Synthesis of isatins by the palladium-catalyzed intramolecular acylation of unactivated aryl C(sp2)–H bonds†
Abstract
Synthesis of isatins from formyl-N-arylformamides is achieved via PdCl2-catalyzed intramolecular acylation. This method shows the possibility of Pd-catalyzed aryl C(sp2)–H bond activation on the synthesis of isatins, affording an array of isatins in good yields. Yet this protocol is operationally simple and atom economical.
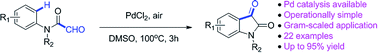

 Please wait while we load your content...
Please wait while we load your content...