Quantitative XRD characterisation and gas-phase photocatalytic activity testing for visible-light (indoor applications) of KRONOClean 7000®†
D. M. Tobaldi*a,
M. P. Seabraa,
G. Otero-Iruruetab,
Y. R. de Miguelc,
R. J. Balld,
M. K. Singhb,
R. C. Pullara and
J. A. Labrinchaa
aDepartment of Materials and Ceramic Engineering, CICECO – Aveiro Institute of Materials, University of Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal. E-mail: david.tobaldi@ua.pt; david@davidtobaldi.org; Tel: +351 234 370 041
bCenter for Mechanical Technology and Automation – TEMA, Department of Mechanical Engineering, University of Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal
cTECNALIA, Parque Tecnológico de Bizkaia, C/Geldo, Edificio 700, E-48160 Derio, Bizkaia, Spain
dBRE Centre for Innovative Construction Materials, Department of Architecture and Civil Engineering, University of Bath, Claverton Down, BA2 7AY, Bath, UK
First published on 26th November 2015
Abstract
Carbon-modified commercial anatase (KRONOClean 7000®) was quantitatively characterised with XRD for the first time – full phase composition (both crystalline and amorphous content) and microstructure. The material was found to be bimodal anatase, mostly ∼4 nm diameter, but with a small amount of a larger fraction between ∼12 and 15 nm. Absorption in the visible range was confirmed by UV-Vis analysis, whilst XPS showed that an aromatic carbon compound is at the origin of that absorption. Also, the photocatalytic activity of this commercial nano-TiO2 was assessed, monitoring the abatement of NOx using a white LED lamp, irradiating exclusively in the visible region. Experiments simulated an indoor environment, highlighting the potential of this nano-TiO2 for adoption as a standard for visible-light photocatalytic activity, i.e. applications for innovative interior eco-building materials.
Introduction
Amongst the various applications of titanium dioxide (TiO2), photocatalysis is one of the most common,1–3 because of its versatility, even degrading persistent pollutants in the gas phase, such as volatile organic compounds (VOCs).4–6 It is well known that, in this regard, the major limit of TiO2 is its wide band-gap (Eg), that restricts the photocatalytic activity (PCA) to the UVA range – representing just 5% of the solar spectrum.7 The actual Eg values for anatase and rutile are, respectively 3.2 and 3.0 eV.8 To overcome the Eg limitation, and thus enhance visible-light absorption, several methods have been proposed in the literature.9–11 A number of commercial photocatalytic TiO2 nanopowders exist in the market. Among them, Evonik Aeroxide TiO2 P25® (P25) has become a de facto standard for photocatalytic reactions under solar light, including the UV region. It also possesses a small visible-light photocatalytic response, but this is attributed to the presence of small rutile crystalline domains (shown to have an average crystalline domain diameter of 19.3 nm by Tobaldi et al.) mixed with ∼15.5 nm anatase domains,12 which behave as an “antenna”, extending the PCA into visible wavelengths.13 Furthermore, it has been recently shown by Scanlon et al. that in anatase–rutile composite materials, an energetic band alignment (type II) of ∼0.4 eV of the band edges of the rutile and anatase polymorphs is present, significantly lowering the effective Eg, and facilitating efficient electron–hole separation, with respect to their individual counterparts.14 Despite this, the photocatalysis of P25 is a greatly reduced when under visible light only, without the UV component. This is exactly the kind of light produced by artificial indoor lighting, also known as white light.For this reason, in this work we report a fully quantitative X-ray characterisation (the crystalline and amorphous content, as well as the crystalline domain shape, size and size distribution), of the commercially available carbon-modified nano-anatase KRONOClean 7000® (K7000), with a view to assessing its ability as a visible-light active photocatalyst for indoor use. Furthermore, the PCA in gas–solid phase was assessed monitoring the degradation of NOx (NO & NO2) using exclusively visible-light irradiation.
