Torularhodin, isolated from Sporidiobolus pararoseus, inhibits human prostate cancer LNCaP and PC-3 cell growth through Bcl-2/Bax mediated apoptosis and AR down-regulation†
Chao Dua,
Yingchao Lia,
Yahui Guoa,
Mei Hanc,
Weiguo Zhangc and
He Qian*ab
aSchool of Food Science and Technology, Jiangnan University, 1800 Lihu Avenue Wuxi, Jiangsu Province 214122, P. R. China. E-mail: amtf168168@126.com; ruindu@163.com; 251405401@qq.com; 601609769@qq.com; Fax: +86-510-8532 9081; Tel: +86-510-8532 8713
bNational Engineering Research Center for Functional Food, Jiangnan University, 1800 Lihu Avenue Wuxi, Jiangsu Province 214122, P. R. China
cSchool of Biotechnology, Jiangnan University, 1800 Lihu Avenue Wuxi, Jiangsu Province 214122, P. R. China. E-mail: hanmei.918521@163.com; zhangwg168@126.com
First published on 7th December 2015
Abstract
Torularhodin is one of the principal carotenoids in Sporidiobolus pararoseus and has a similar structure to that of lycopene. The present work was to elucidate its anti-cancer activity on androgen-dependent prostate cancer LNCaP cells and androgen-independent PC-3 cells using lycopene as a control. WST-1 assay showed that the efficiency of torularhodin was much better than lycopene on both prostate cell lines. Flow cytometry results demonstrated that its apoptotic induction was associated with loss of mitochondrial membrane potential and increased intracellular calcium concentration. Quantitative real-time polymerase chain reaction and western blot data revealed that torularhodin changed the expression ratio of pro-apoptotic to anti-apoptotic Bcl-2 family members. Moreover, we found that torularhodin significantly down-regulated the expression levels of androgen receptor (AR) and prostate-specific antigen (PSA) expression in LNCaP cells. The present results indicate that torularhodin inhibited the growth of LNCaP and PC-3 cells via the mitochondria-mediated pathway and the down-regulation of AR expression.
1. Introduction
The incidence of prostate cancer (PCa), the second leading cause of death among men in the United States, is dramatically increasing, with most cases developing in men over the age of 50 years.1,2 The development and maintenance of PCa are firstly dependent on hormonal regulation by androgens acting through the androgen receptor (AR). Nuclear AR interacts with androgen response elements in the promoters of target genes, such as the prostate-specific antigen (PSA) gene, and stimulates their transcription. Thus, strategies for the treatment of prostate cancer based on the mechanism of action of AR have gained increasing attention to date.3Moreover, drug-induced apoptosis plays more and more important parts in the prevention and treatment of cancer.4 Bcl-2 family proteins play important regulatory roles in apoptosis: Bax is a pro-apoptotic protein that can permeabilizes the mitochondrial outer membrane leading to the loss of mitochondrial integrity and cell death, whereas Bcl-2 promotes the maintenance of mitochondrial integrity and prevents apoptotic cell death.5 Therefore, novel effective alternatives that target both Bcl-2 family proteins and AR expression are getting increasing interest.6,7
Dietary and nutritional factors play a crucial role in the development of prostate cancer.8 Epidemiological studies demonstrate a clear association between diets high in carotenoid-rich fruits and vegetables, particularly tomatoes, and a reduced incidence of prostate cancer.1,8–11 This protective effect of tomatoes is attributed partly to the presence of lycopene, an unsaturated carotenoid with 13 double bonds (11 of which are conjugated) that contribute to the compound's potent antioxidant properties. Torularhodin, one of the most principal carotenoid in Sporidiobolus pararoseus, is a carotenoid with a non-cycled β-ionone ring, which is biosynthesized from γ-carotene via torulene, and a precursor of β-carotene.12,13 The differences of structure between it and lycopene are subtle, but it has not been well-studied with respect to its bioactivity and nutritional purposes.14
The objective of the present study was to elucidate the anti-cancer activity of torularhodin in androgen-dependent prostate cancer LNCaP cells and androgen-independent prostate cancer PC-3 cells in vitro using lycopene as a control. Most importantly, the molecular mechanism of torularhodin-induced growth inhibition and apoptosis was also investigated.
