Mesoporous carbonaceous materials prepared from used cigarette filters for efficient phenol adsorption and CO2 capture
Aibing Chen*a,
Yonglei Lia,
Yifeng Yua,
Yuetong Lia,
Linsong Zhang*b,
Haijun Lva and
Lei Liua
aCollege of Chemistry and Pharmaceutical Engineering, Hebei University of Science and Technology, Shijiazhuang 050018, PR China. E-mail: chen_ab@163.com; Fax: +86 311 8863 2183; Tel: +86 311 8863 2183
bDepartment of Resources and Environmental Engineering, Xingtai Polytechnic College, Xingtai 054000, PR China. E-mail: ls03104126@126.com; Fax: +86 319 2273354; Tel: +86 319 2273354
First published on 1st December 2015
Abstract
Mesoporous carbonaceous materials (MCMs) with a 2-D hexagonal (p6mm) mesostructure are synthesized through evaporation induced self-assembly on the surface of cigarette filters by using phenol/formaldehyde resol as a carbon precursor, triblock copolymer F127 as the template and cigarette filters as the matrix scaffold. The obtained MCMs incorporate the advantages of cigarette filters and phenol/formaldehyde resol, and results in enhanced performance for phenol adsorption and CO2 capture. X-ray diffraction and transmission electron microscopy results indicate that the obtained carbon materials have an ordered p6mm mesostructure and good thermal stability. The MCMs possess a uniform pore size (5.1 nm), large surface area (526 m2 g−1) and pore volume (0.39 cm3 g−1), as well as exhibiting a considerable phenol adsorption (261.7 mg g−1) and CO2 capture (2.48 mmol g−1).
1. Introduction
A large amount of cigarettes are consumed around the world every year, and in most cases the filters are thrown away. Disposed cigarette filters are one of the biggest solid wastes produced. Much attention has to be paid as around 766![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 571 metric tons of cigarette filters are produced each year, which pose a significant environmental contamination threat.1 Environmental hazards may result from the leaching of the toxic components from disposed cigarette filters. This will expose the environment not only to heavy metals but also to ethyl phenol and pesticide residues.2 Cigarette filters made of cellulose acetate contain a high degree of carbon atoms, and can potentially be the initial scaffold materials for the preparation of porous carbon.3 Polarz et al. obtained carbon materials by carbonizing cigarette filters at 1000 °C for 7 h with a temperature ramp of 1 °C min−1, and studied the carbonization of cigarette filters in limited carbonization conditions.4 Unfortunately, a BET specific surface area of only 262 m2 g−1 and a porosity volume of 0.21 cm3 g−1 were obtained.
571 metric tons of cigarette filters are produced each year, which pose a significant environmental contamination threat.1 Environmental hazards may result from the leaching of the toxic components from disposed cigarette filters. This will expose the environment not only to heavy metals but also to ethyl phenol and pesticide residues.2 Cigarette filters made of cellulose acetate contain a high degree of carbon atoms, and can potentially be the initial scaffold materials for the preparation of porous carbon.3 Polarz et al. obtained carbon materials by carbonizing cigarette filters at 1000 °C for 7 h with a temperature ramp of 1 °C min−1, and studied the carbonization of cigarette filters in limited carbonization conditions.4 Unfortunately, a BET specific surface area of only 262 m2 g−1 and a porosity volume of 0.21 cm3 g−1 were obtained.
Ordered mesoporous carbons (OMCs) have received considerable attention owing to their large surface area, tunable pore structure, uniform and adjustable pore size, and mechanical stability.5 These properties make them ideal candidates for applications in adsorption,6–9 supercapacitors,8,9 and catalysis.10 In general, a nanocasting strategy is adopted to fabricate ordered mesoporous materials by employing nanosized ordered mesoporous silica, followed by carbonization and removal of the silica template.11 However, this hard-templating synthesis method is time-consuming, costly, and unsuitable for mass production. A reliable and facile strategy to synthesize OMCs without the use of a hard template is desirable. With this in mind, many studies have been conducted to obtain OMCs, for example an organic–organic assembly method has been employed to synthesize OMCs by using amphiphilic triblock copolymers as a soft template and phenolic resol as a carbon source.12,13 Among these studies, a solvent evaporation induced self-assembly (EISA) process is known as a powerful preparation route for ordered mesoporous carbonaceous films or powders. It can be carried out by solution casting on planar substrates or solid scaffolds by evaporation, thermopolymerization, and carbonization to obtain the carbonaceous materials. In this way, by employing cigarette filters as a solid scaffold, the obtained materials can show a much larger surface area and pore volume than that of scaffold carbon alone, which may result in an enhanced adsorption performance.