Experimental
K7000 was used as received, in three difference batches. X-ray powder diffraction (XRPD) data for the quantitative phase analysis (QPA) were collected using a θ/θ diffractometer (PANalytical X'Pert Pro, NL), equipped with a fast RTMS detector, with Cu Kα radiation (45 kV and 40 mA, 20–80° 2θ range, a virtual step scan of 0.0263° 2θ and virtual time per step of 200 s). Full QPA analyses were assessed using the combined Rietveld and reference intensity ratio (RIR) methods: 10 wt% corundum (NIST SRM 676a) was added to the sample, and treated as an additional phase in the refinements.10,15 Instrumental contribution, was evaluated by means of the NIST SRM 660b standard (LaB6), and taken into account in the refinements. Rietveld refinements were carried out by means of GSAS-EXPGUI software packages.16,17 Microstructure of the received K7000 was solved through the whole powder pattern modelling (WPPM),18,19 as implemented in the PM2K software package.20 The WPPM formalism allows us to describe each observed peak profile as a convolution of instrumental and sample-related physical effects, and refining the corresponding model parameters directly on the observed data. For this purpose, aiming at handling data with a high signal/noise ratio were employed, collected using the same instrument and set-up described above, but in the 20–115° 2θ range, with a virtual step of 0.1° 2θ, and virtual time per step of 500 s. The instrumental contribution was obtained by parameterising the profile of 14 hkl reflections from the NIST SRM 660b standard (LaB6), according to the Caglioti et al. relationship.21 Crystalline domains were approximated to be spherical and their diameter distributed according to a lognormal curve.Optical properties of the specimen were analysed via diffuse reflectance spectroscopy (DRS), performed using a Shimadzu UV 3100 (JP) spectrometer, equipped with a BaSO4 integrating sphere. Spectra of the samples were acquired in the UV-Vis range (250–750 nm) with 0.2 nm resolution, with BaSO4 as reference. Raman spectroscopy was also assessed; spectra were acquired in the 50–1000 cm−1 wavenumber range, with 4 cm−1 resolution, on a Bruker RFS 100/S (DE), equipped with a Nd:YAG laser (1064 nm) as the excitation source. FT-IR was performed with the aim of detecting the occurrence of OH groups and/or water adsorbed on the photocatalyst surface. This was carried out on a Bruker Tensor 27 (DE) spectrometer. The measurements were carried out over the wavenumber range of 4000–350 cm−1, in attenuated total reflectance (ATR) mode.
X-ray photoelectron spectroscopy (XPS) was used to characterise the elemental composition of the sample and the chemical state of the titanium, oxygen and carbon species. XPS spectra were acquired in an Ultra High Vacuum (UHV) system with a base pressure of 2 × 10−10 mbar. The system is equipped with a hemispherical electron energy analyser (SPECS Phoibos 150), a delay-line detector and a monochromatic AlKα (1486.74 eV) X-ray source. High resolution spectra were recorded at normal emission take-off angle and with a pass-energy of 20 eV, which provides an overall instrumental peak broadening slightly better than 0.5 eV. For XPS measurements the sample was diluted in Milli-Q water and a thin film was deposited on silicon by drop coating. Ar+ ion sputtering at 2 kV was used to remove the surface adsorbed species and access to the subsurface region for comparison. The resulting XPS spectra were calibrated in binding energy by referencing to the C 1s peak from contamination at 284.8 eV.
PCA tests were assessed in the gas–solid phase, monitoring the degradation of NOx. The reactor used has been described in detail elsewhere.10,22,23 The initial concentration of NOx (kept stable throughout the experiments, and prepared using synthetic air and NOx gas) was 0.2 ppm. Two mass flow controllers were used to prepare the mixture of air with the desired concentration of NOx, with a flow rate of 1 L min−1; this initial step was necessary to ensure the sample saturation, and ensuring that during the test, measurement of NOx is exclusively due to the photocatalytic process (i.e. no absorption from the sample, nor from the reactor walls).24 The outlet concentration of NOx was measured using a chemiluminescence analyser (AC-30 M, Environment SA, FR).
