Synergistic effect between eosin Y and rhodamine B on a photoelectrode coated with Pt nanoparticle-decorated graphene†
Abstract
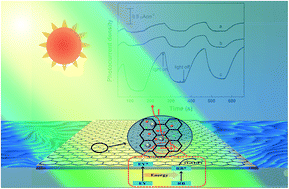
In the present work, a photoelectrochemical system containing eosin Y, rhodamine B and graphene loaded with Pt nanoparticles was fabricated. The synergistic effect between eosin Y and rhodamine B was explored using photoelectrochemical techniques. The results show that there exists an obvious synergistic effect between eosin Y and rhodamine B in the as-fabricated photoelectrochemical system. Compared with the photoelectrochemical systems containing only eosin Y or rhodamine B, the photoelectrochemical system containing eosin Y and rhodamine B exhibits higher photoelectrochemical response. When graphene loaded with Pt nanoparticles is cosensitized by eosin Y and rhodamine B, the photocurrent is about 71% higher than the sum of the photocurrents of the photoelectrochemical system containing eosin Y and the photoelectrochemical system containing rhodamine B. This synergistic effect may be ascribed to the energy transfer from eosin Y to rhodamine B under irradiation. Herein, the Pt nanoparticles play an important role in the photovoltaic performance. The synergistic effect between eosin Y and rhodamine B cannot be observed if the Pt nanoparticles are absent. Moreover, the synergistic effect was investigated as a function of pH, content of Pt, molar ratio of eosin Y to rhodamine B, and total concentration of eosin Y and rhodamine B, respectively.

 Please wait while we load your content...
Please wait while we load your content...