, and 4.21 respectively. After the intervention, their mean scores for the post-models were 3.56, 5.98, and 9.55, respectively (Table 5). The percentages of the actual gains in their mental model scores were 34.20, 36.30, and 35.60 respectively. In addition, the normalized gains for their mental models were 0.54, 0.49, and 0.49, all falling in the medium gain range. The paired-samples T-test analysis indicated that these changes from pre- to post-drawings were statistically significant in all cases. Students obtained a percentage for the pre-mental model score of 37.00 for macroscopic features, much higher than the 23.50 for sub-microscopic features. An explanation of this may be that students find sub-microscopic features difficult to understand due to their intangibility and/or invisibility (Coll and Treagust, 2003; Chandrasegaran et al., 2011). However, after involvement in the corresponding experiments and models, the percentage in the mean post-mental model score regarding sub-microscopic features increased to 59.80. This improvement of 36.30 indicated that the small-scale experiments of electrochemistry in conjunction with the model kit of galvanic cells were effective in the enhancement of the students' mental models.| Criteriab (score) | Pre-models | Post-models | Gain | T | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | % | Mean | SD | % | % Actual | 〈g〉 | ||
| a Statistically different at the significance level of 0.05. b Criteria MacSym A1–A3 and Mol B1–B3 are described in the data analysis. | |||||||||
| MacSym A1 (2) | 0.82 | 0.71 | 41.00 | 1.41 | 0.55 | 70.50 | 29.50 | 0.50 | 3.37a |
| MacSym A2 (2) | 0.65 | 0.63 | 32.50 | 1.42 | 0.49 | 71.00 | 38.50 | 0.57 | 5.74a |
| MacSym A3 (1) | 0.38 | 0.33 | 38.00 | 0.73 | 0.28 | 73.00 | 35.00 | 0.55 | 4.54a |
| MacSym total (5) | 1.85 | 1.11 | 37.00 | 3.56 | 1.30 | 71.20 | 34.20 | 0.54 | 5.66a |
| Mol B1 (4) | 1.06 | 1.04 | 26.50 | 2.48 | 1.31 | 62.00 | 35.50 | 0.49 | 3.92a |
| Mol B2 (4) | 0.85 | 1.08 | 21.25 | 2.30 | 1.21 | 57.50 | 36.25 | 0.47 | 3.88a |
| Mol B3 (2) | 0.44 | 0.50 | 22.00 | 1.20 | 0.60 | 60.00 | 38.00 | 0.50 | 4.23a |
| Mol total (10) | 2.35 | 2.45 | 23.50 | 5.98 | 2.93 | 59.80 | 36.30 | 0.49 | 4.14a |
| Grand total (15) | 4.21 | 2.88 | 28.06 | 9.55 | 4.10 | 63.67 | 35.60 | 0.49 | 4.81a |
Students' conceptual categories in mental models of a galvanic cell
The students were categorized into five groups regarding their information expressed in their mental model drawings. When asked to draw mental models of how they understand what happens at the molecular (or sub-microscopic) level in galvanic cells, the categorization of the students' macroscopic and symbolic (MacSym) information at the pre-stage fell mostly in NU (29.41%), MU (26.47%), and PMU (21.57%), and their molecular information for the same stage was also categorized mostly in NU (47.06%), MU (22.55%), and PMU (20.59%), see Table 6. This indicated that prior to the intervention most students accommodate specific misconceptions at both macroscopic (including symbolic) and sub-microscopic levels in all scientific concepts of galvanic cells (see also Table 2). In addition, there were no students in the SU group at the sub-microscopic level in this stage.| Mental models | Percentage of students (%) | ||||
|---|---|---|---|---|---|
| NU | MU | PMU | PU | SU | |
| Total pre-test | 38.24 | 24.51 | 21.08 | 10.29 | 5.88 |
