Iodine-mediated synthesis of benzopyridothiazines via tandem C–H thiolation and amination†
Liang Weiab,
Li Xub and
Ri-Yuan Tang*ab
aCollege of Chemistry and Materials Engineering, Wenzhou University, Wenzhou 325035, China
bDepartment of Applied Chemistry, College of Materials and Energy, South China Agricultural University, Guangzhou 510642, China. E-mail: rytang@scau.edu.cn
First published on 14th December 2015
Abstract
The first example of an iodine-mediated tandem C–H thiolation/amination strategy for the synthesis of benzopyridothiazines by the reaction of secondary amines with pyridine disulfides is described.
Phenothiazines are important heterocycles that exert numerous effects on biological systems.1 These compounds have been widely used as antibacterial, antifungal, anticancer, antiviral, anti-inflammatory, antimalarial, and antifilarial agents.1 Among this type of compounds, benzopyridothiazines are particularly effective for remedy of various diseases such as asthma, allergic coryza, atopic dermatitis, hives, and hay fever.2 Additionally, phenothiazines are promising biosensor materials in bioelectrochemical systems.3 Despite the importance of phenothiazines, synthetic methods for the formation of phenothiazines are limited.4–8 In recent years, great efforts are made to overcome some drawbacks such as poor regioselectivity, narrow substrate scope, multi-step procedure or harsh conditions in early works.4 Presently, the tandem C–S/C–N bonds cross-coupling reaction strategy serves as a powerful tool for the synthesis of phenothiazines (Scheme 1, eqn (1)–(3)). For example, in 2008, Jørgensen's group demonstrated that palladium-catalyzed three-component coupling reaction of substituted 1-bromo-2-iodobenzenes with primary amines and 2-bromobenzenethiol can efficiently access to phenothiazines (Scheme 1, eqn (1)).5 In 2010, Ma group reported that CuI/L-proline-catalyzed coupling of 2-iodoanilines with 2-bromobenzenethiols is an alternative route to phenothiazines, in which a good regioselectivity can be obtained by controlling the reaction temperature and time (Scheme 1, eqn (2)).6 Recently, copper-(Zeng et al.)7 or iron-catalyzed (Zhang et al.)8 coupling of 2-aminobenzenethiol with 1,2-dihalobenzene is also an effective route to phenothiazines with good regioselectivity (Scheme 1, eqn (3)).
Although great progress has been made for the synthesis of phenothiazines, a long-standing challenge, namely exploring a protocol for tandem C–S/C–N bonds formation by C–H bond disconnection, remains to be addressed. To the best of our knowledge, synthesis of phenothiazines via tandem C–H thiolation/amination strategy has never been evaluated. Additionally, less attention is paid to the synthesis of benzopyridothiazines.9 Guided by the principles of green chemistry,10 the development of an inexpensive and environmental-friendly C–H functionalization strategy for tandem C–S/C–N bonds formation is highly desirable. In recent years, hypervalent iodine(III) reagents have been used in direct C–H amination,11 whereas iodine-mediated nonreactive C–H bond amination remains scarce. Based on our own iodine-mediated C–H thiolation,12 we reasoned that the intramolecular C(sp2)–H amination followed by the ortho C–H thiolation of aniline with diaryl disulfides may be carried out in the presence of iodine, as iodine can react with pyridine to form N-iodopyridinium that may facilitate the amination.13 Herein we report an iodine-mediated tandem C–S/C–N bond formation via C–H bond disconnection leading to a wide range of benzopyridothiazines by the reaction of N-methylanilines with pyridine disulfides (Scheme 1, eqn (4)).