Samples were prepared in the form of a thin layer of powder, with a constant mass (∼0.10 g), and thus approximately constant thickness, in a 6 cm diameter Petri dish. Tests were performed at 18.1 ± 1 °C (temperature inside the reactor) with a relative humidity of 31%. These parameters, controlled by means of a thermocouple that was placed inside the chamber, and a humidity sensor placed in the inlet pipe, remained stable throughout the tests. The light source employed was a LED white light, irradiating only in the visible region (Philips LED Bulb Warm white), placed 28 cm from the photocatalyst, and the light intensity reaching the photocatalyst was 7 W m−2 in the visible range – being nil in the UVA (its emission spectrum being reported in the ESI, Fig. S1†). Once the desired concentration of NOx was attained, the window glass was uncovered, the LED white-lamp turned on, and the PCA reaction started. PCA tests were repeated in triplicate, using the very same sample and with the same protocol of the first test, so as to check the repeatability, recyclability and photostability of the K7000 batches.
Results and discussion
X-ray analysis
QPA data are shown in Table 1; a representative output of a Rietveld refinement is depicted in Fig. S2.† Rietveld–RIR data showed K7000 to consist of anatase as the only crystalline phase, for all three batches examined. Also, all three batches were shown to have a low amount of amorphous phase, varying from 6.5 to 8.1 wt%, depending from the batch used. In any case, the actual mineralogical composition was very close in all the three batches. By contrast, we have previously shown that P25 is composed of only 76.3 wt% anatase, with 10.6 wt% rutile, and 13.0 wt% amorphous phase.12| K7000, batch | No of variables | Agreement factors | Phase composition (wt%) | |||
|---|---|---|---|---|---|---|
| R(F2) (%) | Rwp (%) | χ2 | Anatase | Amorphous | ||
| a Note: there were 2285 observations; the number of anatase reflections in the data set was 32. | ||||||
| 1 | 15 | 3.74 | 4.79 | 2.24 | 91.9 ± 1.3 | 8.1 ± 1.3 |
| 2 | 15 | 3.56 | 4.31 | 1.98 | 93.5 ± 1.1 | 6.5 ± 1.1 |
| 3 | 15 | 3.56 | 4.66 | 2.18 | 93.1 ± 1.2 | 6.9 ± 1.2 |
Individual microstructural analysis and size distribution for the anatase phase present in the three batches of K7000 are shown in Fig. 1a–c and Table 2; a graphical output of the WPPM modelling is shown in Fig. 1a. From this novel XRD analysis, the anatase average crystalline domain diameters were found to be in the range of 2.3–3.3 nm (with a very narrow size distribution for all three batches, the mode being 2.0 nm in all cases, cf. Table 2). Moreover, in all three probability distributions, the right-hand tail is longer than the left, implying that those distributions are skewed to the right (i.e. positive skewness). Thus – assuming a unimodal model for the lognormal size distribution – all the three batches exhibited very similar microstructural characteristics. However, as can be seen in Fig. 1a, in assuming a unimodal model we do not obtain a perfect fit: some features are still present in the difference curve (continuous blue line in Fig. 1a), likely due to a non-ideal distribution of crystalline domains. For this reason, HR-TEM analysis was carried out, to obtain evidence endorsing this suspected non-ideal domain size distribution, cf. Fig. 2a–d. As can be clearly seen in Fig. 2a–d, K7000 shows the simultaneous presence of small and large domains – the small crystalline domains being much more strongly aggregated, cf. Fig. 2a and b. Therefore, the WPPM analysis was performed a second time, considering a bimodal size distribution for anatase. As can be seen in Fig. 1a, the bimodal model led to an almost perfect fit, the resulting difference curve virtually being a straight line (continuous dark grey line in Fig. 1a). Quantitative results of the bimodal size distribution model are depicted in Table 3; introducing a second fraction does not change the unit cell parameter results (sizes and volume are unaltered). As for the domain size, we observe the appearance of a small quantity of a second, larger-sized fraction of anatase (11.9–15.5 nm, Fig. 2c and d), co-existing with the smaller 3.8–4.4 nm domains. Hence, this extra fraction of material is able to explain the presence of the long tail in the actual distribution leading towards anatase larger sizes.