2. Materials and methods
2.1 Cell culture and reagents
S. pararoseus (JD-2 CCTCC M 2010326) was obtained and characterized by our laboratory. Human PCa cell lines LNCaP and PC-3 were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA), 100 IU per mL penicillin, and 100 μg mL−1 streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37 °C.Torularhodin was isolated and purified from the extract of S. pararoseus according to a previously published method. A purity > 96% was achieved, as determined by High Performance Liquid Chromatography with UV detection at 450 nm. The lycopene standard (95% pure) was obtained from Sigma (Shanghai, China). The carotenoids were stored at −80 °C, and 10 mmol stock solutions were freshly prepared with tetrahydrofuran (THF, Sigma) immediately before use.
4′,6-Diamidino-2-phenylindole (DAPI) solution, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl benzimidazolyl-carbocyanine iodide (JC-1), 2′-(4-ethoxyphenyl)-5-(4-methyl-1-piperazinyl)-2,5′-bi-1H-benzimidazole, trihydrochloride/3,8-diamino-5-[3-(diethylmethylammonio)propyl]-6-phenylphenanthridinium diiodide (Hoechst 33342/PI) solution and 4-(6-acetoxymethoxy-2,7-dichloro-3-oxo-9-xanthenyl)-4′-methyl-2,2′-(ethylenedioxy)dianiline-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl) ester (Fluo-3 AM) were purchased from Beyotime Institute of Biotechnology (Haimen, China). Other reagents were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2 Cell viability assay
The proliferation of lycopene and torularhodin treated cells was assessed using a WST-1 Cell Proliferation and Cytotoxicity Assay Kit (Beyotime Institute of Biotechnology, Haimen, China). WST-1 powder was dissolved in 1 mL of the solvent included in the kit and stored at −20 °C before use. The carotenoids were added to cells seeded at a density of 1 × 106 cells per milliliter, with different final concentrations in the 96 well microtiter plates. The plates were incubated for 24 h at 37 °C. Following incubation, 10 μL of the WST-1 solution was added to each well, the plates were incubated at 37 °C for 3 h, and the absorbance of the samples at 450 nm (A450) was measured with a Thermo Multiskan MK3 microplate reader (Thermo, Finland).2.3 Evaluation of morphological changes by Hoechst 33342/PI staining
LNCaP and PC-3 cells were seeded in a 6-well plate and incubated overnight. Cells were treated with different carotenoids at a final concentration of 30 μmol for different time, then washed twice with phosphate-buffered saline (PBS). A total of 1 × 105 cells were stained with Hoechst 33342/PI solution according to the manufacturer's instructions, and images were acquired using a Laser Scanning Confocal Microscope (LSCM, Carl Zeiss AG, Germany).152.4 Evaluation of apoptosis and necrosis by annexin V and PI staining
The apoptotic rate of LNCaP and PC-3 cells was measured using an annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (Beyotime Institute of Biotechnology). Cells were treated with lycopene and torularhodin to a final concentration of 0, 10, 20 and 30 μmol for 24 h. The cells were then harvested, rinsed twice with cold PBS, stained at a density of 2.5 × 105 cells with annexin-V FITC/PI, and analyzed with a FACSCalibur Flow Cytometer (Becton Dickinson, New Jersey, USA).162.5 Mitochondrial membrane potential assessment by JC-1 staining
Changes in the mitochondrial membrane potential (MMP) of LNCaP and PC-3 cells were detected using a mitochondrial membrane potential assay kit with JC-1 (Beyotime Institute of Biotechnology). Cells were treated with lycopene and torularhodin to a final concentration of 0, 10, 20, 30 and 40 μmol for 24 h. The cells were then harvested and resuspended in fresh medium. After the addition of 0.5 mL JC-1 working solution, the cells were incubated in a CO2 incubator for 20 min. The staining solution was removed by centrifugation, and cells were washed twice with JC-1 staining buffer and analyzed using a FACSCalibur Flow Cytometer (Becton Dickinson).172.6 Measurement and analysis of Ca2+ release
