A rhythmic assembly system with fireflies' function based on reversible formation of dynamic covalent bonds driven by a pH oscillator†
Abstract
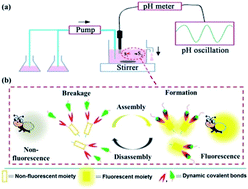
The paper proposed an approach to construct a novel kind of rhythmic assembly system with fireflies' function through combining a pH oscillator based on the hydrogen peroxide/dithionite system with pH-responsive dynamic covalent bonds composed by phenylboronic acid and alizarin red S.

 Please wait while we load your content...
Please wait while we load your content...