| K7000, batch | Agreement factors | Unit cell parameters | Mean crystalline domain diameter (nm) | Skewness of the size distribution (nm) | ||||
|---|---|---|---|---|---|---|---|---|
| Rwp (%) | Rexp (%) | χ2 | a = b (nm) | c (nm) | V (nm3) | |||
| 1 | 4.04 | 2.33 | 1.74 | 0.3793(1) | 0.9524(1) | 0.137(1) | 3.3 ± 0.1 | 2.1 ± 0.1 |
| 2 | 4.87 | 2.28 | 2.15 | 0.3792(1) | 0.9519(1) | 0.137(1) | 2.3 ± 0.1 | 2.5 ± 0.1 |
| 3 | 3.82 | 2.27 | 1.68 | 0.3792(1) | 0.9526(1) | 0.137(1) | 3.3 ± 0.1 | 2.1 ± 0.1 |
| K7000, batch | Agreement factors | Anatase 1 | Anatase 2 | ||||
|---|---|---|---|---|---|---|---|
| Rwp (%) | Rexp (%) | χ2 | Volume (nm3) | Average size (nm) | Volume (nm3) | Average size (nm) | |
| 1 | 3.08 | 2.28 | 1.35 | 0.137(1) | 4.4 ± 0.3 | 0.136(1) | 11.9 ± 0.6 |
| 2 | 3.98 | 2.23 | 1.78 | 0.137(1) | 3.8 ± 0.1 | 0.137(1) | 13.6 ± 0.5 |
| 3 | 4.24 | 2.23 | 1.90 | 0.137(1) | 4.0 ± 0.2 | 0.136(1) | 15.5 ± 0.8 |
Data obtained are thus only partially consistent with those reported by Quesada-Cabrera et al., who stated that K7000 had a “crystallite” size of around 15 nm, obtained using the Scherrer equation, and with a titania content of roughly 95% (though they did not specify whether that remaining 5% was amorphous or not).25 Consequently, they neglected the fraction of the small anatase crystalline domains below 5 nm diameter. In this regard, it is noteworthy that diffraction is sensitive to the volume, therefore to the fraction in that volume, which is the dominant one. A “crystallite” size of ∼15 nm is also given in the data sheet of K7000 manufacturer, though they state a TiO2 content of >87.5%.26
Spectroscopic analyses
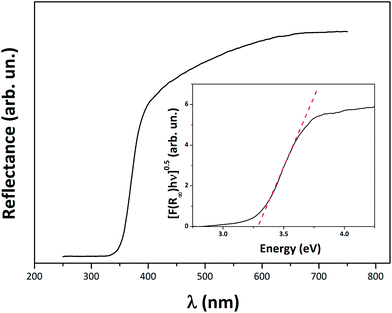
DRS spectrum of K7000 is given in Fig. 3, showing an absorption in the visible range. In nitrogen doped TiO2, this absorption is generally attributed to mid-gap states located just above the valence band.27,28 However, in this particular case, an organic carbon complex is attached to the surface of these TiO2 nanoparticles (NPs), rather than being accommodated in TiO2 lattice. This explains the observed absorption in the visible region – cf. XPS analysis. The optical band gap (Eg) of K7000 was also calculated, taking advantage of the Tauc plot. DRS experimental data were converted to the absorption coefficient α via the Kubelka–Munk equation. The absorption edge of a semiconductor can thus be expressed as: (αhν)1/γ = A(hν − Eg). In this, A is a material constant, h is Planck's constant, ν is the frequency of the light, Eg is the energy band gap of allowed transitions, and the power coefficient γ is characteristic of the type of transition. For TiO2 nanoscale semiconductor materials, the value of γ is accepted to be equal to 2, since for such materials, the transition is assumed to be indirectly allowed.29 Hence, if we plot (αhν)0.5 against hν, we obtain the energy band gap of the semiconductor material (inset of Fig. 3) from the x-axis (α = 0) intercept of the line tangent to the inflection point of the curve. Optical Eg of K7000 was found to be 3.30 ± 0.01 eV, thus in line with the expected value for anatase.8Raman spectroscopy confirmed the XRD results: only Raman modes assignable to anatase were detected in the K7000 spectrum (Fig. S3†). Moreover, the smaller crystalline domain size of anatase in K7000, compared to that in P25, is also confirmed by analysing and comparing the position and full width at half maximum (FWHM) of the symmetric vibrational Eg (O–Ti–O) Raman mode of anatase in K7000 versus P25. The obtained position of the Raman Eg mode in K7000 (147.7 cm−1) is obviously blue shifted compared to that of anatase in P25 (142.7 cm−1, dotted line in the inset of Fig. S3†); the FWHM of K7000 Raman Eg mode (18.7 cm−1) is broader than that of anatase in P25 (11.5 cm−1). This confirms not only the smaller crystalline domain size of K7000 vs. P25, but also its higher disorder.30
An FT-IR spectrum is reported in Fig. S4.† The huge broad band, centred at approximately 3425 cm−1, is assigned to surface-adsorbed hydroxyl groups (this being an advantage for PCA),31 whilst the peak at around 1630 cm−1 corresponds to the bending vibrations of O–H; the band in the range of 400–600 cm−1 clearly belongs to Ti–O–Ti vibrations.