Cytosolic free calcium was detected using Fluo-3 AM (Beyotime Institute of Biotechnology). Cells were treated with lycopene and torularhodin to a final concentration of 0, 10, 20, 30 and 40 μmol for 24 h. The cells were then harvested, resuspended in fresh medium, and incubated with Fluo-3 AM for 30 min in the dark at room temperature. Fluorescence, as an indicator of Ca2+ level, was measured using a FACS Calibur Flow Cytometer (Becton Dickinson) at an excitation wavelength 488 nm.182.7 RNA extraction and quantitative real-time polymerase chain reaction (PCR)
Total RNA was extracted using the TRIzol reagent (Shanghai Generay Biotech Co., Ltd, China). The concentration and purity of the RNA samples were determined spectroscopically. RNA was reverse-transcribed into cDNA using Revert Aid TM M-Mu LV Reverse Transcriptase (Thermo Scientific, Rockford, USA) according to the manufacturer's instructions. For each target mRNA, 1 μL of cDNA was mixed with SYBR Green PCR Premix (TaKaRa, Dalian, China) and 0.8 μL of each specific forward/reverse primer in a final volume of 10 μL. The sequence of the primer sets was shown in Table 1 and synthesized by Generay Biotechnology (Shanghai, China). The PCR amplification was monitored in real time using the ABI 7900 Fast Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA). Real-time PCR conditions were as follows: initial incubation at 95 °C for 10 min, followed by 40 cycles of a two-step RCR, annealing at 95 °C for 3 s and finally extension at 60 °C for 30 s. For relative quantitation, the levels of target genes were determined by the comparative CT method by normalizing to β-actin and relative to a calibrator (2−ΔΔCT), while the purity of the PCR product was verified by melting-curve analysis.19,20| Gene name | Gene bank cDNA accession number | Primer sequence |
|---|---|---|
| LNCaP cells | ||
| Bcl-2 | NM_000633.2 | Forward: 5′-GTGGATGACTGAGTACCTGAACCG-3′ |
| Reverse: 5′-AGACAGCCAGGAGAAATCAAACAGA-3′ | ||
| Bax | NM_004324.3 | Forward: 5′-TTTGCTTCAGGGTTTCATCCA-3′ |
| Reverse: 5′-GAGACACTCGCTCAGCTTCTTG-3′ | ||
| AR | NM_001011645.2 | Forward: 5′-TCTTGTCGTCTTCGGAAATGTTATG-3′ |
| Reverse: 5′-GCCTCTCCTTCCTCCTGTAGTTT-3′ | ||
| PSA | NM_001030048.1 | Forward: 5′-AGCACCCCTATCAACCCCCTATT-3′ |
| Reverse: 5′-GCAACCCTGGACCTCACACCTA-3′ | ||
| β-Actin | NM_001101.3 | Forward: 5′-GGGAAATCGTGCGTGACATTAAGG-3′ |
| Reverse: 5′-CAGGAAGGAAGGCTGGAAGAGTG-3′ | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| PC-3 cells | ||
| Bcl-2 | NM_000657.2 | Forward: 5′-ATGGGATCGTTGCCTTATGC-3′ |
| Reverse: 5′-TCAGTCTACTTCCTCTGTGATGTTG-3′ | ||
| Bax | NM_004324.3 | Forward: 5′-CTCAGGATGCGTCCACCAAGAA-3′ |
| Reverse: 5′-TGTCCACGGCGGCAATCA-3′ | ||
| β-Actin | NM_001101.3 | Forward: 5′-GTTGCGTTACACCCTTTCTTGAC-3′ |
| Reverse: 5′-CTCGGCCACATTGTGAACTTTG-3′ | ||
2.8 Western blot
Following exposure to selected concentrations (0, 10, 20 and 30 μmol) of each carotenoid for 24 h, the cells were washed twice with ice-cold PBS and scraped into 0.5 mL of lysis buffer (10× Cell Lysis Buffer supplemented with 1 mM PMSF). Cells were then centrifuged for 15 min at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 rpm and total protein concentration was determined using the BCA protein assay kit (Beyotime Institute of Biotechnology). For western blotting, equal amounts of protein (50–60 μg) were resolved by 4–15% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and electro-transferred onto a polyvinylidene difluoride membrane. Membranes were washed in Tris-buffered saline with Tween-20 (TBS-T) [10 mM Tris–HCl (pH 8.3), 0.05% Tween-20], blocked in TBS-T containing 5% skim milk overnight at 4 °C, and probed with antibodies against Bcl-2 (2876, Cell Signaling Technology, Massachusetts, USA), Bax (2772), AR (3202), PSA (2475), and β-actin (4967, Cell Signaling Technology) diluted 1