| MacSym criteria | 29.41 | 26.47 | 21.57 | 10.78 | 11.76 |
| MacSym A1 | 23.53 | 32.35 | 17.65 | 8.82 | 17.65 |
| MacSym A2 | 32.35 | 32.35 | 17.65 | 8.82 | 8.82 |
| MacSym A3 | 32.35 | 14.70 | 29.41 | 14.70 | 8.82 |
| Mol criteria | 47.06 | 22.55 | 20.59 | 9.80 | 0.00 |
| Mol B1 | 41.18 | 20.59 | 29.41 | 8.82 | 0.00 |
| Mol B2 | 52.94 | 20.59 | 14.70 | 11.76 | 0.00 |
| Mol B3 | 47.06 | 26.47 | 17.65 | 8.82 | 0.00 |
| Total post-test | 5.88 | 13.72 | 23.04 | 32.84 | 24.51 |
| MacSym criteria | 0.00 | 15.69 | 20.59 | 31.37 | 32.35 |
| MacSym A1 | 0.00 | 17.65 | 20.59 | 29.41 | 32.35 |
| MacSym A2 | 0.00 | 11.76 | 23.53 | 38.24 | 26.47 |
| MacSym A3 | 0.00 | 17.65 | 17.65 | 26.47 | 38.24 |
| Mol criteria | 11.76 | 11.76 | 25.49 | 34.31 | 16.67 |
| Mol B1 | 11.76 | 11.76 | 23.53 | 29.41 | 23.53 |
| Mol B2 | 11.76 | 14.70 | 23.53 | 38.24 | 11.76 |
| Mol B3 | 11.76 | 8.82 | 29.41 | 35.29 | 14.70 |
| Total change | −32.35 | −10.78 | 1.96 | 22.55 | 18.63 |
| MacSym criteria | −29.41 | −10.78 | −0.98 | 20.59 | 20.59 |
| MacSym A1 | −23.53 | −14.70 | 2.94 | 20.59 | 14.70 |
| MacSym A2 | −32.35 | −20.59 | 5.88 | 29.41 | 17.65 |
| MacSym A3 | −32.35 | 2.94 | −11.76 | 11.76 | 29.41 |
| Mol criteria | −35.29 | −10.78 | 4.90 | 24.51 | 16.67 |
| Mol B1 | −29.41 | −8.82 | −5.88 | 20.59 | 23.53 |
| Mol B2 | −41.18 | −5.88 | 8.82 | 26.47 | 11.76 |
| Mol B3 | −35.29 | −17.65 | 11.76 | 26.47 | 14.70 |
After the intervention, their models moved to more correct conceptual understanding categories. For macroscopic and symbolic information, most students were in SU (32.35%) and PU (31.37%) and no students in NU. MacSym A3 (oxidation and reduction half-cells) and MacSym A1 (electrodes, solutions and salt bridges) were the criteria that most students obtained sound understanding (38.24%, 32.35%) over partial understanding (26.47%, 29.41%), while MacSym A2 (particles) was the criterion that most students obtained partial understanding (38.24%) over sound understanding (26.47%). However, there were some students who fell in the MU. The scientific concepts that many students tended to accommodate misconceptions at macroscopic and symbolic levels included (1) switching anode and cathode (Mac), (2) proving incorrect oxidation number for metal ions (Sym), (3) switching oxidation and reduction half-cells (Mac), and (4) providing total oxidation–reaction equation without awareness of mole of electrons (Sym).
For sub-microscopic information, most were categorized in PU (34.31%) and PMU (25.49%), while some of them were in SU (16.67%). Most students obtained partial understanding over partial understanding with specific misconception in all criteria of molecular feature. Mol B1 (position of particles) was the criterion that students tended to have sound understanding over Mol B2 (numbers of particles) and Mol B3 (transfer of particles). However, there were some students who fell in the MU and NU. The scientific concepts that many students tended to accommodate misconceptions at the molecular level included (1) numbers of neutral atoms increases in the anode, while decreases in the cathode, (2) numbers of metal cations increases in the reduction half-cell, while decreases in the oxidation half-cell, (3) proving wrong oxidation number or oxidation state of metal ions in each half-cell, (4) no transfer of salt-generated ions from one to the other half-cell, and (5) no electrolytic anions transfer from one to the other half-cell.