Recently, Zhao’s group prepared carbon–silica composite materials by an EISA route using a decomposable polyether polyol-based polyurethane (PU) foam scaffold.14 Afterwards, this group prepared hierarchically porous carbonaceous monolith materials with 3-D cubic (Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) and 2-D hexagonal (p6mm) mesostructures using a foam scaffold.15 It is important to note that a good foam scaffold plays a key role in the preparation of hierarchically ordered porous carbons (HOPC). However, it is time-consuming to obtain the foam scaffold and requires the allocation of large resources to fabricate, and produces lots of unfavourable residues to the environment.16,17 In this case, it is also not an eco-friendly way to prepare HOPC by using plenty of PU foam. Cigarette filters, one of the most common solid wastes, are often thrown away in most cases. It is an important task to reuse these solid wastes as a new resource. Nevertheless, the carbonaceous materials obtained by carbonizing used cigarette filters couldn’t be widely used due to its lower specific surface area and pore volume. In this paper, a strategy for the preparation of MCMs is presented by using solid waste, i.e. cigarette filters as a scaffold. The resulting materials obtained using an EISA method have the advantages of both cigarette filters and phenol/formaldehyde resol, and possess a uniform pore structure, large surface area and pore volume, as well as exhibiting considerable CO2 capture (2.48 mmol g−1) and phenol adsorption (261.7 mg g−1).
m) and 2-D hexagonal (p6mm) mesostructures using a foam scaffold.15 It is important to note that a good foam scaffold plays a key role in the preparation of hierarchically ordered porous carbons (HOPC). However, it is time-consuming to obtain the foam scaffold and requires the allocation of large resources to fabricate, and produces lots of unfavourable residues to the environment.16,17 In this case, it is also not an eco-friendly way to prepare HOPC by using plenty of PU foam. Cigarette filters, one of the most common solid wastes, are often thrown away in most cases. It is an important task to reuse these solid wastes as a new resource. Nevertheless, the carbonaceous materials obtained by carbonizing used cigarette filters couldn’t be widely used due to its lower specific surface area and pore volume. In this paper, a strategy for the preparation of MCMs is presented by using solid waste, i.e. cigarette filters as a scaffold. The resulting materials obtained using an EISA method have the advantages of both cigarette filters and phenol/formaldehyde resol, and possess a uniform pore structure, large surface area and pore volume, as well as exhibiting considerable CO2 capture (2.48 mmol g−1) and phenol adsorption (261.7 mg g−1).
2. Experimental
2.1. Samples
Smoked cigarettes were collected, and the coating was separated from the remaining filter materials. The filters were washed with deionized water and dried for future use.2.2. Experimental methods
Preparation of the carbon precursor. The carbon precursor was prepared according to the literature.18 For a typical procedure, phenol (6.1 g, 65 mmol) was completely melted at 42 °C in a flask, then a 20 wt% NaOH solution (1.3 g, 32.5 mmol) was slowly added with stirring. After that, 37 wt% formalin (10.5 g, 130 mmol), which is an aqueous solution of formaldehyde, was added, and the mixture was heated at 70 °C for 1 h. After cooling to room temperature, the pH was adjusted to 7.0 using 2.0 M HCl solution. After removing the water under vacuum, the product was dissolved in ethanol (40 wt%).
Preparation of the ordered mesoporous carbon (FDU-15). The ordered mesoporous materials were prepared according to the literature method.18 The typical synthetic procedure was as follows: the triblock copolymer poly(ethyleneoxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO, pluronic 127, 1.0 g) was dissolved in ethanol (20.0 g), then the carbon precursor containing phenol (0.61 g, 6.50 mmol) and formaldehyde (0.39 g, 13.0 mmol) was added into the solution and stirred for 10 min. After that, the solution was poured into a dish to evaporate the ethanol at room temperature over 8 h to obtain a transparent membrane. The membrane was then kept at 100 °C for 24 h to thermopolymerize the phenolic resins. Pyrolysis was carried out in a tubular furnace under a N2 atmosphere at 350 °C for 2 h and then 600 °C for 2 h with a ramp rate of 1 °C min−1.