XPS analysis
Fig. 4 shows high resolution XPS spectra obtained for Ti 2p (a), O 1s (b) and C 1s (c) core levels of K7000, after a soft etching with argon ions. Furthermore, the best fits are also included for the O 1s and C 1s core levels. | ||
| Fig. 4 High resolution XPS of Ti 2p (a), O 1s (b) and C 1s (c) core levels of the as received samples (bottom spectra in black) and after ion etching (upper spectra in red). | ||
Two main features dominate the Ti 2p spectrum, appearing at binding energies (BE) of (458.8 ± 0.1) eV and (464.6 ± 0.1) eV, that correspond to Ti 2p3/2 and Ti 2p1/2, respectively. These values are in good agreement with previously reported BEs for TiO2 NPs.32 Consequently, a main component is observed in the O 1s region at (530.1 ± 0.1) eV and ascribed to the oxygen atoms of the TiO2 NPs.32 The relatively small component that appears at (531.3 ± 0.1) eV is usually assigned to adsorbed surface species such as hydroxyl groups,33 and/or carbonate-like species in carbon-doped TiO2 samples. On the other hand, in the case of the C 1s core level, three components were necessary for the fitting of the spectrum. The first component is centred at a BE of (284.8 ± 0.1) eV and is usually ascribed to the graphitic-like C–C bond, while the other two components, at (286.3 ± 0.1) and (288.9 ± 0.1) eV, are ascribed to C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.33 Our XPS data for the C 1s core level are very close to those reported in the literature for carbon-doped TiO2 NPs,33–35 in which the peaks ascribed to C–O and C
O bonds, respectively.33 Our XPS data for the C 1s core level are very close to those reported in the literature for carbon-doped TiO2 NPs,33–35 in which the peaks ascribed to C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds were assigned to an arylcarboxylate group, whilst the peak centred at 286.3 eV was assigned to a bidentately-bound arylcarboxylate. Thus, by analogy with those literature reports, we assigned the C 1s core level to an aromatic carbon compound.
O bonds were assigned to an arylcarboxylate group, whilst the peak centred at 286.3 eV was assigned to a bidentately-bound arylcarboxylate. Thus, by analogy with those literature reports, we assigned the C 1s core level to an aromatic carbon compound.
Moreover, Zhang et al.33 recently performed ion etching on similar samples in order to remove the adsorbed surface species, and access the bulk composition. XPS spectra obtained after having followed the same approach on our samples are presented in the upper panel of Fig. 4 (red spectra). The O 1s core level is very similar to the spectrum obtained before the ion etching, but an important change appears in the Ti 2p3/2 core level. In this peak, the main component is still related to the Ti4+ atoms of TiO2 (see Fig. 4a), while the new small and broad component near 457.1 eV is ascribed to Ti3+ from the formation of oxygen vacancies induced by the ion bombardment.33 Likewise, the C 1s core level was fitted by the same three components discussed above but, interestingly, the intensity of the signal significantly diminished. Furthermore, Fig. S5† shows a comparison of the normalised C 1s spectra before and after the ion etching. The shape, BEs and relative intensities of the main features are virtually the same before and after the erosion with argon ions. This indicates that, even after having partially cleaned the surface of the NPs, “new” carbon species inside the bulk of the TiO2 NPs do not appear. Thus, this effect suggests a preferential covering of the surface of the NPs with carbon species, rather than their accommodation inside the TiO2 lattice (neither interstitial nor substitutional).
