Surface PEGylation on PLA membranes via micro-swelling and crosslinking for improved biocompatibility/hemocompatibility
Abstract
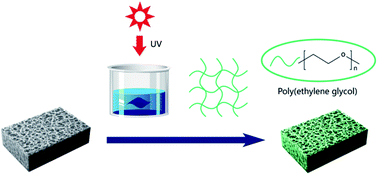
Poly(lactic acid) (PLA) has attracted growing attention as a sustainable and environmentally benign membrane material. The good biocompatibility/hemocompatibility is essential for hemodialysis membranes. To circumvent the inadequate hydrophilicity/biocompatibility/hemocompatibility, we have developed a feasible strategy that enables the persistent PEGylation on PLA membranes via micro-swelling and subsequent UV-initiated crosslinking of poly(ethylene glycol)diacrylate (PEGDA). The content of DMSO and PEGDA in the reaction solution was crucial to control the surface PEGylation kinetics. Besides, the influence of PEGylation on membrane chemistry, morphology, hydrophilicity, water flux and dynamic fouling resistance to BSA was investigated. It was demonstrated that the biocompatibility/hemocompatibility was significantly improved by the surface PEGylation in terms of reduced BSA adsorption, extended APTT and alleviative platelet adhesion.

 Please wait while we load your content...
Please wait while we load your content...