For the conceptual changes, the majority of students moved from the less understanding (NU + MU) to the more understanding (PU + SU) categories in the macroscopic features. The order of NU + MU decreases was MacSym A2 (52.94%), MacSym A1 (38.23%), and MacSym A3 (29.41%), respectively. On the other hand, the order of PU + SU increases was MacSym A2 (47.06%), MacSym A3 (41.17%), and MacSym A1 (35.29%), respectively. In other words, the conceptual changes from the less understanding (NU + MU) to the more understanding (PU + SU) categories of MacSym A2, A1, and A3 were 100%, 73.52% and 70.58%. This finding indicated that this intervention promoted students' conceptual changes at the macroscopic level in scientific concepts of MacSym A2 over concepts of MacSym A1 and MacSym A3. For the sub-microscopic features, the order of NU + MU decreases was Mol B3 (52.94%), Mol B2 (47.06%), and Mol B1 (38.23%), respectively. On the other hand, the order of PU + SU increases was Mol B1 (44.12%), Mol B3 (41.17%), and Mol B2 (38.23%), respectively. In other words, the conceptual changes from the less understanding (NU + MU) to the more understanding (PU + SU) categories of Mol B3, B2, and B1 were 94.11%, 85.29% and 82.35%. This finding indicated that this intervention promoted students' conceptual changes at the sub-microscopic level in scientific concepts of Mol B3 over concepts of Mol B2 and Mol B1.
The improvement of students' mental models of galvanic cells and the changes of their mental model categories to the more correct categories may arise from the fact that the model of galvanic cells provided students a chance to access the sub-microscopic level to direct perception. The students can construct or transform their own mental models based on the sub-microscopic information obtained from the model and macroscopic information from the experiments (Glynn and Duit, 1995; Briggs and Bodner, 2005; Doymus et al., 2010; Dixon and Johnson, 2011). This supported students to relate macroscopic and symbolic information to sub-microscopic information. They then generated reasonable mental (or conceptual) models and used these models to achieve full understanding of these intangible electrochemistry concepts (Johnstone, 1993; Doymus et al., 2010; Dixon and Johnson, 2011; Duis, 2011).
Examples of students' mental models of galvanic cells
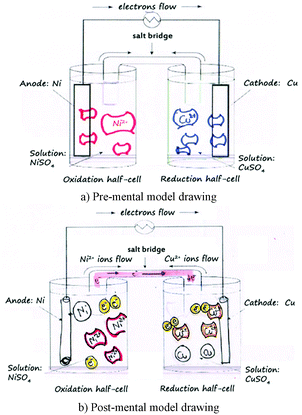
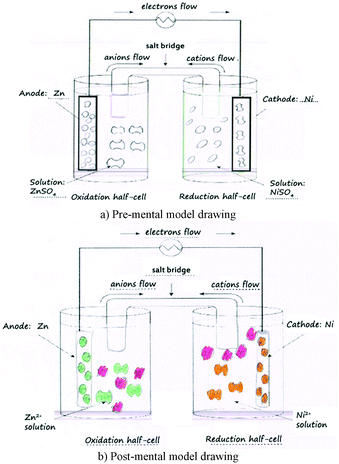
Consider the mental model drawings of a Ni–Cu galvanic cell of Student A. Prior to the involvement of the experiment, Student A provided partial understanding (PU) information that Ni2+ and Cu2+ ions appear in solution, as shown in Fig. 6a.However, she provided incomplete information, no Ni and Cu atoms present. After involvement of the corresponding experiment, she noticed her incomplete information and changed her post-mental model to the more correct understanding (Fig. 6b). However, she provided new mis-understanding (MU) information that Ni2+ and Cu2+ ions transferred from one to the other half-cell and electrons transferred via the salt bridge. She also provided mis-understanding (MU) information that when Cu2+ ions received 2 electrons they became the Cu atoms and appeared in the solution instead of the cathode electrode. She provided partial understanding (PU) information that when Ni atoms gave two electrons they became Ni2+ ions and appeared in solution.