Photocatalytic NOx abatement
It is well-established that the reaction path for NOx abatement is mediated by OH˙ radicals, and HNO3 is generated, according to:| TiO2 + hν → eCB− + hVB+ | (1) |
| hVB+ + H2O(ads) → OH˙ + H+ | (2) |
| eCB− + O2 → O2˙− | (3) |
| NO + OH˙ → NO2 + H+ | (4) |
| NO + O2˙− → NO3− | (5) |
| NO2 + OH˙ → NO3− + H+ → HNO3 | (6) |
Such HNO3 is produced on the surface of the catalyst, and might act as a physical barrier, inhibiting the photocatalytic reaction. As a consequence, a plateau is generally observed in the NOx abatement vs. time curve.36,37 This explains why the PCA tests were carried out for up to 20 min visible-light irradiation time. Nevertheless, a test up to 100 min visible-light irradiation time was assessed, and is depicted in Fig. S6.† In this, it is shown that the photocatalytic NOx abatement is attenuated at ∼40 min reaction time, and after this the photocatalytic reaction proceeds slowly. This likely means that the product of the photocatalytic NOx reaction is insufficient to act as a barrier that can “deactivate” the photocatalyst – in any case, the formed HNO3 can be easily eluted from TiO2 into water.38
NOx abatement PCA results are shown in Fig. 5. It is seen that, in the first photocatalytic run, after 20 min reaction time the NOx degradation by all the three batches is in the range of 20–23%. Furthermore, these PCA tests with K7000 were shown to be repeatable, the PCA in the second and third tests being approximately the same as in the first run. This clearly shows that a complete reuse cycle is possible for K7000, which proved to be very stable after three consecutive photocatalytic runs for all the three batches used, and suitable for continued reuse. Also, K7000 was shown to possess a considerably higher PCA (∼30% higher) compared to the well-known P25, under white (visible-only) indoor light (Fig. 5).
 | ||
| Fig. 5 Photocatalytic NOx abatement test in triplicate of the three K7000 batches, and P25 as comparison, using the white LED lamp. | ||
Such a superior, and very stable, PCA under white-light exposure in K7000 has been attributed to the presence of an organic sensitiser (i.e. aryl carboxylate species), cf. spectroscopic and XPS sections, as suggested in the literature.33–35 Interestingly, these species clearly seem to be photostable, and thus resistant to photodegradation, which explains the stability and reusability of K7000 (in all the three batches) when using white-light. Also, the much higher specific surface area (SSA) of K7000 compared to that of P25 (229.2 versus ∼50 m2 g−1, respectively) and, consequently, its smaller crystalline domain size, could account for its higher PCA with regards to that of P25 – high SSA is actually connected to a high number of active centres on the photocatalyst's surface.39 Furthermore, although we do not have any experimental evidence that upon visible-light irradiation K7000 exhibits a better charge separation/transfer, it has been recently reported that carbon-modified TiO2 shows improved charge separation and transfer ability.40,41 These findings would make K7000 a more consistent commercial standard for comparing the indoor PCA of materials made only of the anatase TiO2 phase.
Conclusions
A commercial carbon-modified nano-anatase photocatalyst, KRONOClean 7000®, was characterised via advanced X-ray methods. Results showed the only crystalline phase to be anatase, with amorphous phase content between 6.5–8.1 wt%. They also highlighted the bimodal nature of K7000, with a bimodal size distribution of anatase crystalline domains in all three batches studied. This consisted of a smaller fraction of large anatase domains (11.9–15.5 nm), co-existing with a much larger quantity of smaller domains between 3.8 and 4.4 nm. XPS analysis was in agreement with results previously reported in the literature, i.e. that an aromatic compound was at the origin of the visible-light absorption seen in the UV-Vis spectrum.PCA of the commercial material (in three batches) was assessed against NOx abatement, simulating an indoor environment (pollutant concentration, and intensity of the white-light entering the reactor). It was found that the K7000 photocatalyst showed a higher PCA (∼30% higher) compared to that of the well-known P25. Furthermore, K7000 exhibited a very stable PCA after three consecutive photocatalytic runs, and proved suitable for reuse. K7000 is highlighted as a promising commercial anatase standard for indoor (visible-light) PCA applications.
