Fabrication of binary components based on a poly(ionic liquid) through “grafting” and “clicking” and their synergistic antifouling activity†
Abstract
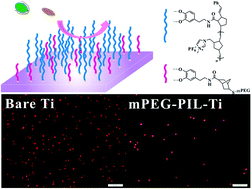
The rational design of an effective antifouling component is challenging but important for many fundamental and applied applications. Herein, we report the synergistic antifouling effects of nonionic, cationic and anionic compounds in combination with a poly(ionic liquid) toward suppressing marine fouling. Firstly, nonionic, cationic and anionic compounds were respectively grafted onto substrate surfaces by a light-induced click reaction of mPEG-SH, bromohexadecyl nicotinate (1-sulfydryl) ethyl ester and sodium 2-mercaptoethane sulfonate. Subsequently the poly(ionic liquid) brushes were grafted onto as-prepared surfaces via surface-initiated ring-opening metathesis polymerizations to obtain binary components modified surfaces. The antifouling properties of the sole component and binary components modified surfaces were evaluated by an algae adhesion assay using Navicula spores and Dunaliella tertiolecta. The antifouling results show that the binary compound modified substrate surfaces exhibit better anti-adsorption performance than the sole component, which is owing to the synergistic antifouling effect of the binary components.

 Please wait while we load your content...
Please wait while we load your content...