Long-lived photoluminescence and high quantum yield of copper(ii) complexes with novel nanostructures†
Abstract
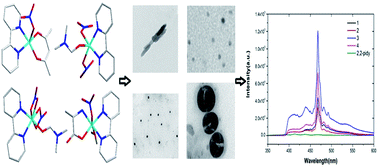
We report four crystalline mixed-ligand complexes with high luminescence intensity, long-lived photoluminescence and quantum yield, which are rarely researched in the field of bivalent copper ion. Three kinds of novel nanostructures were prepared from the CuII-based bulk crystals, and they are the first mixed-ligand copper(II) complexes to be studied in the direction of nanostructures. The research shows that crystal stacking, the π system and rigid flat structure are important for the photophysics of copper complexes.

 Please wait while we load your content...
Please wait while we load your content...