200 rpm and total protein concentration was determined using the BCA protein assay kit (Beyotime Institute of Biotechnology). For western blotting, equal amounts of protein (50–60 μg) were resolved by 4–15% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and electro-transferred onto a polyvinylidene difluoride membrane. Membranes were washed in Tris-buffered saline with Tween-20 (TBS-T) [10 mM Tris–HCl (pH 8.3), 0.05% Tween-20], blocked in TBS-T containing 5% skim milk overnight at 4 °C, and probed with antibodies against Bcl-2 (2876, Cell Signaling Technology, Massachusetts, USA), Bax (2772), AR (3202), PSA (2475), and β-actin (4967, Cell Signaling Technology) diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 in TBS-T with 5% skim milk at 4 °C overnight, followed by incubation with secondary antibody (anti-rabbit IgG, 1
1000 in TBS-T with 5% skim milk at 4 °C overnight, followed by incubation with secondary antibody (anti-rabbit IgG, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 v/v) for at least 1 h at room temperature and three washes in TBST for 10 min between each step. Following the addition of enhanced chemiluminescence reagent (ECL Plus, Solarbio, Beijing, China), membranes were exposed to a chemiluminescence imaging analysis system (Protein Simple, California, USA).2,21,22
1000 v/v) for at least 1 h at room temperature and three washes in TBST for 10 min between each step. Following the addition of enhanced chemiluminescence reagent (ECL Plus, Solarbio, Beijing, China), membranes were exposed to a chemiluminescence imaging analysis system (Protein Simple, California, USA).2,21,22
2.9 Statistical analysis
Data represent the results of at least three independent experiments and are expressed as the mean ± standard deviation (SD). Experimental data were analyzed by one-way analysis of variance (ANOVA) using SPSS version 21.0. A p-value < 0.05 was considered to be statistically significant.3. Results
3.1 Effects of torularhodin on the viability of LNCaP and PC-3 cells
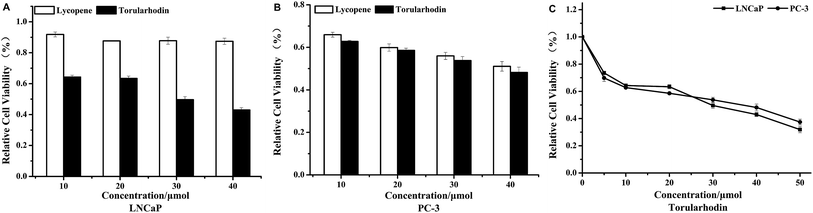
The WST-1 assay was used to assess the effects of torularhodin on cancer cell proliferation using lycopene as the control. The carotenoid exerted different cytotoxic effect on both prostate cancer cell lines in dose-dependent manner (Fig. 1A and B). At 30 μmol concentration, torularhodin decreased cell viability approx. 50% on androgen-dependent LNCaP cells and androgen-independent PC-3 prostate cancer cells. And as shown in Fig. 1C, torularhodin had similar inhibitory effect on both cell lines.4,83.2 Morphological changes caused by torularhodin
As shown in Fig. 2A, torularhodin induced markedly morphological changes at 24 h, including cell shrinkage and loss of the confluent monolayer, whereas at 12 h, the carotenoid had a mild effect on the morphology of LNCaP and PC-3 cells. The inhibitory effect of torularhodin was time-dependent, inducing marked cell shrinkage and rounding at 48 h and progressively leading to the loss of adhesion and ultimately cell death, as evidenced by the floating of the majority of cells in the medium.4Apoptotic changes in cells, including karyorrhexis and karyopyknosis, were observed in carotenoid-treated groups after Hoechst 33342/PI staining (Fig. 2B). Apoptotic nuclei in the carotenoid-treated groups appeared split into several nuclear apoptotic bodies. Viable cells stained bright blue, whereas cells undergoing apoptosis in response to torularhodin treatment stained red.