Preparation of the mesoporous carbonaceous materials (MCMs). The mesoporous composites were prepared according to the literature method using resol, the F127 triblock copolymer and cigarette filters.15 In a typical synthetic procedure, F127 (1.0 g) was dissolved in ethanol (8.5 g) and stirred at 40 °C for 1 h to afford a clear solution. A resol solution (2.5 g) of the above precursors was slowly added with stirring over 1 h. After that, an amount of used cigarette filter was immersed in the obtained homogeneous solution. Air bubbles inside the cigarettes filters were removed by squeezing the scaffolds with a glass rod. It took 8 h to evaporate the solvent at room temperature in a drafty closet, and then 24 h at 100 °C in an oven for the following thermopolymerization. Mesoporous materials were obtained by carbonizing the as-made samples at 600 °C for 2 h in N2 with a ramp rate of 1 °C min−1.
Preparation of the porous carbonaceous materials (PCMs) using used cigarette filters. The PCMs were obtained by carbonizing the used cigarette filters without adding phenolic resin. The filters were carbonized at 600 °C for 2 h in N2 with a 1 °C min−1 heating rate.
Phenol adsorption measurements. Since phenol is toxic and corrosive, experiments were conducted in a fume hood while wearing rubber gloves and a gas mask. The adsorption performance of the three adsorbents (MCMs, FDU-15, PCMs) was investigated. In each adsorption experiment, 0.20 g of adsorbent was added to a 100 mL phenol solution with different initial phenol concentrations (100–700 mg L−1). The resulting mixture was continuously stirred at 25 °C for a certain time until equilibrium was reached. The phenol concentration in the supernatant was analyzed using a UV spectrophotometer (UV-1700, Shimadzu), with the wavelength at 270 nm. Prior to analysis, centrifugation was used to avoid potential interference from suspended scattering particles in the UV analysis. The amount of phenol adsorbed qe (mg g−1) was calculated as
 | (1) |
CO2 capture measurements. CO2 adsorption measurements were carried out using a Micromeritics TriStar 3020 volumetric adsorption analyzer at 25 °C and pressures of CO2 from 0 to 101 kPa. The temperature was controlled using a circulating bath. Before measurement the samples were degassed at 120 °C in a vacuum for 24 h to remove any moisture and CO2 molecules adsorbed in the pores.
2.3. Characterization techniques
Small-angle X-ray diffraction (XRD) patterns were collected using a Rigaku D/MAX-2500 X-ray diffraction system (Cu Kα radiation, λ = 0.15406 nm) operated at 40 kV and 40 mA. Scanning electron microscopy (SEM) was performed on a HITACHI S-4800-I scanning electron microscope. The samples were mounted on an aluminum stub using adhesive carbon tape and SEM images were obtained at different magnifications. High-resolution transmission electron micrographs (HR-TEM) were obtained using a JEOL JEM-2010 electron microscope. Samples for HR-TEM studies were prepared by placing a drop of the suspension of the sample in ethanol onto a carbon-coated copper grid, followed by evaporating the solvent. Nitrogen adsorption–desorption isotherm measurements were performed using a Micromeritics TriStar 3020 volumetric adsorption analyzer at −196 °C. The samples were degassed at 100 °C overnight before the measurement. The Brunauer–Emmett–Teller (BET) method was utilized to calculate the specific surface area of each sample and the average pore size distribution was derived from the desorption branch of the corresponding isotherm using the Barrett–Joyner–Halenda (BJH) method. The total pore volume was estimated from the N2 amount adsorbed at a relative pressure of P/P0 = 0.97. The specific surface area was calculated from the relative pressure of P/P0 = 0.05–0.35.3. Results and discussion
3.1. XRD characterization
The small-angle X-ray diffraction (XRD) patterns of the carbon materials are shown in Fig. 1. It shows that FDU-15 has two well-defined diffraction peaks which can be indexed as the (100) and (200) reflections of 2D hexagonal (p6mm) symmetry, indicating a highly ordered mesostructure.18 In comparison, the MCMs show a less resolved diffraction peak due to the introduction of the disordered cigarette filter carbon. Only one diffraction peak is seen for the MCMs. According to the literature, this diffraction peak could be indexed as the (100) reflection of a 2D hexagonal mesostructure (p6mm space group).18,19 It suggests that the products obtained are imperfect with degradation of the ordered mesostructure, and may contain ordered mesostructured carbonaceous shells and amorphous carbon of the cigarette filters. It also shows that the ordered mesostructure of the MCMs is thermally stable during the carbonization process.14,18 The ordered mesostructure is perfectly retained, despite the removal of a large part of the template on calcination.3.2. SEM and TEM characterization