Consider the mental model drawings of a Zn–Ni galvanic cell of Student B. Prior to the involvement of the electrochemistry experiment, Student B provided sound understanding (SU) information in the oxidation half-cell that Zn atoms appear in the Zn anode, while Zn2+ ions appear in the solution, as shown in Fig. 7a. However, she provided partial understanding and mis-understanding (PMU) information in the reduction half-cell that Zn2+ ions appear in the Zn anode, while Zn atoms appear in the solution. After involvement of the electrochemistry experiment, she noticed her mis-understanding and changed her post-mental model to the more correct understanding (Fig. 7b).
Most students provided more complete macroscopic information than molecular information at both the pre- and post-stages as the former is not difficult to understand due to images shown in learning materials and more obvious observations of changes in the experiments. The reason for the students' higher post-stage score may be due to the fact that after the experience of the experiments, the students obtained relevant information by observation of the experiments, leading to modification of their mental models to provide more reasonable explanations of what happens at the molecular level of the given galvanic cells. However, some students' modified models may still contain mis-conceptions (Piquette and Heikkinen, 2005).
Students' alternative- and mis-conceptions encountered in their mental model drawings of a galvanic cell at a sub-microscopic level in this study were mostly consistent with the summaries by Karsli and Çalik (2012) and by Sanger and Greenbowe (1997b). For example, they understood that the cathode is an oxidation half-cell that loses electrons, and decreases mass over time, while the anode is a reduction half-cell that gains electrons, and increases mass over time (Karsli and Çalik, 2012). Some of them thought that the salt bridge allows electrons to travel from the anode to the cathode without assistance from the ions (Sanger and Greenbowe, 1997b) and allows the electrolytic cations migrate toward the anode electrode, whereas the electrolytic anions migrate towards the cathode electrode (Karsli and Çalik, 2012). Some students understood that electrons move through solution from one to the other by attaching themselves to ions (Sanger and Greenbowe, 1997b), while cations in the electrolyte solution transfer from the cathode to the anode by accepting electrons (Sanger and Greenbowe, 1997b), and so on.
In addition, the analysis of mental models of galvanic cells together with the informal unstructured interview regarding their models revealed some potential causes that can lead to misconceptions at the sub-microscopic level of galvanic cells. These causes were shown below.
(1) Number of neutral atoms. Many students misunderstood that the number of neutral atoms increases in the anode, while it decreases in the cathode. This arose from the confusion between the changes of anode and cathode electrodes. Many of them thought that the number remains constant because the experiments that they conducted may not be long enough to obviously notice the change of any metal electrodes although the model kit illustrated this change.
(2) Number of metal cations. Many students misunderstood that the number of metal cations increases in the reduction half-cell, while it decreases in the oxidation half-cell. This arose from the confusion between the changes of oxidation and reduction half-cells. Some of them thought that the number remains constant in both half-cells, or changes (increases or decreases) only in the oxidation or reduction half-cell. This could arise from the fact that some galvanic cells obviously changed colour only in one half-cell (i.e., the colour change can be observed only in the Cu reduction half-cell of the Zn–Cu cell). Therefore, they thought that the number of ions must be constant in the unchanged solution. The number of metal cations and electrons as well as neutral atoms model activity should be more emphasized to minimize the first and second issues.
(3) Oxidation number. Many students identified an incorrect oxidation number for metal cations in each half-cell. This occurred because they could not provide the correct dissolution equation of salt in water, which led to the incorrect oxidation states. Some of them just misremember the oxidation states or for each metal ion and electrolytic anion. The latter case was considered as a mistake rather than a misconception.
(4) Transfer of salt-generated ions. Many students misunderstood that salt-generated cations transferred from the reduction to oxidation half-cell, while anions transferred from the oxidation to reduction half-cell. Some of them thought that no ions transfer but electrons. This arose because the model activity sometimes allowed students to omit salt-generated ions. Therefore, they may not able to notice this change.
(5) Transfer of electrolytic anions. Many of them did not notice the transfer of electrolytic anions from the reduction to oxidation half-cell to balance the new generated metal cations. This arose because they thought that salt-generated ions already transferred from one to the other half-cell. Therefore, electrolytic anions should remain in their half-cell.