3.3 Torularhodin induced apoptotic cell death in LNCaP and PC-3 cells
The effect of torularhodin on the percentage of early and late apoptotic LNCaP and PC-3 cells is shown in Fig. 3. Treatment with torularhodin at 0–30 μmol decreased the number of viable cells and increased the number of apoptotic cells, including early apoptotic (annexin V-FITC +/PI−) and late apoptotic cells (annexin V-FITC −/PI+). After treatment for 24 h with 30 μmol torularhodin, total fraction of apoptotic cells increased from 0.59 (control) to 9.68 in LNCaP cells and from 2.84 (control) to 66.46 in PC-3 cells. But more necrosis cells emerged in LNCaP cells (34.49) than PC-3 cells (10.07) treated with torularhodin. These results suggested that torularhodin exerted more obvious apoptotic effect in PC-3 cells.23.4 Disruption of mitochondrial membrane potential in cells treated with torularhodin
Alterations in mitochondrial function play a crucial role in apoptosis; therefore, the effects of torularhodin on MMP were investigated.2 As shown in Fig. 4A, treatment with 30 μmol torularhodin decreased the MMP of both cells, with an approximately 55.97% reduction in MMP in LNCaP cells and a 16.54% reduction in PC-3 cells compared with the control group. Therefore, torularhodin resulted more effective on LNCaP cells than PC-3 cells in the reduction of MMP.3.5 Increase of intracellular Ca2+ in response to torularhodin treatment
Calcium is a vital intracellular second messenger that plays a role in many different cell functions. To determine the effects of torularhodin and torulene in the intracellular Ca2+ level, the intracellular fluo-3 fluorescence intensity was measured in both cells treated with torularhodin at different concentrations. Ca2+ levels were increased in both cells, and the increase in Ca2+ level was more evident in LNCaP cells compared with the control group (Fig. 4B).183.6 Torularhodin downregulate Bcl-2 and upregulate Bax expression
To clarify the apoptotic mechanisms of torularhodin, the mRNA and protein expression of Bcl-2 and Bax was measured in LNCaP and PC-3 cells (Fig. 5). Expression levels of anti-apoptotic Bcl-2 mRNA decreased to 0.17- and 0.35-fold control levels after 24 h treatment with 30 μmol torularhodin in LNCaP and PC-3 cells, respectively. In contrast, expression levels of pro-apoptotic Bax mRNA were increased to 4.65- and 3.74-fold control levels after the same treatment. A significant shift (p < 0.05) in the ratio of Bcl-2 and Bax was observed in response to treatment with torularhodin for 24 h, which was consistent with the changes in protein expression and indicated the induction of apoptosis.2 Overall the ratio between pro-apoptotic and anti-apoptotic Bcl-2 family members was higher in LNCaP cells following torularhodin treatment, which suggested that torularhodin caused more efficient apoptotic induction in androgen-dependent prostate cancer cells.3.7 Torularhodin down-regulate AR and PSA expression in LNCaP cells
Downregulation of AR inhibits the proliferation of LNCaP cells. To investigate whether the inhibition by torularhodin was due to the downregulation of AR, cells were subjected to quantitative PCR and western blot analysis. As shown in Fig. 6, torularhodin down-regulated the expression of AR and PSA in LNCaP cells in both mRNA and protein levels. Torularhodin treatment at 30 μmol for 24 h down-regulated the mRNA expression of AR by 80%, whereas the mRNA expression of PSA was down-regulated by 70% compared with the control, respectively.4. Discussion
PCa is one of the most common malignancies and the second leading cause of death in men in US, with an estimated 241![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 new cases and 28
740 new cases and 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170 deaths in 2012 according to the National Cancer Institute.19 Chemoprevention has been proposed as an effective approach to reduce the incidence of PCa,8 and lycopene was shown to be associated with a reduced risk of PCa in a number of studies.1,9–11 Torularhodin is one of the principal carotenoids in S. pararoseus.14 Despite having similar structures, torularhodin and lycopene show different anti-cancer effects on human prostate cancer cells, and the mechanisms underlying the variation in the effects of these carotenoids remain unclear.
170 deaths in 2012 according to the National Cancer Institute.19 Chemoprevention has been proposed as an effective approach to reduce the incidence of PCa,8 and lycopene was shown to be associated with a reduced risk of PCa in a number of studies.1,9–11 Torularhodin is one of the principal carotenoids in S. pararoseus.14 Despite having similar structures, torularhodin and lycopene show different anti-cancer effects on human prostate cancer cells, and the mechanisms underlying the variation in the effects of these carotenoids remain unclear.