After extensive study, the reaction of N,4-dimethylaniline 1a with 1,2-di(pyridin-2-yl)disulfane 2a was selected as a model reaction (Table 1). Several solvents including DMSO, acetone, MeCN, MeNO2, DMF, and 1,4-dioxane were examined in the presence of I2 (20 mol%) at 80 °C for 12 hours, respectively (entries 1–6). Among these solvents, acetone is ineffective for this reaction (entry 2); and MeNO2 appears effective to give the target product 3a in 45% yield (entry 4). In the presence of NIS (N-iodosuccinimide) instead of I2, the reaction also proceeded smoothly to afford product 3a in 40% yield (entry 7). We are expecting to improve the yield of 3a by increasing the loading of I2. However, the reaction with one equivalent of I2 appears no change on the yield of 3a (entry 8). Reducing the loading of I2 to 10 mol% lowered the yield of 3a to 31% (entry 9). To our delight, the addition of FeF3 (20 mol%) enhanced the yield of 3a to 56% in the presence of I2 (20 mol%) at 80 °C for 12 hours (entry 10). Encouraged by this result, other iron salts such as FeBr3, FeCl3, Fe(OAc)2, Fe(OTf)3, and Fe2O3 were investigated (entries 11–15). However, these iron salts were less effective than FeF3. The reaction temperature evaluation indicates that either lower or higher temperature than 80 °C cannot improve the yield of 3a (entry 16–18). It was pleasing to find that prolonging the reaction time to 24 hours would increase the yield of 3a to 63% (entry 19). Finally, the amount of FeF3 was examined. Loading 50 mol% of FeF3 decreased the yield of 3a to 50% (entry 20). No target product was observed when the reaction was conducted in the absence of I2 (entry 21).
| Entry | Catalyst (mol%) | Solvent | Isolated yield (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.15 mmol), solvent (2 mL) at 80 °C for 12 h.b At 60 °C.c At 100 °C.d At 120 °C.e For 24 h. | |||
| 1 | I2 (20) | DMSO | 26 |
| 2 | I2 (20) | Acetone | 0 |
| 3 | I2 (20) | CH3CN | 37 |
| 4 | I2 (20) | CH3NO2 | 45 |
| 5 | I2 (20) | DMF | 30 |
| 6 | I2 (20) | 1,4-Dioxane | 34 |
| 7 | NIS (20) | CH3NO2 | 40 |
| 8 | I2 (100) | CH3NO2 | 47 |
| 9 | I2 (10) | CH3NO2 | 31 |
| 10 | I2 (20)/FeF3 (20) | CH3NO2 | 56 |
| 11 | I2 (20)/FeBr3 (20) | CH3NO2 | 49 |
| 12 | I2 (20)/FeCl3 (20) | CH3NO2 | 44 |
| 13 | I2 (20)/Fe(OAc)2 (20) | CH3NO2 | 46 |
| 14 | I2 (20)/Fe(OTf)3 (20) | CH3NO2 | 50 |
| 15 | I2 (20)/Fe2O3 (20) | CH3NO2 | 52 |
| 16b | I2 (20)/FeF3 (20) | CH3NO2 | 51 |
| 17c | I2 (20)/FeF3 (20) | CH3NO2 | 55 |
| 18d | I2 (20)/FeF3 (20) | CH3NO2 | 53 |
| 19e | I2 (20)/FeF3 (20) | CH3NO2 | 63 |
| 20e | I2 (20)/FeF3 (50) | CH3NO2 | 50 |
| 21e | FeF3 (20) | CH3NO2 | 0 |
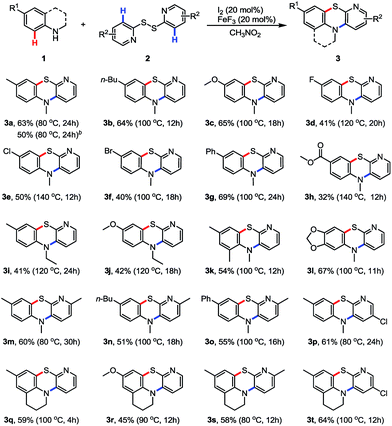
With the optimized reaction conditions in hand (Table 1, entry 19), the scope of the tandem C–H thiolation/amination of N-methylanilines with 1,2-di(pyridin-2-yl)disulfane was investigated (Table 2). N-Methylanilines 1 bearing either an electron-withdrawing or -donating substituents on the benzene ring worked well to give the corresponding products in moderate yields (products 3a–3l). Generally, the electron-donating groups favored the transformation and gave better yields. For example, the butyl-substituted substrate gave product 3b in 64% yield at 100 °C for 12 hours. The substrate bearing a methoxy group afforded product 3c in 65% yield at 100 °C for 18 hours. Whereas the halogen groups exhibit less effective for this transformation, giving the corresponding products in lower yields (products 3d, 3e and 3f). Interestingly, the substrate with a phenyl group performed well to afford product 3g in 69% yield at 100 °C for 24 hours. The substrate with an ester group was also tolerated, albeit in low yield (product 3h). It is noteworthy that further modification can be easily made on the ester group. Replacing methyl with ethyl on the nitrogen atom of anilines leads to a decrease on the yield of products (e.g. 3i