These misconceptions were consistent with the previous studies (Sanger and Greenbowe, 1997b; Karsli and Çalik, 2012). However, these encounter misconceptions will be further studied in attempt to minimize them and change them to the more correct conceptions.
The model kit demonstration together with class discussion could diminish the misconceptions about the numbers of neutral atoms and metal cations and the transfer of salt-generated ions and electrolytic anions. In addition, class discussion about the dissolution equations of common salts in water can decrease misconceptions about oxidation states or oxidation numbers. Once students can provide correct states for both cations and anions, they are expected to provide correct oxidation numbers for each metal ion.
In short, the corresponding small-scale experiments allowed students to observe what occurs at a macroscopic level and relate the macroscopic observation to a symbolic level (chemical formulas and equations). This Green chemistry based experiment can diminish the amounts of chemicals used, toxic chemicals, and generated-wastes, while preserve concepts of the experiments, and necessary laboratory techniques and skills (Poliakoff and Licence, 2007; Martin and Gilbert, 2011). Moreover, the corresponding model of galvanic cells, which was inexpensive, portable and flexible, can diminish the difficulty in sub-microscopic visualization and allow students to link the macroscopic experiment observation and symbolic levels to the sub-microscopic level. Once students were able to visualize and relate among the macroscopic, symbolic and sub-microscopic representations, their conceptual understandings of electrochemistry concepts were effectively improved (Chittleborough and Treagust, 2007; Çalik et al., 2010). In addition, the 5E inquiry learning approach also actively engaged students to scientific questions and to explore the answers for these questions through an inquiry process (Deters, 2005). This study also verified that discussion in a small group and in a class with the instructor facilitation effectively enhanced students' conceptual understanding as they gained their understanding and corrected their alternative conceptions while discussing with their peers (Cullen and Pentecost, 2011).
Conclusion and implications
The study results verified that the intervention of the low-cost and small-scale experiments of electrochemistry in conjunction with the inexpensive, portable, reproducible, and flexible model kit by using the 5E inquiry learning approach was effective to enhance students' conceptual understanding and mental models of corresponding concepts. The students obtained a mean post-conceptual test score statistically higher than the pre-conceptual test score. The majorities of the pre-conceptual test were from the choice part but after the intervention, the explanation part played a more important role in their post- than in their pre-conceptual test scores. Before the intervention, most students were in the partial understanding with specific misunderstanding (PMU) to no understanding (NU) categories, but after the intervention they moved to the more correct scientific conceptions, partial understanding (PU) to partial understanding with specific misunderstanding (PMU) categories. For the mental models, the students obtained a mean post-mental model score statistically higher than the pre-mental model score. The majorities of the pre-experiment scores were from the macroscopic part in their mental models, but the sub-microscopic part played more important role in their post-experiment scores than in the pre-experimental scores. Prior to the intervention, the majority of students were in the partial understanding with specific misunderstanding (PMU) to no understanding (NU) categories, but they moved to the better scientific conceptions, partial understanding (PU) to partial understanding with specific misunderstanding (PMU) categories, after the intervention. The major misconceptions encountered in students' mental models of galvanic cells included (1) the number of neutral atoms increases in the anode, while it decreases in the cathode, (2) the number of metal cations increases in the reduction half-cell, while it decreases in the oxidation half-cell, (3) identified incorrect oxidation state for metal cations in each half-cell, (4) salt-generated cations transferred from the reduction to oxidation half-cell, while anions transferred from the oxidation to reduction half-cell, and (5) unaware of transfer of electrolytic anions from the reduction to oxidation half-cell.This study may have implications for chemistry instructors in that teaching or directing students to perform an experiment might be not enough to help students understand important concepts at the molecular level. Chemistry instructors should consider using a corresponding model featuring the sub-microscopic level or various tools such as jigsaws, simulations, animations, virtual laboratory (Hawkins and Phelps, 2013) or other visualization tools (Osman and Tien Lee, 2014) to help students visualize concepts at the molecular level and then connect these concepts to the corresponding macroscopic experiment observations (Doymus et al., 2010). The use of a cooperative learning approach should be considered to let students learn and understand the concepts from their peers (Acar and Tarhan, 2007). As a result, students may achieve a complete and lasting conceptual understanding (Doymus et al., 2010). It is advisable that numbers of neutral atoms, metal cations, and electrons should be emphasized in regard to mole concepts.