In the present study, we confirmed that torularhodin inhibit growth and induce apoptosis in androgen-dependent LNCaP and androgen-independent PC-3 prostate cancer cells. Morphologic analysis was performed to further investigate the inhibitory effects of torularhodin on cancer cells. Torularhodin induced the characteristic morphological changes of apoptosis in both cells, including cell shrinkage, membrane blebbing, and nuclear condensation and fragmentation,4 which taken together with the results of proliferation assays, indicated the induction of apoptosis.
To further explore the mechanism underlying the cytotoxic effect of torularhodin on human prostate cancer LNCaP and PC-3 cells, the mitochondrial membrane potential (MMP) was measured in cells after exposure to the carotenoid. The mitochondrion is one of the most important organelles involved in programmed cell death. Because cell injury can be associated with mitochondrial dysfunction, we detected changes in the MMP of cells. The results showed that treatment with torularhodin decreased the MMP of both LNCaP and PC-3 cells, with torularhodin showing a stronger effect on LNCaP cells. The results of the MMP assay demonstrated that early damage to the mitochondrial membrane may further activate the intrinsic pathway of apoptosis.1
Cytosolic free calcium, which is known to play vital roles in cell apoptosis, was also measured. Intracellular Ca2+ is an important secondary messenger that regulates many cellular processes including cell growth, proliferation, and signal-transduction. Previous reports demonstrated that disturbance of Ca2+ balance triggers diverse abnormal cellular processes, including apoptosis or necrosis.18 Our results showed a rapid rise of cytosolic Ca2+ levels in both LNCaP and PC-3 cells in response to treatment with different concentrations of torularhodin, which may have been caused by the release of Ca2+ from the endoplasmic reticulum and mitochondria.
Multiple mechanisms are involved in apoptosis, such as the regulation of death receptors located on the cell surface or the intrinsic pathway, which includes the transmission of apoptotic signals from the mitochondria. The Bcl-2 family plays a crucial role in apoptosis and it includes both anti-apoptotic members such as Bcl-2 and pro-apoptotic members such as Bax. Several anti-cancer agents have been shown to induce apoptosis in human prostate cancer cells through the Bcl-2 family pathway.2,23,24 Furthermore, the Bax/Bcl-2 ratio plays an important role in determining whether cells will undergo apoptosis under experimental conditions that promote cell death.4 Our results clearly demonstrated that torularhodin down-regulated the expression of Bcl-2 and up-regulated the expression of Bax in both type of prostate cancer cells, which suggested that modulation of Bcl-2 family proteins is a potential mechanism underlying the induction of apoptosis by torularhodin in human prostate cancer cells. It is noteworthy that, although the apoptotic effect of torularhodin was more efficient in LNCaP cell lines, the cell viability decreased by it was almost the same in both cell lines. For this reason, further research is required to understand the functional role of torularhodin on other signaling pathways in prostate cancer cell lines.
Therapeutic strategies based on targeting AR expression or blocking AR-mediated signaling has been developed for the treatment of prostate cancer. AR plays an important role in the development and progression of prostate cancer, is expressed in almost all prostate cancers, and downregulation of AR, which inhibits cell proliferation, is currently used as an effective strategy to treat prostate cancer.3,7 PSA, a key AR target gene, is a common biomarker for PCa screening and one of the most important indicators of treatment efficacy. Therefore, we investigated whether AR and PSA are molecular targets of torularhodin. Our results showed that torularhodin down-regulated AR and PSA mRNA expression in a dose-dependent manner. These findings suggested that torularhodin may exert its anti-proliferative effects by inhibiting the expression of AR and PSA.6 However, further molecular mechanisms about how the carotenoids affected the level of AR and PSA in human prostate cancer LNCaP cells still need an in-depth study.
The present study showed that torularhodin, which was isolated from S. pararoseus, led to the loss of mitochondrial transmembrane potential, increased intracellular calcium concentration, and contributed to the regulation of Bcl-2 family members in androgen-dependent prostate cancer LNCaP cells and androgen-independent prostate cancer PC-3 cells. Furthermore, its anti-proliferation effect on LNCaP cells also had relationship with the down-regulation of the expression of AR and PSA. These results provided a potential molecular mechanism for torularhodin-induced apoptosis in LNCaP and PC-3 cells and suggested the potential beneficial effects of torularhodin in the treatment of prostate cancer, which may be even superior to those of lycopene. However, the anti-cancer effect of torularhodin was found different in each cell type, which reflected that the modulation of apoptotic mechanism was different. Thus, further investigation is needed to clarify its molecular mechanisms and assess the in vivo anticancer activity of it.