vs. 3a, 3j vs. 3c). The dimethyl- and dioxyl-substituted substrates are also suitable for this reaction, giving the corresponding products 3k and 3l in 54% and 67% yields, respectively. Next, other pyridine disulfides such as 1,2-bis(6-methylpyridin-2-yl)disulfane and 1,2-bis(5-chloropyridin-2-yl)disulfane were also investigated. Both appear to have good tolerance with the reaction conditions to afford products 3m and 3p in 60% and 61% yields, respectively. Significantly, 1,2,3,4-tetrahydroquinolines were well tolerated to give novel benzopyridothiazines in moderate yields ranging from 45% to 64% (products 3q–3t). These fused heterocycles, containing pyridine, thiazine and tetrahydroquinoline rings, were constructed for the first time. It was pleasing to find that pyridine-2-thiol can react with 1a to afford product 3a in 50% yield under the standard conditions. This because pyridine-2-thiol can be oxidized to 1,2-di(pyridin-2-yl)disulfane in situ for the next transformation.
To gain insight into the mechanism, several control experiments were conducted (see the ESI†). During the development of this reaction, a thiolated intermediate B (Scheme 2) was observed on GC-MS.14 It was shown that intermediate B could undergo an intramolecular amination to provide product 3a in 65% yield under the standard conditions. In the absence of I2, the amination did not take place. Whereas the treatment of intermediate B with I2 alone gave product 3a in 53% yield. The NMR study demonstrated that I2 could interact with intermediate B in CD3CN at room temperature. In comparison with the 1H NMR of intermediate B in CD3CN without I2, The addition of I2 led the proton peaks shift to low field, but the proton peak of the amino disappeared (see the ESI†). These results suggest that I2 may be chelated with pyridine to form pyridine–iodine complexes C.13 After stirring for 24 hours, the mixture of intermediate B and I2 in CD3CN at room temperature was also detected on a HRMS instrument (ESI-Q-TOF). Unfortunately, only the intermediate B was observed under the electrospray ionization conditions. According to the present results, I2 may play two roles in the amination step: (1) activate the reactivity of the pyridine ring; (2) help to abstract the hydrogen atom and enhance the nucleophilicity of the amino group. According to the present results and previous reports,12,13 a possible mechanism is proposed (Scheme 2). Disulfide 2a first reacts with I2 to yield intermediate A in situ, followed by a thiolation with compound 1a to generate an intermediate B. Intermediate B was then reacted with I2 to produce intermediate C. Intermediate C undergoes an hydrogen iodide elimination to afford an intermediate D. Finally, intermediate D undergoes an amination process to afford target product 3a. Hydrogen iodide can be oxidized by FeF3 or CH3NO2 to regenerate I2 for next cycle.
Conclusions
In summary, we have developed an iodine-mediated tandem C–H thiolation/amination strategy for the synthesis of benzopyridothiazines. Significantly, this unprecedented protocol allows 1,2,3,4-tetrahydroquinolines to give novel benzopyridothiazines with unique structure (products 3q–3t) that may attract great attention in medicine chemistry. Although the progress of C–H functionalization strategy for the formation of thiazine ring is made, the direct synthesis of phenothiazines by tandem C–H thiolation/amination of anilines with common diaryldisulfides remains to be addressed.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21202121).Notes and references
- (a) K. Pluta, B. Morak-Młodawska and M. Jeleń, Eur. J. Med. Chem., 2011, 46, 3179–3189 CrossRef CAS PubMed; (b) L. Rodrigues, J. A. Ainsa, L. Amaral and M. Viveiros, Recent Pat. Anti-Infect. Drug Discovery, 2011, 6, 118–127 CrossRef CAS; (c) J. J. Aaron, M. D. G. Seye, S. Trajkovska and N. Motohashi, Top. Heterocycl. Chem., 2009, 16, 153–231 CAS.