There were some limitations in this study. One of these was about the use of a two-tier multiple choice test with the open explanation/reason in the second tier. The author found it difficult to encourage students to supply their reasons for their responses in the first tier. The use of a two-tier test with multiple choices or other forms of test may be considered to diminish this limitation. In addition, using students' explanations to construct 2-tier multiple-choice items is advisable to avoid this limitation. Another limitation was that the same pre- and post tests were used in this study. This was considered as a weak methodology because improvements could be observed with almost any other learning approach. The parallel test or equivalence test should be used to avoid this limitation. The last limitation was about one group pre-test/post-test design without control group. This could be questionable about the effectiveness of this intervention. The design with control and treatment group is advised to diminish this limitation.
For the further study, the information about students' conceptual understanding of electrochemistry and about mental models of a galvanic cell will be used in the design and development of a molecular animation to support students' acquisition to understand electrochemistry concepts or to generate the more correct mental models (Markman, 1999). The content taught to students will be designed to be more contextualized in real situations to promote students to connect between the content and everyday life contexts. The small-scale experiments incorporated with corresponding molecular animation will be implemented to investigate how they impact students' conceptual understandings and mental models of electrochemistry.
Acknowledgements
This study was part of the TRG5680024 project entitled “Development of Inquiry Chemistry Experiments in conjunction with Molecular Animations (ICEMA) to Promote High School Students' Conceptual Understanding and Conceptual Change at the Molecular Level”, co-funded by Thailand Research Fund (TRF) and Ubon Ratchathani University (UBU). The author thanks Richard E. Coll at University of Fiji for a detailed review of the manuscript. The author also thanks Bob Tremayne of the Office of International Relations at Ubon Ratchathani University (UBU) for assistance with English editing and thanks Nutjaree Supasorn (class instructor) and her grade 12 students during academic year 2014 at Srimuang Wittayakhan School for their fruitful participation.References
- Acar B. and Tarhan L., (2007), Effect of cooperative learning strategies on students' understanding of concepts in electrochemistry, Int. J. Sci. Math. Educ., 5, 349–373.
- Ahtee M., Asunta T. and Palm H., (2002), Student teachers problems in teaching electrolysis with a key demonstration, Chem. Educ. Res. Pract., 3, 317–326.
- Aydeniz M. and Krbulut Z. D., (2011), Assessing pre-service science teachers' topic specific pedagogical content knowledge (PCK): pre-service science teachers' pck of electrochemistry, in Psillos D. and Sperandeo R. M. (ed.), Proceedings of the European Science Education Research Association (ESERA 2011): Science Learning and Citizenship (Part 12: Pre-service science teacher education), ESERA, pp. 1–7, retrieved Oct. 10, 2014 from http://www.esera.org/.
- Balci S., Cakiroglu J. and Tekkaya C., (2006), Engagement, exploration, explanation, extension, and evaluation (5E learning cycle and conceptual change text as learning tools, Biochem. Mol. Biol. Educ., 34(3), 99–102.
- Briggs M. W. and Bodner G. M., (2005), A model of molecular visualization, in Gilbert J. K. (ed.), Visualization in science education, Netherlands: Springer, pp. 61–73.
- Bybee R. W., Taylor J. A., Gardner A., Van Scotter P., Powell J. C., Westbrook A. and Landes N., (2006), The BSCS 5E instructional model: origins, effectiveness, and applications, Colorado Springs: BSCS.
- Çalik M., Kolomuc A., and Karagolge Z., (2010), The effect of conceptual change pedagogy on students' conceptions of rate of reaction, J. Sci. Educ. Technol., 19(5), 422–433.
- Chandrasegaran A. L., Treagust D. F. and Mocerino M., (2007), The development of a two-tier multiple-choice diagnostic instrument for evaluating secondary school students' ability to describe and explain chemical reactions using multiple levels of representation, Chem. Educ. Res. Pract., 8, 293–307.
- Chandrasegaran A. L., Treagust D. F. and Mocerino M., (2011) Facilitating high school students' use of multiple representations to describe and explain simple chemical reactions, Teaching Science, 57(4), 13–20.
- Chittleborough G. and Treagust D. F., (2007), The modelling ability of non-major chemistry students and their understanding of the sub-microscopic level, Chem. Educ. Res. Pract., 8(3), 274–292.
- Coll R. K. and Treagust D. F., (2003), Learners' mental models of metallic bonding: cross-age study, Sci. Educ., 87, 685–707.
- Cullen D. M. and Pentecost T. C., (2011), A Model Approach to the Electrochemical Cell: An Inquiry Activity, J. Chem. Educ., 88(11), 1562–1564.
- Demirbaş M. and Ertuǧrul N., (2014), A study on preschoolers' conceptual perceptions of states of matter: a case study of Turkish students, S. Afr. J. Educ., 34(3), 1–13.
- Deters K. M., (2005), Student opinions regarding inquiry-based labs, J. Chem. Educ., 82(8), 1178–1180.
- Dixon R. A. and Johnson S. D., (2011), Experts vs. novices: differences in how mental representations are used in engineering design, J. Technol. Educ., 23(1), 47–65.
- Doymus K., Karacop A. and Simsek U., (2010), Effects of Jigsaw and animation techniques on students' understanding of concepts and subjects in electrochemistry, Educ. Technol. Res. Dev., 5(6), 671–691.
- Duis J. M., (2011), Organic chemistry educators' perspectives on fundamental concepts and misconceptions: an exploratory study, J. Chem. Educ., 88(3), 346–350.
- Ekiz B., Kutucu E. S., Akkus H. and Boz Y., (2011), Pre-service chemistry teachers' understanding of electrolytic cells, in Psillos D. and Sperandeo R. M., Proceedings of the European Science Education Research Association (ESERA 2011): Science Learning and Citizenship (Part 12: Pre-service science teacher education), ESERA, pp. 51–54, retrieved Oct 10, 2014 from http://www.esera.org/.
- Garnett P. J. and Treagust D. F., (1992), Conceptual difficulties experienced by senior high school students of electrochemistry: electrochemical (galvanic) and electrolytic cells, J. Res. Sci. Teach., 29(10), 1079–1099.
- Glynn S. M. and Duit R., (1995), Learning science meaningfully: constructing conceptual model, in Glynn S. M. and Duit R. (ed.), Learning science in the schools: research reforming practice, Lawrence Erlbaum, pp. 3–33.
- Hake R. R., (1998), Interactive engagement vs. traditional methods: a six-thousand-student survey of mechanics test data for introductory physics courses, Am. J. Phys., 61(1), 64–74.
- Hawkins I. and Phelps A. J., (2013), Virtual laboratory vs. traditional laboratory: which is more effective for teaching electrochemistry? Chem. Educ. Res. Pract., 14, 516–523.
- Hoffmann R. and Laszlo P., (1991), Representation in chemistry, Angew. Chem., Int. Ed., 30(1), 1–16.
- Huddle P. A., White M. D. and Rogers F., (2000), Using a teaching model to correct known misconceptions in electrochemistry, J. Chem. Educ., 77(1), 104–110.
- Ibrahim B. and Rebello N. S., (2013), Role of mental representations in problem solving: students' approaches to nondirected tasks, Phys. Rev. ST Phys. Educ. Res., 9(2), 1–16.
- Johnstone A. H., (1993), The development of chemistry teaching: a changing response to changing demand, J. Chem. Educ., 70(9), 701–704.
- Karsli F. and Çalik M., (2012), Can freshman science student teachers' alternative conceptions of ‘electrochemical cells’ be fully diminished? Asian J. Chem., 24(2), 485–491.
- Khattiyavong P., Jarujamrus P., Supasorn S. and Kulsing C., (2014), The Development of Small Scale and Low-Cost Galvanic Cells as a Teaching Tool for Electrochemistry, Journal of Research Unit on Science, Technology and Environment for Learning, 5(2), 146–154.
- Liu Y., Hou H., Chiu H. L. and Treagust D. F., (2014), An exploration of secondary students' mental state when learning about acids and bases, Res. Sci. Educ., 44(1), 133–154.
- Markman A., (1999), Knowledge representation, Lawrence Erlbaum Associates.
- Martin S. F. and Gilbert J. C., (2011), Organic chemistry experiments: miniscale and microscale, 5th edn, Canada: Brooks/Cole Cengage Learning.
- Miller G., (2014), Addressing students' difficulties and misconceptions about electrochemistry, in AP Central, California: University of California, retrieved Oct 10, 2014 from http://apcentral.collegeboard.com/.
- Mulford D. R. and Robinson W. R., (2002), An inventory for alternate conceptions among first-semester general chemistry students, J. Chem. Educ., 79(6), 739–744.
- Niaz M., (2002), Facilitating conceptual change in students' understanding of electrochemistry, Int. J. Sci. Educ., 24(4), 425–439.
- Niaz M. and Chacón E., (2003), A conceptual change teaching strategy to facilitate high school students' understanding of electrochemistry, J. Sci. Educ. Technol., 12(2), 129–134.
- Ökaya A. R., (2002), Conceptual difficulties experienced by prospective teachers in electrochemistry: half-cell potential, cell potential, chemical, and electrochemical equilibrium in galvanic cells, J. Chem. Educ., 79(6), 735–738.
- Osman K. and Tien Lee T., (2014), Impact of interactive multimedia module with pedagogical agents on students' understanding and motivation in the learning of electrochemistry, Int. J. Sci. Math. Educ., 12, 395–421.
- Piquette J. S. and Heikkinen H. W., (2005), Strategies reported used by instructors to address student alternate conceptions in chemical equilibrium, J. Res. Sci. Teach., 42, 1112–1134.
- Poliakoff M. and Licence P., (2007), Sustainable technology (QandA): green chemistry, Nature, 450(6), 810–812.
- Sanger M., (2009), How does inquiry-based instruction affect teaching majors' views about teaching and learning science? J. Chem. Educ., 85(2), 297–302.
- Sanger M. J. and Greenbowe T. J., (1997a), Common student misconceptions in electrochemistry: galvanic, electrolytic, and concentration cells, J. Res. Sci. Teach., 34(4), 377–398.
- Sanger M. J. and Greenbowe T. J., (1997b), Common student misconception in electrochemistry: current flow in electrolyte solutions and the salt bridge, J. Chem. Educ., 74(7), 819–823.
- Sözbilir M., Pınarbaşı T. and Canpolat N., (2010), Prospective chemistry teachers' conceptions of chemical thermodynamics and kinetics, Eurasia Journal of Mathematics, Science, and Technology Education, 6(2), 111–120.
- Stears M. and Gopal N., (2010) Exploring alternative assessment strategies in science classrooms, African J. Educ., 30, 2014, 591–604.
- Supasorn S., Khattiyavong P., Jarujamrus P. and Promarak V., (2014), Small-scale inquiry-based experiments to enhance high school students' molecular conceptual understanding of electrochemistry, Int. Proc. Econ. Dev. Res., 81, 85–91.
- Taber K., (2013), Three levels of chemistry educational research, Chem. Educ. Res. Pract., 14, 151–155.
- Treagust D. F., (1988), Development and use of diagnostic tests to evaluate students' misconceptions in science, Int. J. Sci. Educ., 10, 159–169.
- Yakmaci-Guzel B., (2013), Preservice chemistry teachers in action: an evaluation of attempts for changing high school students' chemistry misconceptions into more scientific conceptions, Chem. Educ. Res. Pract., 14, 95–104.
| This journal is © The Royal Society of Chemistry 2015 |