Conflict of interest
The authors declare that they have no competing interests.Acknowledgements
This study was supported by the National Key Technology R&D Program in the 12th Five-year Plan of China (No. 2014BAD04B03, No. 2012BAD36B02 and No. 2015BAD16B01) and 2015 Independent Research Program of Jiangnan University – Key Projects (JUSRP51501).References
- H. L. Hantz, L. F. Young and K. R. Martin, Physiologically Attainable Concentrations of Lycopene Induce Mitochondrial Apoptosis in LNCaP Human Prostate Cancer Cells, Exp. Biol. Med., 2005, 230(3), 171–179 CAS.
- S. Fang, C. Hsu, H. Lin and G. Yen, Anticancer effects of flavonoid derivatives isolated from Millettia reticulata Benth in SK-Hep-1 human hepatocellular carcinoma cells, J. Agric. Food Chem., 2010, 58(2), 814–820 CrossRef CAS PubMed.
- S. Murthy, M. Wu, V. U. Bai, Z. Hou, M. Menon, E. R. Barrack, G. Sahn-Ho Kim and P.-V. Reddy, Role of androgen receptor in progression of LNCaP prostate cancer cells from G1 to S phase, PLoS One, 2013, 8(2), 1–10 Search PubMed.
- Y. Wei, Y. Xu, X. Han, Y. Qi, L. Xu, Y. Xu, L. Yin, H. Sun, K. Liu and J. Peng, Anti-cancer effects of dioscin on three kinds of human lung cancer cell lines through inducing DNA damage and activating mitochondrial signal pathway, Food Chem. Toxicol., 2013, 59, 118–128 CrossRef CAS.
- C. Feng, L. Zhou, T. Yu, G. Xu, H. Tian, J. Xu, H. Xu and K. Luo, A new anticancer compound, oblongifolin C, inhibits tumor growth and promotes apoptosis in HeLa cells through Bax activation, Int. J. Cancer, 2012, 131(6), 1445–1454 CrossRef CAS PubMed.
- H. Zhao, C. Zhu, C. Qin, T. Tao, J. Li, G. Cheng, P. Li, Q. Cao, X. Meng, X. Ju, P. Shao, L. Hua, M. Gu and C. Yin, Fenofibrate down-regulates the expressions of androgen receptor (AR) and AR target genes and induces oxidative stress in the prostate cancer cell line LNCaP, Biochem. Biophys. Res. Commun., 2013, 432(2), 320–325 CrossRef CAS PubMed.
- L. Kong, Q. Yuan, H. Zhu, Y. Li, Q. Guo, Q. Wang, X. Bi and X. Gao, The suppression of prostate LNCaP cancer cells growth by selenium nanoparticles through Akt/Mdm2/AR controlled apoptosis, Biomaterials, 2011, 32(27), 6515–6522 CrossRef CAS PubMed.
- L. Tang, T. Jin, X. Zeng and J.-S. Wang, Lycopene Inhibits the Growth of Human Androgen-Independent Prostate Cancer Cells In Vitro and in BALB/c Nude Mice, J. Nutr., 2005, 135(2), 287–290 CAS.
- C. Yang, I. Lu, H. Chen and M. Hu, Lycopene inhibits the proliferation of androgen-dependent human prostate tumor cells through activation of PPARgamma-LXRalpha-ABCA1 pathway, J. Nutr. Biochem., 2012, 23(1), 8–17 CrossRef CAS.
- N. I. Ivanov, S. P. Cowell, P. Brown, P. S. Rennie, E. S. Guns and M. E. Cox, Lycopene differentially induces quiescence and apoptosis in androgen-responsive and -independent prostate cancer cell lines, Clin. Nutr., 2007, 26(2), 252–263 CrossRef CAS PubMed.
- Y. Tang, B. Parmakhtiar, A. R. Simoneau, J. Xie, J. Fruehauf, M. Lilly and X. Zi, Lycopene Enhances Docetaxel's Effect in Castration-Resistant Prostate Cancer Associated with Insulin-like Growth Factor I Receptor Levels, Neoplasia, 2011, 13(2), 108–119 CrossRef CAS.