Acknowledgements
Authors are grateful to the ECO-SEE project (European Union's Seventh Framework Programme funding, grant agreement no 609234). This work was developed in the scope of the project CICECO – Aveiro Institute of Materials (Ref. FCT UID/CTM/50011/2013), financed by national funds through the FCT/MEC and when applicable co-financed by FEDER under the PT2020 Partnership Agreement. R. C. Pullar acknowledges the support of FCT grant SFRH/BPD/97115/2013. M. Ferro and RNME – University of Aveiro, FCT Project REDE/1509/RME/2005 – are kindly acknowledged for HR-TEM analysis. Dr C. Scherer (Fraunhofer-Institut für Bauphysik, Germany) is kindly acknowledged for his helpful discussion. G. Otero-Irurueta would like to thank FCT from Portugal for his Post-Doctoral research grant (SFRH/BPD/90562/2012).Notes and references
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- P. V. Kamat, J. Phys. Chem. Lett., 2011, 2, 839–840 CrossRef CAS.
- X. Chen and A. Selloni, Chem. Rev., 2014, 114, 9281–9282 CrossRef CAS PubMed.
- Y. Zhang, Z.-R. Tang, X. Fu and Y.-J. Xu, Appl. Catal., B, 2011, 106, 445–452 CrossRef CAS.
- S. Liu, Z.-R. Tang, Y. Sun, J. C. Colmenares and Y.-J. Xu, Chem. Soc. Rev., 2015, 44, 5053–5075 RSC.
- N. Zhang, M.-Q. Yang, S. Liu, Y. Sun and Y.-J. Xu, Chem. Rev., 2015, 115, 10307–10377 CrossRef CAS PubMed.
- Z. Şen, Solar energy fundamentals and modeling techniques: atmosphere, environment, climate change, and renewable energy, Springer, London, 2008 Search PubMed.
- S.-D. Mo and W. Y. Ching, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 51, 13023–13032 CrossRef CAS.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- D. M. Tobaldi, C. Piccirillo, R. C. Pullar, A. F. Gualtieri, M. P. Seabra, P. M. L. Castro and J. A. Labrincha, J. Phys. Chem. C, 2014, 118, 4751–4766 CAS.
- D. M. Tobaldi, R. C. Pullar, A. S. Škapin, M. P. Seabra and J. A. Labrincha, Mater. Res. Bull., 2014, 50, 183–190 CrossRef CAS.
- D. M. Tobaldi, R. C. Pullar, M. P. Seabra and J. A. Labrincha, Mater. Lett., 2014, 122, 345–347 CrossRef CAS.
- D. C. Hurum, A. G. Agrios, K. A. Gray, T. Rajh and M. C. Thurnauer, J. Phys. Chem. B, 2003, 107, 4545–4549 CrossRef CAS.
- D. O. Scanlon, C. W. Dunnill, J. Buckeridge, S. A. Shevlin, A. J. Logsdail, S. M. Woodley, C. R. A. Catlow, M. J. Powell, R. G. Palgrave, I. P. Parkin, G. W. Watson, T. W. Keal, P. Sherwood, A. Walsh and A. A. Sokol, Nat. Mater., 2013, 12, 798–801 CrossRef CAS PubMed.
- A. F. Gualtieri, J. Appl. Crystallogr., 2000, 33, 267–278 CrossRef CAS.
- A. C. Larson and R. B. von Dreele, General Structure Analysis System (GSAS), Los Alamos National Laboratory Report LAUR, 2004 Search PubMed.
- B. H. Toby, J. Appl. Crystallogr., 2001, 34, 210–213 CrossRef CAS.
- P. Scardi and M. Leoni, Acta Crystallogr., Sect. A: Found. Crystallogr., 2002, 58, 190–200 CrossRef CAS.
- Diffraction Analysis of the Microstructure of Materials, ed. E. J. Mittemeijer and P. Scardi, Springer, 2004th edn, 2004 Search PubMed.
- M. Leoni, T. Confente and P. Scardi, Z. Geomorphol., Suppl., 2006, 23, 249–254 Search PubMed.