The scanning electron microscopy (Fig. 2a–c) images show that the struts of the carbonaceous materials are not hollow, but are irregular rods with some grooves. The SEM image of the PCMs (Fig. 2a) fabricated with used cigarette filters shows that several impurities appear on the surface of the irregular rods, which result from residual impurities of tobacco burning. As shown in Fig. 2c, much carbon from phenolic resins (red circle) coats the surface of the MCMs. It is noted that the surface transforms into a folded structure due to the presence of schistose carbonaceous materials, which come from the polymerization and carbonization of the phenolic resins. In addition, it also shows the presence of residual impurities (blue circle). Transmission electron microscopy (TEM) images reveal more refined structural features. The MCMs show large domains of stripe-like patterns (Fig. 2d), further confirming the ordered mesostructure. In addition, lots of micropores appear on the edges of the carbonaceous materials or the walls of the mesoporous channels (Fig. 2e and f), that is to say, the main mesoporous channels are interconnected through micropores inside the walls of the main channels, which is beneficial for CO2 capture.203.3. N2 adsorption–desorption measurements
N2 sorption isotherms of the carbon materials are shown in Fig. 3. It can be seen that FDU-15 and the MCMs both exhibit representative type IV curves with obvious capillary condensation steps at P/P0 = 0.4–0.75 (Fig. 3a), which is consistent with the literature.15,18 The adsorption and desorption branches of the MCMs deviate at low relative pressure, which is related to the asymmetric shrinkage of the pores.15 Compared with FDU-15, the capillary condensation step of the MCMs is shifted slightly to a lower relative pressure, which is related to the slight reduction in aperture size to 5.1 nm, resulting from the framework shrinkage.15 The MCMs show a BET surface area of 526 m2 g−1 and a narrow pore size distribution with a mean value of 5.1 nm calculated from the adsorption branch based on the BJH model (Table 1). The H1 hysteresis loop of the MCMs suggests that it has imperfect cylindrical channels, implying the presence of partial asymmetric pore shrinkage.14 Compared with the PCMs and FDU-15, an increase in surface area (526 m2 g−1) and pore volume (0.39 cm3 g−1) of the MCMs is obtained after the template is removed. This can be attributed to the formation of micropores resulting from the cigarette filter carbonization or the edges of the carbonaceous materials and the walls of the mesoporous channels.21| Sample | SBET (m2 g−1) | VBJH (cm3 g−1) | DBJH (nm) |
|---|---|---|---|
| MCMs | 526 | 0.39 | 5.1 |
| FDU-15 | 460 | 0.22 | 6.6 |
| PCMs | 296 | 0.18 | — |
3.4. Phenol adsorption measurements
 | (2) |
 | ||
| Fig. 5 (a) The Langmuir isotherms of phenol adsorption over the three adsorbents; (b) the Freundlich isotherms of phenol adsorption over the MCMs, FDU-15 and PCMs. | ||
| Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Q0 (mg g−1) | b (L mg−1) | R2 | KF ((mg g−1) (L mg−1)1/n) | n | R2 | |
| MCMs | 257.5 | 0.19 | 0.998 | 45.02 | 3.502 | 0.947 |
| FDU-15 | 143.9 | 0.11 | 0.994 | 17.17 | 2.704 | 0.920 |
| PCMs | 99.1 | 0.05 | 0.990 | 6.39 | 2.232 | 0.911 |
The Freundlich model is an empirical equation assuming heterogeneous adsorptive energies on the surface, and can be shown as:25
| qe = KFC1/ne | (3) |
The parameters are also calculated and summarised in Table 2. It can be seen that the Langmuir isotherms of the three adsorbents fit quite well with the experimental data, compared to the Freundlich isotherms which have lower correlation coefficients. This is comparable to the result for phenol adsorption on activated carbon reported in the literature.25 In addition, compared to the other adsorbents, the MCMs exhibit a much larger monolayer adsorption capacity (257.5 mg g−1) of phenol, which is 2.59 and 1.79 times that of the PCMs and FDU-15, respectively. This should be attributed to its large surface area and large pore volume.23 It should also be noted that the MCMs show the best adsorption capacity of phenol among the adsorbents reported in the literature (1.48–165.80 mg g−1).26–28