- H. Sakaki, T. Nakanishi, K.-Y. Satonaka, W. Miki, T. Fujita and S. Komemushi, Properties of a high-torularhodin-producing mutant of Rhodotorula glutinis cultivated under oxidative stress, J. Biosci. Bioeng., 2000, 89(2), 203–205 CrossRef CAS PubMed.
- H. Sakaki, H. Nochide, S. Komemushi and W. Miki, Effect of active oxygen species on the productivity of torularhodin by Rhodotorula glutinis No. 21, J. Biosci. Bioeng., 2002, 93(3), 338–340 CrossRef CAS PubMed.
- E. D. Simova, G. I. Frengova and D. M. Beshkova, Synthesis of carotenoids by Rhodotorula rubra GED8 co-cultured with yogurt starter cultures in whey ultrafiltrate, J. Ind. Microbiol. Biotechnol., 2004, 31(3), 115–121 CrossRef CAS PubMed.
- H. Xu, P. Wang, Y. Fu, Y. Zheng, Q. Tang, L. Si, J. You, Z. Zhang, Y. Zhu, L. Zhou, Z. Wei, B. Lin, L. Hu and X. Kong, Length of the ORF, position of the first AUG and the Kozak motif are important factors in potential dual-coding transcripts, Cell Res., 2010, 20(4), 445–457 CrossRef CAS PubMed.
- J. Han, Z. Tao, S. Chen, Y. Kong and B. Xiao, Adenovirus-mediated transfer of tris-shRNAs induced apoptosis of nasopharyngeal carcinoma cell in vitro and in vivo, Cancer Lett., 2011, 309(2), 162–169 CrossRef CAS PubMed.
- X. Su, X. Zheng and J. Ni, Lanthanum citrate induces anoikis of Hela cells, Cancer Lett., 2009, 285(2), 200–209 CrossRef CAS PubMed.
- H. Jin, P. Yang, J. Cai, J. Wang and M. Liu, Photothermal effects of folate-conjugated Au nanorods on HepG2 cells, Appl. Microbiol. Biotechnol., 2012, 94(5), 1199–1208 CrossRef CAS PubMed.
- Y. Cui, Q. Ye, H. Wang, Y. Li, X. Xia, W. Yao and H. Qian, Aloin protects against chronic alcoholic liver injury via attenuating lipid accumulation, oxidative stress and inflammation in mice, Arch. Pharmacal Res., 2014, 37(12), 1624–1633 CrossRef CAS PubMed.
- A. E. Damla, O. Pinar, C.-G. Ajda, C. Annarica, A. Enzo and U. N. Palavan, CDK Inhibitors Induce Mitochondria-mediated Apoptosis Through the Activation of Polyamine Catabolic Pathway in LNCaP, DU145 and PC3 Prostate Cancer Cells, Curr. Pharm. Des., 2014, 20(2), 180–188 CrossRef.
- M. Ono, T. Higuchi, M. Takeshima, C. Chen and S. Nakano, Antiproliferative and apoptosis-inducing activity of curcumin against Human Gallbladder Adenocarcinoma Cells, Anticancer Res., 2013, 33(5), 1861–1866 CAS.
- R. R. Kaini and C.-A. A. Hu, Synergistic killing effect of chloroquine and androgen deprivation in LNCaP cells, Biochem. Biophys. Res. Commun., 2012, 425(2), 150–156 CrossRef CAS PubMed.
- A. Palumbo Jr, L. B. Ferreira, P. A. V. R. D. Souza, F. L. D. Oliveira, B. Pontes, N. B. Viana, D. E. Machado, C. Y. Palmero, L. M. Alves, E. R. P. Gimba and L. E. Nasciutti, Extracellular matrix secreted by reactive stroma is a main inducer of pro-tumorigenic features on LNCaP prostate cancer cells, Cancer Lett., 2012, 321(1), 55–164 CrossRef PubMed.
- N. Takeo, Y. Mutsushi, H. Kenichi, I. Toru, S. Ryuta, M. Keiko, M. Masatsugu, S. Fuminori and M. Hiromitsu, Targeting the Vav3 oncogene enhances docetaxel-induced apoptosis through the inhibition of androgen receptor phosphorylation in LNCaP prostate cancer cells under chronic hypoxia, Mol Cancer, 2013, 12(1), 1–15 CrossRef PubMed.
Footnote |
| † The research was conducted by the School of Food Science and Technology of Jiangnan University. |
| This journal is © The Royal Society of Chemistry 2015 |