- G. Caglioti, A. Paoletti and F. P. Ricci, Nucl. Instrum. Methods, 1960, 9, 195–198 CrossRef CAS.
- D. M. Tobaldi, R. C. Pullar, R. Binions, A. Belen Jorge, P. F. McMillan, M. Saeli, M. P. Seabra and J. A. Labrincha, Catal. Sci. Technol., 2014, 4, 2134 CAS.
- D. M. Tobaldi, R. a. S. Ferreira, R. C. Pullar, M. P. Seabra, L. D. Carlos and J. A. Labrincha, J. Mater. Chem. C, 2015, 3, 4970–4986 RSC.
- U. I. Gaya and A. H. Abdullah, J. Photochem. Photobiol., C, 2008, 9, 1–12 CrossRef CAS.
- R. Quesada-Cabrera, A. Mills and C. O'Rourke, Appl. Catal., B, 2014, 150–151, 338–344 CrossRef CAS.
- http://kronostio2.com/en/component/jdownloads/finish/99/288, accessed October 2015.
- V. N. Kuznetsov and N. Serpone, J. Phys. Chem. C, 2009, 113, 15110–15123 CAS.
- F. Spadavecchia, G. Cappelletti, S. Ardizzone, C. L. Bianchi, S. Cappelli, C. Oliva, P. Scardi, M. Leoni and P. Fermo, Appl. Catal., B, 2010, 96, 314–322 CrossRef CAS.
- N. Serpone, D. Lawless and R. Khairutdinov, J. Phys. Chem., 1995, 99, 16646–16654 CrossRef CAS.
- X. Xue, W. Ji, Z. Mao, H. Mao, Y. Wang, X. Wang, W. Ruan, B. Zhao and J. R. Lombardi, J. Phys. Chem. C, 2012, 116, 8792–8797 CAS.
- G. Balasubramanian, D. D. Dionysiou, M. T. Suidan, I. Baudin and J.-M. Laîné, Appl. Catal., B, 2004, 47, 73–84 CrossRef CAS.
- D. M. Tobaldi, R. C. Pullar, A. F. Gualtieri, G. Otero-Irurueta, M. K. Singh, M. P. Seabra and J. A. Labrincha, J. Solid State Chem., 2015, 231, 87–100 CrossRef CAS.
- L. Zhang, M. S. Tse, O. K. Tan, Y. X. Wang and M. Han, J. Mater. Chem. A, 2013, 1, 4497 CAS.
- P. Ząbek, J. Eberl and H. Kisch, Photochem. Photobiol. Sci., 2009, 8, 264–269 Search PubMed.
- J. Zhuang, Q. Tian, H. Zhou, Q. Liu, P. Liu and H. Zhong, J. Mater. Chem., 2012, 22, 7036 RSC.
- J. Lasek, Y.-H. Yu and J. C. S. Wu, J. Photochem. Photobiol., C, 2013, 14, 29–52 CrossRef CAS.
- Y. Ohko, Y. Nakamura, A. Fukuda, S. Matsuzawa and K. Takeuchi, J. Phys. Chem. C, 2008, 112, 10502–10508 CAS.
- T. Ibusuki and K. Takeuchi, J. Mol. Catal., 1994, 88, 93–102 CrossRef CAS.
- K. A. Michalow, D. Logvinovich, A. Weidenkaff, M. Amberg, G. Fortunato, A. Heel, T. Graule and M. Rekas, Catal. Today, 2009, 144, 7–12 CrossRef CAS.
- Y. Zhang, Z.-R. Tang, X. Fu and Y.-J. Xu, ACS Nano, 2010, 4, 7303–7314 CrossRef CAS PubMed.
- J. Su, P. Geng, X. Li, Q. Zhao, X. Quan and G. Chen, Nanoscale, 2015, 7, 16282–16289 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Emission spectrum of the lamp used for the photocatalytic experiments; graphical output of Rietveld–RIR QPA refinement; Raman and FT-IR spectra; normalised XPS C 1s core level spectra; PCA up to 100 min visible-light irradiation time. See DOI: 10.1039/c5ra22816f |
| This journal is © The Royal Society of Chemistry 2015 |