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (4) |
The pseudo-second-order model is shown as:30
 | (5) |
The adsorption constants of the two models over the three adsorbents are listed in Table 3. It can be seen that the pseudo-first-order model gives poor fitting with low R2 values and notable differences between experimental and theoretical uptakes. The pseudo-second-order fits the experimental data well for all samples. The R2 values are close to unity and the experimental and theoretical uptakes are in good agreement, which agrees with the report that pseudo-second-order models are suitable for the adsorption of lower molecular weight adsorption on smaller adsorption particles.31
| Adsorbent | qe,exp (mg g−1) | Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|---|---|
| k1 | R2 | qe,cal (mg g−1) | k2 | R2 | qe,cal (mg g−1) | ||
| MCMs | 261.7 | 0.418 | 0.952 | 120.79 | 0.039 | 0.997 | 265.3 |
| FDU-15 | 148.9 | 0.327 | 0.965 | 93.86 | 0.026 | 0.996 | 152.7 |
| PCMs | 72.4 | 0.172 | 0.616 | 25.08 | 0.019 | 0.992 | 74.2 |
3.5. CO2 adsorption measurements
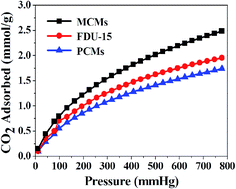
The capture of CO2 has attracted considerable attention in recent years because it is the main anthropogenic contributor to climate change. A comparative analysis of the CO2 adsorption isotherms (measured at 25 °C and 1.0 bar) of the three carbonaceous materials is presented in Fig. 7. It can be seen that the CO2 adsorption capacities of the MCMs with a large surface area (526 m2 g−1) is 2.48 mmol g−1. The value is higher than or at least comparable with those of reported N-doped porous carbon for CO2 adsorption.6,32 In contrast, the PCMs and FDU-15 only show CO2 capacities of 1.74 mmol g−1 and 1.95 mmol g−1 with surface areas of 296 m2 g−1 and 460 m2 g−1, respectively. It should be noted that the MCMs display a much larger pore volume (see Table 1) than that of FDU-15 and the PCMs. This demonstrates that a large surface area and pore volume are beneficial to CO2 capture. Some previous studies have also reported that microporosity in materials is beneficial for CO2 capture and storage.33,34 This is due to micropores having a high adsorption potential that enhances the adsorption performance of CO2 molecules.23 In addition, pore channels with a high specific surface area and large pore volume can accelerate the diffusion and interaction of CO2 molecules during the sorption process.35 This is confirmed by the CO2 capture performance of the MCMs. In addition, no saturation is observed up to a pressure of 1.0 bar, suggesting that a higher CO2 adsorption capacity can be achieved at a higher pressure.4. Conclusions
Porous carbon materials with an ordered mesostructure were prepared successfully using the EISA method with phenol/formaldehyde resol as the carbon precursor, triblock copolymer F127 as the mesostructural template, and cigarette filters as the carbon matrix scaffold. The resulting products possess an ordered p6mm mesostructure, large surface area and uniform pore size. In addition, it is noted that the MCMs incorporate the advantages of ordered mesostructured carbon and amorphous carbon of cigarette filters. The obtained materials exhibited a considerable performance for phenol adsorption (261.7 mg g−1) and CO2 capture (2.48 mmol g−1), and provides a new way of recycling of solid waste and cigarette filters.Acknowledgements
This work was financially supported by the Natural Science Foundation of China (20906019), Science and Technology Research Projects in Hebei Universities (QN20131069, QN2014142, ZD20131032 and Z2013001), and Five Platform Open Fund Projects of Hebei University of Science and Technology (2014PT86).References
- E. A. Smith and T. E. Novotny, Tobac. Contr., 2011, 20, 2–9 CrossRef PubMed.
- E. T. Novotny, S. N. Hardin, L. R. Hovda, D. J. Novotny, M. K. McLean and S. Shan, Tobac. Contr., 2011, 20(1), 17–20 CrossRef PubMed.
- Y. S. Kazemi, S. S. Masoudi and S. Hossein, Adv. Mater. Res., 2012, 587, 88–92 CrossRef.
- S. Polarz, B. Smarsly and J. H. Schattka, Chem. Mater., 2002, 14, 2940–2945 CrossRef CAS.
- W. Li, Q. Yue, Y. Deng and D. Zhao, Adv. Mater., 2013, 25, 5129–5152 CrossRef CAS PubMed.
- J. Wei, D. D. Zhou, Z. K. Sun, Y. H. Deng, Y. Y. Xia and D. Y. Zhao, Adv. Funct. Mater., 2013, 23, 2322–2328 CrossRef CAS.