- M. Miyamoto, T. Yoshiuchi, K. Sato, M. Soejima, T. Sato, K. Kikuchi, H. Yoshimura, K. Moriya, Y. Sakuma, S. Akasofu, K. Yamada, S. Yamada and R. Yamada, US Pat., 6583138, 2003.
- (a) M. Ma, Z. Miao, D. Zhang, X. Du, Y. Zhang, C. Zhang, J. Lin and Q. Chen, Biosens. Bioelectron., 2015, 64477–64484 Search PubMed; (b) H. Puzanowska-Tarasiewicz, J. Karpinska and L. Kuzmicka, Anal. Chem., 2011, 63–77 CAS.
- (a) M. Mayer, P. T. Lang, S. Gerber, P. B. Madrid, I. G. Pinto, R. K. Guy and T. L. James, Chem. Biol., 2006, 13, 993–1000 CrossRef CAS PubMed; (b) N. Sharma, R. Gupta, M. Kumar and R. R. Gupta, J. Fluorine Chem., 1999, 98, 153–157 CrossRef CAS; (c) M. Sailer, A. W. Franz and T. J. J. Müller, Chem.–Eur. J., 2008, 14, 2602–2614 CrossRef CAS PubMed; (d) R. Y. Lai, X. Kong, S. A. Jenekhe and A. J. Bard, J. Am. Chem. Soc., 2003, 125, 12631–12639 CrossRef CAS PubMed.
- T. Dahl, C. W. Tornøe, B. Bang-Andersen, P. Nielsen and M. Jørgensen, Angew. Chem., Int. Ed., 2008, 47, 1726–1728 CrossRef CAS PubMed.
- D. Ma, Q. Geng, H. Zhang and Y. Jiang, Angew. Chem., Int. Ed., 2010, 49, 1291–1294 CrossRef CAS PubMed.
- C. Dai, X.-F. Sun, X.-Z. Tu, L. Wu, D. Zhan and Q.-L. Zeng, Chem. Commun., 2012, 48, 5367–5369 RSC.
- W.-Y. Hu and S.-L. Zhang, J. Org. Chem., 2015, 80, 6128–6132 CrossRef CAS.
- B. Morak-Młodawska, K. Pluta, M. Zimecki, M. Jeleń, J. Artym and M. Kocięba, Med. Chem. Res., 2015, 24, 1408–1418 CrossRef PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, NY, 1998 Search PubMed.
- Reviews for hypervalent iodine(III)-mediated amination, see: (a) R. Narayan, S. Manna and A. P. Antonchick, Synlett, 2015, 26, 1785–1803 CrossRef CAS , and references therein cited; (b) P. Finkbeiner and B. J. Nachtsheim, Synthesis, 2013, 45, 979–999 CrossRef CAS , and references therein cited.
- (a) X.-M. Ji, S.-J. Zhou, F. Chen, X.-G. Zhang and R.-Y. Tang, Synthesis, 2015, 47, 659–671 CrossRef CAS; (b) X.-L. Fang, R.-Y. Tang, P. Zhong and J.-H. Li, Synthesis, 2009, 4183–4189 CAS.
- (a) E. M. Karlsen and J. Spanget-Larsen, Chem. Phys. Lett., 2009, 473, 227–232 CrossRef CAS; (b) S. Aronson, S. B. Wilensky, T.-I. Yeh, D. Degraff and G. M. Wieder, Can. J. Chem., 1986, 64, 2060–2063 CrossRef CAS.
- Reaction conditions for intermediate B: 1a (1 mmol), 2a (1.5 mmol), I2 (20 mol%), FeF3 (20 mol%), in DMF (2 mL) at 90 °C for 24 h; yield: 48%.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra22855g |
| This journal is © The Royal Society of Chemistry 2015 |