- M. Barczak, K. Michalak-Zwierz, K. Gdula, K. Tyszczuk-Rotko, R. Dobrowolski and A. Dabrowski, Microporous Mesoporous Mater., 2015, 211, 162–173 CrossRef CAS.
- H. Liu, W. Cui, L. Jin, C. Wang and Y. Xia, J. Mater. Chem., 2009, 19, 3661–3667 RSC.
- Q. Shi, R. Zhang, Y. Lv, Y. Deng, A. A. Elzatahrya and D. Zhao, Carbon, 2015, 84, 335–346 CrossRef CAS.
- K. Kwon, Y. Sa, J. Cheon and S. Joo, Langmuir, 2012, 28, 991–996 CrossRef CAS PubMed.
- G. Mane, S. Talapaneni, C. Anand, S. Varghese, H. Iwai and Q. Ji, et al., Adv. Funct. Mater., 2012, 22, 3596–3604 CrossRef CAS.
- C. Liang and S. Dai, J. Am. Chem. Soc., 2006, 128, 5316–5317 CrossRef CAS PubMed.
- F. Zhang, D. Gu, T. Yu, F. Zhang, S. Xie and L. Zhang, et al., J. Am. Chem. Soc., 2007, 129, 7746–7747 CrossRef CAS PubMed.
- C. Xue, B. Tu and D. Zhao, Adv. Funct. Mater., 2008, 18, 3914–3921 CrossRef CAS.
- C. F. Xue, B. Tu and D. Y. Zhao, Nano Res., 2009, 2, 242–253 CrossRef CAS.
- G. Behrendt and B. Naber, Metallurgy, 2009, 44, 3–23 CAS.
- J. H. Janik and M. Marzec, Mater. Sci. Eng., C, 2015, 48, 586–591 CrossRef PubMed.
- Y. Meng, D. Gu, F. Zhang, Y. Shi, H. Yang and Z. Li, et al., Angew. Chem., Int. Ed., 2005, 44, 7053–7059 CrossRef CAS.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS PubMed.
- L. Wang and R. Yang, J. Phys. Chem. C, 2011, 115, 21264–21272 CAS.
- G. Chandrasekar, W. Son and W. Ahn, J. Porous Mater., 2009, 16, 545–551 CrossRef CAS.
- J. He, K. Ma, J. Jin, Z. Dong, J. Wang and R. Li, Microporous Mesoporous Mater., 2009, 121, 173–177 CrossRef CAS.
- B. Hameed and A. Rahman, J. Hazard. Mater., 2008, 160, 576–581 CrossRef CAS PubMed.
- K. Hall, L. Eagleton, A. Acrivos and T. Vermeulen, Ind. Eng. Chem. Fundam., 1996, 5, 212–223 CrossRef.
- H. Freundlich, J. Phys. Chem., 1906, 57, 385–470 CAS.
- I. Vázquez, J. Rodríguez-Iglesias, E. Marañón, L. Castrillón and M. Álvarez, J. Hazard. Mater., 2007, 147, 395–400 CrossRef PubMed.
- A. Kumar, S. Kumar and D. Gupta, J. Hazard. Mater., 2007, 147, 155–166 CrossRef CAS PubMed.
- B. Özkaya, J. Hazard. Mater., 2006, 129, 158–163 CrossRef PubMed.
- E. Tutem, R. Apak and C. Unal, Water Res., 1998, 32, 2315–2324 CrossRef CAS.
- E. Haque, J. Jun, S. Talapaneni, A. Vinu and S. Hung, J. Mater. Chem., 2010, 20, 10801–10803 RSC.
- F. Wu, R. Tseng, S. Huang and R. Juang, Chem. Eng. J., 2009, 151, 1–9 CrossRef CAS.
- G. Hao, Z. Jin, Q. Sun, X. Zhang, J. Zhang and A. Lu, Energy Environ. Sci., 2013, 6, 3740–3747 CAS.
- V. Zelenak, D. Halamova, L. Gaberova, E. Bloch and P. Llewellyn, Microporous Mesoporous Mater., 2008, 116, 358–364 CrossRef CAS.
- C. Martín, M. Plaza, S. García, J. Pis, F. Rubiera and C. Pevida, Fuel, 2011, 90, 2064–2072 CrossRef.
- H. Yang, Y. Yuan and S. Tsang, Chem. Eng. J., 2012, 185–186, 374–379 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |