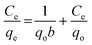Prospective application of Aspergillus species immobilized in sodium montmorillonite to remove toxic hexavalent chromium from wastewater
T. Sathvikaa,
Manasib,
Vidya Rajeshb and
N. Rajesh*a
aDepartment of Chemistry, Birla Institute of Technology and Science, Pilani-Hyderabad Campus, Jawahar Nagar, Shameerpet Mandal, R.R. Dist 500 078, India. E-mail: nrajesh05@gmail.com; Fax: +91 40 66303998; Tel: +91 40 66303503
bDepartment of Biological Sciences, Birla Institute of Technology and Science, Pilani-Hyderabad Campus, Jawahar Nagar, Shameerpet Mandal, R.R. Dist 500 078, India
First published on 7th December 2015
Abstract
An interdisciplinary approach involving chemistry and biotechnology offers greener solutions to mitigate heavy metal pollution originating from wastewaters. Aspergillus species (fungi) were isolated from bread and immobilized in sodium montmorillonite (an inorganic clay material). This biosorbent has good ability to remove toxic Cr(VI) from an acidic medium with a Langmuir adsorption capacity of 45.72 mg g−1. FTIR, SEM-EDAX, optical imaging and TGA techniques were used to explore the characteristics of the biosorbent before and after Cr(VI) adsorption. Optimum pH and temperature for Cr(VI) biosorption were 2.0 and 30 °C, respectively and the kinetics followed the pseudo second order model. The biosorbent regeneration was accomplished using sodium hydroxide. As a proof of concept, the method was validated in an industrial effluent wastewater sample of BCR-715, a certified reference material. The biosorbent can also be very useful to treat tannery and electroplating wastewaters discharging chromium.
1. Introduction
The deleterious effect on the biosphere due to the release of toxic heavy metals from industrial wastewaters is a major concern.1–3 Primarily, chromium is released from electroplating, tannery and dye effluents.4 Cr(III) is required for carbohydrate metabolism5 and hexavalent chromium is more mobile, genotoxic and carcinogenic.6 The USEPA stipulates a stringent regulatory limit7 of 0.05 mg L−1 for the discharge of Cr(VI). Therefore, it is important to devise effective and sustainable solutions to remove chromium from wastewater so as to bring down the concentration within the tolerance limit. Conventionally, precipitation, liquid–liquid extraction, and membrane based techniques have been utilized in the removal of various metals8,9 including chromium. Considering the multiple drawbacks such as higher operational capital costs, use of organic solvents and sludge disposal problems, adsorption techniques provide a more viable and sustainable option for treating wastewaters containing heavy metals. Adsorption can be performed using batch and column methods. The ease of regeneration of the adsorbent and relative cost effectiveness are some of the inherent advantages associated with the process.10,11Chemically modified polymeric resins,12 graphene oxide13 and activated carbon14 based adsorbents are known to remove chromium from wastewater. In order to improve upon some of the problems encountered with certain adsorbents, other processes have emerged as greener alternatives. Biosorption is regarded as an environmentally benign option to remove metal ions from wastewater at varying concentrations.15 Microorganisms such as bacteria, fungi and algae are known for their excellent ability to adsorb heavy metals from diverse wastewaters.16,17 The functional groups (carboxyl, amino, hydroxyl) present in the cell surface shows good binding affinity towards heavy metals through complexation, ion exchange and surface precipitation interactions.18
The well-studied bacterial and fungal species for metal adsorption includes Bacillus, Pseudomonas, Aspergillus and Rhizopus respectively.19–21 Immobilizing the microorganism in a polymeric matrix such as polyacrylamide22 and cellulose23 improves the porosity and increases the rigidity thereby enhancing the removal efficiency considerably. A bacterial species namely Burkholderia species24 immobilized in a silica matrix showed enhanced performance for the ability to reduce Cr(VI) in the range 50–500 μg g−1. Calcium alginate–Rhodamine conjugate gel bead has shown excellent ability as a fluorogenic sensor and also in the extraction of chromium in its lower oxidation state from aqueous medium.25 Removal of chromium(VI) using microbes involves principally adsorption26 and partial reduction to the trivalent state.27 More recently, yeast immobilized in cellulose matrix28 has been reported to adsorb hexavalent chromium with an adsorption capacity of 23.6 mg g−1.
Fungal species can be obtained in considerable amount as by product from industrial fermentation processes. These are known to have good potential in removing toxic metals such as cadmium, lead, copper etc. from aqueous medium. Aspergillus species finds applications in biotechnological processes such as citric acid production, transformations of progesterone, citral etc. Aspergillus is also used to synthesize extracellular enzymes such as amylase, lipase and pectinase and the FDA has recognised these enzymes to be safe and non-toxic.16
Filamentous fungi such as Aspergillus sp. are known for their excellent potential to remediate hexavalent chromium.29 Pretreatment of Aspergillus niger with CTAB followed by immobilization in a polysulfone matrix30 gives a biosorption capacity of 3.1 mg g−1. A removal efficiency of 29% has been reported using dead mass of the same fungal species.31
Smectite type of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 clays such as montmorillonite which principally contains two silica tetrahedral sheets and an alumina octahedral are also very useful to adsorb heavy metals.32 Clay–polymer nanocomposites and type of clays such as kaolinite, bentonite etc. are equally efficient in the treatment of wastewater.33,34 Biocomposites such as cellulose–clay35 and starch–montmorillonite36 effectively adsorb chromium in the presence of diverse ionic constituents. Acid activated kaolinite gives a Langmuir adsorption capacity of 13.9 mg g−1 for Cr(VI) adsorption in acidic pH medium.37 Surfactant modified montmorillonite38 has the ability to remove anionic chromium(VI) from water with an adsorption capacity of 18 mg g−1. The ability of dithionite reduced clays in the transformation of Cr(VI) to Cr(III) has been correlated with the ferrous content present in montmorillonite.39
1 clays such as montmorillonite which principally contains two silica tetrahedral sheets and an alumina octahedral are also very useful to adsorb heavy metals.32 Clay–polymer nanocomposites and type of clays such as kaolinite, bentonite etc. are equally efficient in the treatment of wastewater.33,34 Biocomposites such as cellulose–clay35 and starch–montmorillonite36 effectively adsorb chromium in the presence of diverse ionic constituents. Acid activated kaolinite gives a Langmuir adsorption capacity of 13.9 mg g−1 for Cr(VI) adsorption in acidic pH medium.37 Surfactant modified montmorillonite38 has the ability to remove anionic chromium(VI) from water with an adsorption capacity of 18 mg g−1. The ability of dithionite reduced clays in the transformation of Cr(VI) to Cr(III) has been correlated with the ferrous content present in montmorillonite.39
Clay minerals are recognized to be good supports and offer a defensive environment for microbes by forming a biofilm.40 Hence, the microbe–clay combination can even degrade pollutants such as volatile organic compounds. Clay minerals and microorganisms are known to thrive in similar temperature environment and hence the microbes can precipitate or dissolve in clay and alter some of the properties of clays. Typically, iron reducing bacteria as well as fungi can reduce the Fe(III) in montmorillonite to Fe(II), thereby augmenting the reduction of Cr(VI) to Cr(III) over a period of time. The microorganisms help in maintaining the Fe2+/Fe3+ balance through the adsorption coupled reduction mechanisms. Furthermore, clay minerals are endowed with good particle aggregation and low-oxygen penetration41 and hence serves as an ideal matrix to immobilize the microorganism and subsequently for the removal of chromium.
Biosorption encompasses the use of living as well as non-living microorganisms. The removal of pollutants by living microorganisms involves principally a metabolism dependent active bioaccumulation process.17 The bioaccumulation depends on a range of physicochemical and biological mechanisms (extra and intracellular processes). The extracellular polysaccharides generated by the microorganism plays an important role in the biosorption using living cells. Living microorganisms in soil and other clay rich environments are more useful to degrade organic pollutants such as polycyclic aromatic hydrocarbons.40 The utility of living microorganisms is more significant in metabolism dependent processes such as sewage treatment, anaerobic digestion etc. However, there are practical limitations in the application of living organisms for heavy metal removal. This is due to the fact that at higher metal ion concentrations and when adequate amount of metal ions are taken up by the microorganisms, the metabolism is disturbed leading to the death of the microbes.42 Hence, with living cells, the biosorption processes are prone to the pH variation, other metabolites and also dependent on the stress response generated by the toxic metal ion. These drawbacks are overcome when non-living microorganisms are used for the removal of metal ions.42
The removal of metal ions by non-living microorganisms involves a metabolism independent, passive biosorption mechanism. The binding of metals involves the coordination with the various active chelating functional groups in the cell surface. There are considerable advantages of using dead cells for biosorption over the living cells. In the case of dead cells, the growth of microbe and its application for metal removal are two distinct processes. These can be controlled more effectively thereby increasing the removal of metal ion by the biosorbent. Dead cells can be stored and used over a longer time period and are not affected by the metal toxicity and furthermore an uninterrupted nutrient supply to preserve the microbe is not required. With dead cells, the adsorption parameters such as isotherms, kinetics etc. can be studied easily by taking a known weight of the biosorbent. Living cells in the wet condition have larger water content and are prone to decay over a time period.43 It has been reported that the live Aspergillus niger shows adsorption capacity for heavy metals such as Pb, Cu and Cd in the range 0.75–2.3 mg g−1, while pre-treated and immobilized Aspergillus niger in matrices such as polyurethane matrix44,45 shows adsorption capacities greater than 20 mg g−1.
Hence, non-living microorganisms are easily amenable for surface modification using appropriate matrix support. In the non-living state, they can be easily immobilized onto the interlayers of clay. Therefore, the immobilization of dead microbes onto a suitable support has more mechanical strength and is also effective for column operations to treat a large sample volume, thereby enhancing the ease of regeneration of the biosorbent using simple desorbing agents.16
Literature reports on the immobilization of living fungi in a clay matrix for Cr(VI) adsorption are scarce. Considering the advantages of non-living microorganisms towards enhancing the adsorption capacity as well as efficiency, we felt it worthwhile to investigate the combination of inorganic materials such as clay and a non-living fungal species to adsorb hexavalent chromium. This concept provides a good illustration of the convergence of chemistry and biotechnology towards the application to remove heavy metals. In this work, we demonstrate the potential of a filamentous fungal strain (isolated as Aspergillus BRVR) in sodium montmorillonite for the effective adsorption of Cr(VI) from wastewater. Systematic characterization of the biosorbent, batch studies and preliminary column tests has been undertaken towards the development of this novel methodology.
2. Materials and methods
2.1 Materials
Analytical grade reagents were used in the optimization of all the experimental conditions. The aqueous solutions were prepared using Millipore water. Montmorillonite was procured from Fluka. A 1.0 g L−1 stock solution of Cr(VI) was prepared from G.R grade potassium dichromate (Merck). Working solution of 30 mg L−1 Cr(VI) for batch adsorption study was prepared accordingly by further dilution. The certified industrial effluent wastewater sample (BCR-715) is provided by IRRM-European Commission Joint Research Centre, Belgium.The clay–Aspergillus biosorbent was characterized through standard physicochemical characterization techniques. Mixing of fungi with clay was done using a sonication bath procured from Biotechnics, India. The preparation of samples for FT-IR studies was done by grinding 1 mg of the biosorbent with 100 mg of spectroscopy grade KBr and the spectra were recorded on a Jasco-4200 FT-IR spectrometer covering the range 400 to 4000 cm−1. Cr(VI) concentration was measured as its red-violate chelate with diphenylcarbazide formed in the post column derivatization at 540 nm using a 883 Basic IC plus Ion chromatography (Metrohm, 887 UV visible detector, Switzerland). SEM images of the biosorbent were taken using a S3400N Scanning electron microscope (Hitachi) attached with an EDAX analysis system (Thermo Electron Corporation). Optical images were recorded for Aspergillus species at 40× objective and for the biosorbent at 10× objective using an Olympus CH20i model optical microscope. Thermogravimetric analysis was done using a Shimadzu (DTG-60) thermal analyzer wherein the samples were heated in the range 30–800 °C at the rate of 10 °C min−1 under nitrogen atmosphere. The surface area and pore size of the biosorbent were analysed using a Smart Sorb 92/93 model surface area analyser (Smart Instruments Company Private Limited, India).
2.2 Production and identification of Aspergillus species
Aspergillus species was isolated from bread and maintained on Sabaroud Dextrose Agar and broth46 having dextrose as carbon source and peptone as the respective carbon and nitrogen sources. After autoclaving and inoculation, the flasks were incubated at 27 °C for 4–5 days. The morphological identification of Aspergillus culture was done by staining the fungi with lactophenol cotton blue47 (Fig. 1). Harvesting of the fungi was done through filtration and subsequently dried overnight at 70 °C and the powdered form was used for the adsorption of chromium. | ||
| Fig. 1 Photographic image of a three day old culture of Aspergillus BRVR on SDA and optical image of Aspergillus BRVR under 40× objective. | ||
2.3 Isolation of fungal 18S rDNA and cultivation
Fungal 18S rDNA was isolated using the Mollers et al.48 method as reported previously. The cultivated fungal culture was freeze dried overnight at −80 °C. 50 mg of dried material was taken and 500 μL of Tris–EDTA–sodium dodecyl sulphate (TES) buffer was added and mixed thoroughly. 60 μg of Proteinase K was added from the stock solution and incubated for at 60 °C for 60 min with intermittent mixing. The salt concentration was adjusted using NaCl followed by the addition of 1 mL of 10% CTAB and incubated for 15 min. Extraction was accomplished using chloroform: isoamyl alcohol and precipitated with isopropanol and the DNA pellet was washed with ethanol and suspended in Tris–EDTA (TE) for further studies.2.4 PCR amplification of 18S rDNA
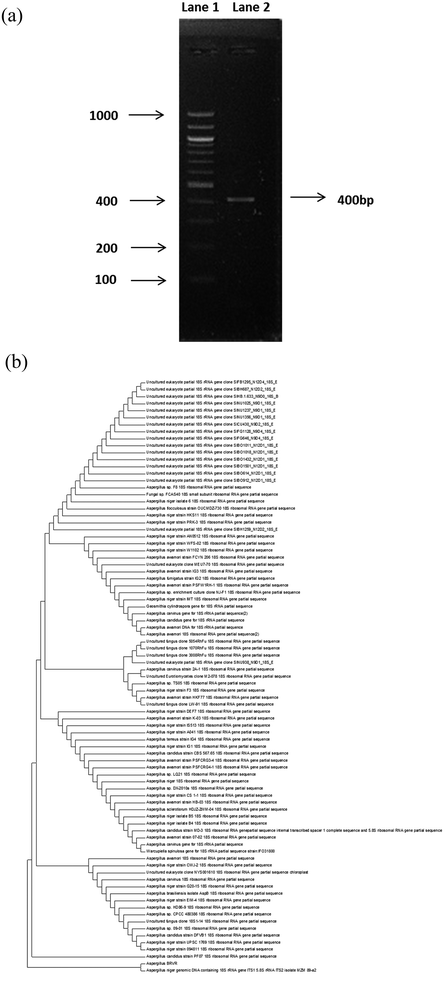
The PCR amplification was performed in a 25 μL reaction mixture using 100 ng per μL genomic DNA as a template having 200 ng of forward [nu-ssu-0817-59 5′(TTAGCATGGAAT AATGCAATAGG)] and reverse primers [nu-ssu-1196 5′(TCTGGACCTGGTGAGTTTCC)], 200 μM dNTP, 1 μL of Taq polymerase, 2.5 μL of 10× PCR buffer, containing 1.5 mM of MgCl2.49,50 The PCR programme incorporates an initial denaturation at 95 °C for 3 min and 32 cycles of denaturation at 95 °C for 1 min, annealing at 60 °C for 1 min, extension at 72 °C for 3 min with a final extension at 72 °C for 5 min. DNA of 400 bp was observed as a single band when resolved on 1% agarose gel (Fig. 2). Commercial sequencing of the gel purified samples was done at Bioserve Pvt. Ltd, Hyderabad, India using ABI 3730 xls Genetic analyser (Applied Biosystems). The sequences were identified through the BLAST search in the Gene Bank database (Nucleotide Blast) after the chromatogram verification.2.5 Preparation of biosorbent
The Na+ form of montmorillonite was prepared from montmorillonite as described in the literature.51 The powdered fungi was mixed with sodium montmorillonite in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in aqueous medium and sonicated for about 5 min to facilitate the immobilization of Aspergillus in clay matrix. The obtained biosorbent was filtered, washed with water and dried at 40 °C in a vacuum oven and utilized for the adsorption of chromium(VI).
1 ratio in aqueous medium and sonicated for about 5 min to facilitate the immobilization of Aspergillus in clay matrix. The obtained biosorbent was filtered, washed with water and dried at 40 °C in a vacuum oven and utilized for the adsorption of chromium(VI).
2.6 Batch adsorption procedure
The preliminary biosorption studies were conducted at 30 °C by equilibrating 0.2 g of the fungal–clay biosorbent with 30 mL of 30 mg L−1 chromium(VI) solution taken in an Erlenmeyer flask at pH 2.0–2.5 using an orbital incubator shaker (Biotechnics, India) at 120 rpm for varying time intervals. The concentration of chromium remaining in the solution phase was estimated using the post column derivative ion chromatography technique.52 The amount of chromium adsorbed (mg g−1) after the attainment of equilibrium (qe) was calculated using the expression
 | (1) |
3. Results and discussion
3.1 Molecular identification of the isolated fungal strain
The microscopic examination of Aspergillus stained with lactophenol cotton blue showed the presence of transparent hyphae and conidiophores with a bulging end known as ‘vesicule’ that produces significant amounts of conidia or spores (Fig. 1). The confirmation of the isolated fungal strain was acquired through 18S rDNA sequencing. Comparison of 18S rDNA gene sequence of the isolated strain with the database (Gen Bank) showed 98% similarity with the Aspergillus genera. The phylogenetic tree was constructed by neighbour joining method using MEGA version 5.0 software to analyse the evolutionary relationships among the various sequences of isolated fungal strain and its nearest neighbours (Fig. 2). The data obtained confirms that the fungal strain isolated (Aspergillus BRVR) belongs to the Aspergillus genera. The fungal strain sequence is assigned the accession number KT699195 upon submission to NCBI Gen Bank database.3.2 Characterisation of biosorbent
The fungi cell wall has diverse functional groups such as amine, hydroxyl, carboxyl and the FT-IR spectrum (Fig. 3) indicates the characteristic bands corresponding to the functional groups in Aspergillus as well as montmorillonite.53–55 The band near 3603 cm−1 is attributed to the silanol groups (Si–OH), while the broader band near 3402 cm−1is ascribed to the merging of N–H and O–H stretching vibrations in the fungus as well as the Al–O–H stretching vibration bands of montmorillonite clay. An intense peak at 1016 cm−1can be correlated to the C–O group of polysaccharide present in the cell walls of Aspergillus and Si– O–Si stretching vibration band of montmorillonite. The other peaks that are typical for montmorillonite were observed at 774 cm−1 (Si–O stretching of quartz and silica), 663 cm−1 (Si–O deformation), respectively.56 The peak at 1627 cm−1is linked to the amide-I band of the protein–peptide bond of the fungi and also the H–OH bonding in water. The participation of the hydroxyl groups of the fungi and the clay surface as well as the amine groups in Aspergillus are reflected in the spectral shift to 3418 cm−1 after the adsorption of hexavalent chromium along with the appearance of a sharp characteristic Cr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond vibration at 912 cm−1.57 The thermogravimetric analysis (TGA) (Fig. 4) gives an insight into the thermal stability of the clay–Aspergillus biosorbent and the first step corresponding to the temperature range 70–100 °C relates to water desorption from clay, the second one at 300 °C corresponds to dehydration of hydrated cation in the interlayer and last step at 450 °C is attributed to the dehydroxylation of aluminosilicate of montmorillonite.58
O bond vibration at 912 cm−1.57 The thermogravimetric analysis (TGA) (Fig. 4) gives an insight into the thermal stability of the clay–Aspergillus biosorbent and the first step corresponding to the temperature range 70–100 °C relates to water desorption from clay, the second one at 300 °C corresponds to dehydration of hydrated cation in the interlayer and last step at 450 °C is attributed to the dehydroxylation of aluminosilicate of montmorillonite.58
The interaction between the Aspergillus cell surface and the clay matrix depends on physicochemical (electrostatic, hydrophobic, hydrogen bonding, van der Waals) and biological phenomena involving production of enzymes, polysaccharides etc.59 The surface area of sodium montmorillonite clay (NaMMT) used to immobilize the Aspergillus sp. was found to be 195.17 m2 g−1 with a pore volume of 0.0841 cm3 and a pore size of 1.72 nm indicating the microporous nature60 of the clay.
After the addition of fungi, the surface area of the biosorbent was found to be 69.1 m2 g−1 with a pore size of 6.575 nm indicating the mesoporous nature of biosorbent with a pore volume of 0.1136 cm3 g−1. The enzymes produced by Aspergillus sp. contain functional groups that can interact with the surface silanol hydroxyl groups of NaMMT through hydrogen bonding. Hence, they can act as effective sites for linkage with the enzymes produced by the fungal species. After intercalation of Aspergillus sp., the immobilized enzymes block the pores in the clay matrix thereby altering the surface area after immobilization.61
The morphological features in the fungi immobilized clay matrix shows assorted clusters and the hyphae (Fig. 3). The accompanying energy dispersive X-ray spectrum of the Aspergillus immobilized clay surface indicated the adsorption of chromium in the range 5–6 keV (Fig. 3) and also supported well through the optical images. The Aspergillus immobilized clay was spread on a glass slide and the images were taken at 10× objective (Fig. 3). The addition of diphenyl carbazide28 as a complexing agent to the biosorbent containing chromium oxo anion showed a characteristic red violet color indicating the presence of adsorbent Cr(VI) onto the fungi immobilized clay surface.
3.3 pH effect and interaction of biosorbent with Cr(VI)
The pH of aqueous phase is quite important in knowing about the existence of chromium as HCrO4−, Cr2O72− and CrO42− oxy anions.28 At a concentration of 10 mg L−1 and in the acidic pH range 2.0–2.5, more than 94% of Cr(VI) was adsorbed onto the fungi immobilized clay surface (Fig. 5). As a matter of fact, between pH 2.0–2.5 hexavalent chromium is present as it's HCrO4− (hydrochromate) oxy anion since this pH range is also lower than the pKa (∼6) of the oxy anion. Representing the equilibrium as 2HCrO4− ⇌ Cr2O72− + H2O and when the pH is in the range 1.0–2.0, the existence of dichromate anion would be more probable. The hydrochromate anion is involved in the electrostatic interaction with the protonated functional groups (amine, carboxyl and hydroxyl) of Aspergillus as well as the silanol and aluminol groups (SiOH2+⋯HCrO4− and AlOH2+⋯HCrO4−) on the clay surface.35 The conceptual illustration depicting the immobilization of Aspergillus in the clay matrix and subsequent interaction with hexavalent chromium is shown in Fig. 6. Hence, these interactions involving the clay matrix as well as the fungal cell surface collectively helps in influencing the adsorption performance of Cr(VI). At higher pH, the surface of the microbe begins to attain a negative charge coupled with the deprotonation of the silanol and aluminol groups of clay leading to the repulsion with the chromium oxy anion. This results in the lowering of adsorption with increase in pH.The amount of biosorbent was also varied in the range 0.1–0.6 g (Fig. 5) using 30 mg L−1 chromium(VI) solution. Hexavalent chromium was adsorbed very effectively with as low as 0.2 g of the biosorbent in 30 mL aqueous phase. The presence of active surface adsorption sites augments the biosorption process with 0.2 g of the biosorbent and beyond this amount saturation is attained.
3.4 Equilibrium adsorption isotherms and kinetics
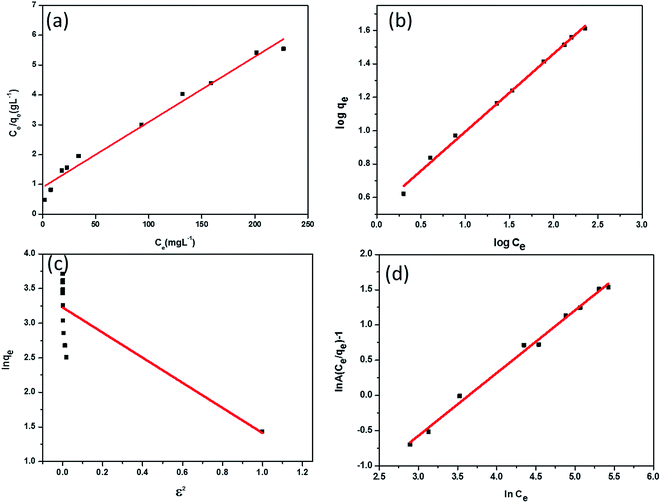
Biosorption is generally classified under a sub category of adsorption wherein the adsorbent is a microrganism. Biosorption is primarily a metabolically independent physicochemical process. The solution pH, temperature, effect of initial Cr(VI) concentration, speed of agitation can significantly affect the biosorption. In addition to adsorption, complexation, ion exchange and precipitation mechanisms are also equally important. The biosorption process involves a rapid, reversible equilibrium adsorption process involving the binding of HCrO4− oxy anion from aqueous solution onto the protonated functional groups present in the fungal cell surface. The biological part thus plays a vital role in the adsorption. Hence, adsorption equilibrium, kinetics and thermodynamics are of equal importance in biosorption processes as well. By choosing an appropriate concentration range of the metal ion, it is therefore possible to extend the commonly used isotherms (Langmuir, Freundlich, etc.) to biosorption in a manner similar to other conventional adsorption processes.16,62 The experimental biosorption data were fitted using four well established isotherm models namely, Langmuir, Freundlich, Redlich–Peterson (R–P) and Dubinin Radukskevich (D–R)63,64 respectively (Table 1). The respective isotherm plots are shown in Fig. 7. Each of these gives adequate information regarding the mode of adsorption. The Langmuir isotherm which principally accounts for monolayer adsorption is one of the time tested models that is used to relate the equilibrium adsorption capacity (qe) with the equilibrium concentration Ce and the Langmuir adsorption capacity (qo) respectively. Freundlich isotherm is yet another empirical model that gives a logarithmic relationship between the adsorption capacity, adsorption intensity (n) and the constant KF.| Dubinin Radushkevich | qm (mg g−1) | β (mol2 kJ−2) | E (kJ mol−1) | r2 | χ2 |
|---|---|---|---|---|---|
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qm − βε2 qm − βε2 |
25.196 | 1.816 | −0.524 | 0.62 | 3.378 |
The Langmuir adsorption capacity was found to be 45.72 mg g−1 with a correlation coefficient of 0.978. Also, the applicability to this model was established through a dimensionless parameter RL65 given as RL = 1/1 + bCo, wherein b indicates the energy of adsorption. The RL value was higher than unity for the biosorption process and shows the validity of this particular isotherm in correlating the biosorption data quite well. The Freundlich constant KF and n were obtained as 3.37 mg1−1/n g−1 L1/n and 2.14 respectively with a correlation coefficient of 0.99. The value of n in the range 1–10 also indicates favourable biosorption and the implication of Freundlich model as well. The statistical chi square values for both these isotherm models were also quite low indicating their good fit towards describing the biosorption data. The R–P isotherm model has striking similarity with Langmuir equation. The g value (0.89) which is near unity as obtained from the three parameter R–P isotherm model also indicates that the biosorption data can be explained reasonably well using the Langmuir model. The electrostatic interaction between the protonated functional groups in the biosorbent surface and Cr(VI) is reflected through the mean free energy of adsorption (−0.524 kJ mol−1) obtained from D–R isotherm model. The D–R isotherm predicts that when the mean free energy of adsorption is less than 8 kJ mol−1 the adsorption is more likely to be associated as physisorption.
The kinetics associated with the interaction between Cr(VI) and Aspergillus immobilized montmorillonite was studied using the pseudo first and second order kinetic models respectively66,67 given as
 | (2) |
 | (3) |
The removal of chromium Cr(VI) by the fungi immobilized clay attained its maximum within 60 min. The data obtained from the kinetic plots (Fig. 8) at 30 mg L−1 Cr(VI) concentration shows effective correlation to the second order model as shown in Table 2 with the experimental and calculated qe values as 4.19 and 4.30 mg g−1. Generally, particle, film diffusion and surface adsorption are the processes that affect the transport of Cr(VI) during agitation from solution phase to the Aspergillus immobilized clay surface. Intraparticle diffusion influences the kinetics at higher concentrations of the metal ion and at lower levels film diffusion governs the adsorption kinetics of Cr(VI).28 The Weber–Moris intraparticle diffusion model68 that connects qt and √t as a straight line fit (Fig. 8) results in a finite intercept indicating the influence of boundary layer phenomenon in accounting for the adsorption of Cr(VI) by the fungi–clay biosorbent surface.
 | ||
Fig. 8 (a) Pseudo first order kinetic plot (b) pseudo second order kinetic plot (c) intra particle diffusion (d) plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K against 1/T. K against 1/T. | ||
| Co (mg L−1) | qe (mg g−1) | k2 (g mg−1 min−1) | R2 | k1 (min−1) | R12 | kint (mg g−1 min−0.5) |
|---|---|---|---|---|---|---|
| 30 | 4.301 | 0.115 | 0.998 | 0.065 | 0.897 | 0.115 |
3.5 Biosorption thermodynamics
The assessment of the spontaneity and the energetics of interaction between the host clay–biosorbent matrix and the guest (hexavalent chromium) were obtained from the Gibb's free energy (ΔGo), enthalpy (ΔHo) and entropy (ΔSo) values. The ratio of the concentrations of Cr(VI) present as HCrO4− at equilibrium on the clay–Aspergillus surface and the aqueous phase gives the equilibrium constant (K) at different temperatures.28 The equilibrium constant is correlated with the Gibb's isotherm equation (ΔGo = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K) and from the straight line Van't Hoff plot of ln
K) and from the straight line Van't Hoff plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K against 1/T (Fig. 8) we obtain the ΔH and ΔS changes for the biosorption process. The above extensive thermodynamic properties arise due to primary and secondary host–guest interactions. Montmorillonite acts as a primary host in immobilizing the fungi (guest) and together they act as secondary host in inviting hexavalent chromium as guest. The respective thermodynamic properties could be expressed as
K against 1/T (Fig. 8) we obtain the ΔH and ΔS changes for the biosorption process. The above extensive thermodynamic properties arise due to primary and secondary host–guest interactions. Montmorillonite acts as a primary host in immobilizing the fungi (guest) and together they act as secondary host in inviting hexavalent chromium as guest. The respective thermodynamic properties could be expressed as| (ΔG) = ΔGclay–Aspergillus + ΔGclay–Aspergillus–chromium |
| (ΔS) = ΔSclay–Aspergillus + ΔSclay–Aspergillus–chromium |
| (ΔH) = ΔHclay–Aspergillus + ΔHclay–Aspergillus–chromium |
The concentration gradient across the clay–fungi biosorbent surface and the viscosity of the medium influences the transport of chromium from the aqueous phase. Accordingly, the free energy change in the biosorbent surface is greater than in the solution phase as evident from the negative values of free energy obtained at various temperatures. The free energy is also associated with the solvation of the host (biosorbent) and guest (HCrO4−) resulting in the lowering of chemical potential. Therefore, the enthalpically favourable exothermic biosorption that gives a relatively more negative entropy change signifies decreased randomness at the biosorbent–solution interface. The negative values of the average activation energy, Ea = ΔHadso + RT (ref. 28) also reflects this exothermic interaction (Table 3) between hexavalent chromium and the fungi immobilized clay matrix. The enthalpy values obtained are quite high to account for a total physisorption process and are also relatively low for pure chemisorption.69 Hence, the interaction between the functional groups on the clay–fungal adsorbent is conceptualized as a physicochemical adsorption phenomenon.
| Temperature (Kelvin) | ΔGo (kJ mol−1) | ΔSo (J mol−1 K −1) | ΔHo (kJ mol−1) | Ea (kJ mol−1) |
|---|---|---|---|---|
| 303 | −6.663 | −182.675 | −61.867 | −61.602 |
| 313 | −4.460 | |||
| 323 | −2.483 | |||
| 333 | −1.211 |
3.6 Column studies
The applicability of the biosorbent material for Cr(VI) removal was examined in a higher sample volume through preliminary laboratory scale column studies. For this purpose, a 1.5 g clay–fungal biosorbent was packed onto a short glass column of 2 cm diameter upto a bed height of 3 cm. A 50 mL volume of 5 mg L−1 Cr(VI) solution at pH 2.0 was loaded onto the column at a flow rate of 6 mL min−1. The concentration of chromium(VI) that emerged out of the column was checked periodically (for every 10 mL collected in the eluate) with diphenyl carbazide as the derivatizing agent using ion chromatography. There was no trace of chromium detectable in the solution phase and this shows that the adsorption onto the biosorbent was quantitative for an initial concentration (5 mg L−1) of chromium and a sample volume of 50 mL.The number of bed volumes, empty bed residence time (EBRT) and adsorbent exhaustion rate (AER) were also obtained for this fixed-bed adsorption process.
The AER was found to be 7.5 g L−1 and EBRT indicated that the Cr(VI) solution was in contact with the biosorbent for approximately 1.6 min corresponding to 21.23 bed volumes. With larger amount of the biosorbent in the packed bed, there is more potential to treat still higher sample volumes with lower AER and higher EBRT values.70
3.7 Regeneration of biosorbent
The reusability of biosorbent is quite important in order to understand the economics of the entire adsorption process. Sodium hydroxide was explored as a potential reagent for desorption since in alkaline medium, hexavalent chromium forms the yellow tetraoxochromate (CrO42−) oxy anion. The concentration of NaOH was varied between 0.5–3.0 mol L−1 and we observed the elution of chromium as sodium chromate28 from the biosorbent surface with 2.0–3.0 mol L−1 NaOH to be quite high as shown in Fig. 9. The biosorbent surface was washed with water and after mild acid conditioning was subjected to further use. The Aspergillus immobilized clay adsorbent could be reused without any apparent decrease in the efficiency for 4 adsorption–desorption cycles. A regeneration efficiency of 86% and 78% were observed in the fifth and sixth cycles respectively Fig. 9. The repetitive alkaline and acid treatment of the biosorbent surface resulted in the reduction in the regeneration efficiency beyond four cycles. The eluted chromium(VI) containing solution was utilized for the other ongoing biosorption methods that are in progress and a part of the eluate was also diluted considerably. This solution was converted to the less toxic Cr(III) and collected separately. The process was undertaken so as to minimize the disposal of higher concentrations of the hexavalent form.The reduction of hexavalent chromium to the trivalent state was also not apparent after biosorption on the clay–fungi surface. Although, microbes have the ability to reduce Cr(VI), we observed the partial reduction to occur quite gradually after 4–5 days and this was seen in the form of a light green coloration (due to Cr(III)) appearing on the clay–Aspergillus biosorbent surface. This phenomena was quite similar as observed earlier with yeast immobilized cellulose28 as the adsorbent for chromium(VI). Hence, the Aspergillus immobilized clay biosorbent is more likely to adsorb chromium quantitatively in its hexavalent form than the instant coupled reduction to the +3 state.
3.8 Application to a certified wastewater sample
The effect of some familiar ionic constituents known to be present in the industrial wastewater samples were probed independently in a synthetic wastewater containing 20 mg L−1 concentrations of Fe(II), Cu(II), Ni(II), Cd(II), Pb(II), Mn(II), Zn(II) and chloride, nitrate, sulfate and phosphate ions and 10 mg L−1 Cr(VI). A 9.53 ± 0.02 mg L−1 of Cr(VI) could be retained onto the biosorbent surface in the presence of these diverse ions.Following this, the methodology was tested in an industrial effluent wastewater sample BCR 715 which is a certified mixture having low concentration of various ions such as As, Cd, Cr, Cu, Fe, Mn, Ni, Pb, Se and Zn of which chromium concentration is (1.0 ± 0.09) mg L−1. A 5 mL volume of the waste water sample (corresponding to an amount of 5 μg Cr) was converted to Cr(VI) by gentle oxidation using sodium hydroxide and hydrogen peroxide.12 The solution was then diluted to 100 mL volume and passed through the biosorbent column (1.5 g) at pH 2.0. Cr(VI) was completely adsorbed onto the column and this was confirmed by finding the concentration of chromium left in the solution phase through ion chromatography. The adsorbed Cr(VI) was also eluted effectively using 10 mL of 2.0 mol L−1 sodium hydroxide.
With 5 μg of chromium in 1.5 g of the biosorbent, the adsorption of chromium was found to be quantitative. Chromium was not detectable in the solution phase and this was verified by measuring the chromium concentration using ion chromatography in the solution phase after loading 100 mL of the sample. Chromium(VI) adsorbed on the biosorbent was eluted using 10 mL of 2.0 mol L−1 NaOH solution. The corresponding chromatogram and the concentration of chromium eluted are shown in Fig. 9. The effective concentration of chromium in the eluate was found to be 0.5 mg L−1 corresponding to a retention time of 2.46 min thereby resulting in a preconcentration factor of 10 in the certified waste water sample. The results obtained indicate that the biosorbent has good potential to remove chromium from industrial waste water samples.
Electroplating effluents that discharge hexavalent chromium are quite acidic and the industrial effluents are usually in the pH range 2.0–3.0.27,71 In this pH range Cr(VI) exists as hydrochromate (HCrO4−) anion and interacts very well with the protonated biosorbent surface. Hence, the clay–Aspergillus biosorbent could be well suited to treat such highly acidic actual industrial waste waters.
3.9 Comparison of adsorption capacity against allied fungal strains and clays
The isolated Aspergillus BRVR fungal strain as such showed an adsorption capacity of 13.5 mg g−1 and the Aspergillus immobilized sodium montmorillonite (NaMMT) showed a relatively higher adsorption capacity of 45.75 mg g−1. The original clay material (montmorillonite) and sodium modified montmorillonite showed adsorption capacities of only 8.14 mg g−1 and 9.16 mg g−1 respectively. Hence, the synergistic or co-operative effect is manifested through the effective microbe–NaMMT interaction with the hexavalent chromium.Furthermore, the biosorbent also shows relatively higher adsorption capacity in comparison with other allied fungal strains and some clay composites35,72–76 as well (Table 4). The above results quantify the improvement in chromium removal performance. The good adsorption capacity shows that this biosorbent has the potential to remove chromium from wastewaters quite efficiently.
| Adsorbents | Adsorption capacity (mg g−1) | pH | Temperature |
|---|---|---|---|
| Agaricus bisporus72 | 8.0 | 1.0 | 20 °C |
| Rhizopus arrhizus73 | 23.92 | 1.3 | 30 °C |
| Aspergillus sydoni74 | 9.07 | 2.0 | 25 °C |
| Lentinus sajor-caju75 | 22.10 | 2.0 | 40 °C |
| Chitosan–sodium montmorillonite–Fe3O4 microspheres76 | 58.82 | 5.0 | 30 °C |
| Cellulose–sodium35 montmorillonite | 22.20 | 3.8–5.5 | 45 °C |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| (Present studies) | |||
| Aspergillus BRVR | 13.50 | 2.0 | 30 °C |
| Montmorillonite | 8.14 | 2.0 | 30 °C |
| Na+ montmorillonite | 9.16 | 2.0 | 30 °C |
| Aspergillus BRVR immobilized in sodium montmorillonite | 45.72 | 2.0 | 30 °C |
4. Conclusions
This study has illustrated the combined influence of fungi and sodium montmorillonite for the prospective removal of hexavalent chromium. The use of clay as a support reinforces the interaction between the functional groups present in the cell wall of Aspergillus and chromium. The inorganic support imparts good stability thereby giving a high adsorption capacity of 45.72 mg g−1. Second order model is more appropriate in describing the adsorption kinetics of chromium(VI). The thermodynamics of biosorption process indicates the spontaneous and exothermic interaction. The preliminary laboratory scale column tests have indicated the prospects of this biosorbent in the scale up operations to a higher sample volume. The detailed fixed bed column modeling aspects which we plan to undertake as a further study would certainly enhance the worth of this biosorbent. The ability of the biosorbent to treat industrial wastewater and the regeneration using sodium hydroxide highlights the convergence of chemistry and biotechnology in formulating sustainable solutions to address heavy metal contamination.Acknowledgements
We thankfully acknowledge Department of Science and Technology (DST), India (Project No: SR/S1/IC-06-2012) for financial support. We are also grateful to ARCI, Hyderabad, India, for their technical assistance in characterization of biosorbent.References
- J. Hu, H. Chen, B. Lan, J. Geng, H. Li and X. Xing, Environ. Sci.: Water Res. Technol., 2015, 1, 199–203 CAS.
- R. Lakshmipathy and N. C. Sarada, Environ. Sci.: Water Res. Technol., 2015, 1, 244–250 CAS.
- O. Sayer, M. M. Amini, H. Moghadamzadeh, O. Sadeghi and S. J. Khan, Microchim. Acta, 2013, 180, 227–233 CrossRef.
- Z. Song, W. Li, W. Liu, Y. Yang, N. Wang, H. Wang and H. Gao, RSC Adv., 2015, 5, 13028–13035 RSC.
- J. B. Vincent, Acc. Chem. Res., 2000, 33, 503–510 CrossRef CAS PubMed.
- P. F. Shi, B. Zhao, G. Xiong, Y. L. Hou and P. Cheng, Chem. Commun., 2012, 48, 8231–8233 RSC.
- USEPA Analytical feasibility support document for the six year review of existing national primary drinking water regulation, office of ground water and drinking water EPA 815R 03; EPA: Washington DC, March 2003.
- S. Kalidhasan, S. Sricharan, M. Ganesh and N. Rajesh, J. Chem. Eng. Data, 2010, 55, 5627–5633 CrossRef CAS.
- L. Yuan, W. Zhi, Q. Xie, X. Chen and Y. Liu, Environ. Sci.: Water Res. Technol., 2015, 1, 814–822 Search PubMed.
- V. Camel, Spectrochim. Acta, Part B, 2003, 58, 1177–1233 CrossRef.
- J. Lalley, C. Han, G. R. Mohan, D. D. Dionysiou, T. F. Speth, J. Garland and M. N. Nadagouda, Environ. Sci.: Water Res. Technol., 2015, 1, 96–107 CAS.
- N. Rajesh, A. S. K. Kumar, S. Kalidhasan and V. Rajesh, J. Chem. Eng. Data, 2011, 56, 2295–2304 CrossRef CAS.
- A. S. K. Kumar and N. Rajesh, RSC Adv., 2013, 3, 2697–2709 RSC.
- J. Zhang, T. Shang, X. Jin, J. Gao and Q. Zhao, RSC Adv., 2015, 5, 784–790 RSC.
- N. Xafenias, Y. Zhang and C. J. Banks, Environ. Sci. Technol., 2013, 47, 4512–4520 CrossRef CAS PubMed.
- J. Wang and C. Chen, Biotechnol. Adv., 2009, 27, 195–226 CrossRef CAS.
- M. Fomina and G. M. Gadd, Bioresour. Technol., 2014, 160, 3–14 CrossRef CAS PubMed.
- W. W. Li and H. Q. Yu, Bioresour. Technol., 2014, 160, 15–23 CrossRef CAS PubMed.
- L. Li, N. Hu, D. Ding, X. Xin, Y. Wang, J. Xue, H. Zhang and Y. Tan, RSC Adv., 2015, 5, 65827–65839 RSC.
- K. Vijayaraghavan and Y. S. Yun, Biotechnol. Adv., 2008, 26, 266–291 CrossRef CAS PubMed.
- J. Samuel, M. L. Paul, M. Pulimi, M. J. Nirmala, N. Chandrasekaran and A. Mukherjee, Ind. Eng. Chem. Res., 2012, 51, 3740–3749 CrossRef CAS.
- J. R. Dodson, H. L. Parker, A. M. Garcia, A. Hicken, K. Asemave, T. J. Farmer, H. He, J. H. Clark and A. J. Hunt, Green Chem., 2015, 17, 1951–1965 RSC.
- Z. Xu, W. Bae, A. Mulchandani, R. K. Mehra and W. Chen, Biomacromolecules, 2002, 3, 462–465 CrossRef CAS.
- G. S. Alvarez, M. L. Foglia, D. E. Camporotondi, M. V. Tuttolomondo, M. F. Desimone and L. E. Diaz, J. Mater. Chem., 2011, 21, 6359–6364 RSC.
- S. Saha, M. U. Chhatbar, P. Mahato, L. Praveen, A. K. Siddhanta and A. K. Das, Chem. Commun., 2012, 48, 1659–1661 RSC.
- W. Xu, S. Wang, Y. Liu, G. Zeng, B. Zheng, X. Tan, T. Li, H. Wang, F. Guo and M. Zhang, RSC Adv., 2015, 5, 24009–24015 RSC.
- D. Park, Y. S. Yun, J. H. Jo and J. M. Park, Water Res., 2005, 39, 533–540 CrossRef CAS PubMed.
- T. Sathvika, Manasi, V. Rajesh and N. Rajesh, Chem. Eng. J., 2015, 279, 38–46 CrossRef CAS.
- Y. Gu, W. Xu, Y. Liu, G. Zeng, J. Huang, X. Tan, H. Jian, X. Hu, F. Li and D. Wang, Environ. Sci. Pollut. Res., 2015, 22, 6271–6279 CrossRef CAS PubMed.
- D. P. Mungasavalli, T. Viraraghavan and Y. C. Jin, Colloids Surf., A, 2007, 301, 214–223 CrossRef CAS.
- D. Park, Y. S. Yun and J. M. Park, Process Biochem., 2005, 40, 2559–2565 Search PubMed.
- S. S. Gupta and K. G. Bhattacharyya, Phys. Chem. Chem. Phys., 2012, 14, 6698–6723 RSC.
- E. I. Unuabonah and A. Taubert, Appl. Clay Sci., 2014, 99, 83–92 CrossRef CAS.
- M. Matlok, R. Petrus and J. K. Warchol, Ind. Eng. Chem. Res., 2015, 54, 6975–6984 Search PubMed.
- A. S. K. Kumar, S. Kalidhasan, V. Rajesh and N. Rajesh, Ind. Eng. Chem. Res., 2012, 51, 58–69 CrossRef CAS.
- Y. Koriche, M. Darder, P. Aranda, S. Semsari and E. R. Hitzky, Dalton Trans., 2014, 43, 10512–10520 RSC.
- K. G. Bhattacharyya and S. S. Gupta, Ind. Eng. Chem. Res., 2006, 45, 7232–7240 CrossRef CAS.
- M. C. Brum, J. L. Capitaneo and J. F. Oliveira, Miner. Eng., 2010, 23, 270–272 CrossRef CAS.
- R. W. Taylor, S. Shen, W. F. Bleam and S. I. Tu, Clays Clay Miner., 2000, 48, 648–654 CAS.
- B. Biswas, B. Sarkar, R. Rusmin and R. Naidu, Environ. Int., 2015, 85, 168–181 CrossRef CAS PubMed.
- H. Dong, Elements, 2012, 8, 113–118 CrossRef CAS.
- D. W. Darnall and J. M. Hosea, Emerging technologies: Bio-recovery systems removal and recovery of metal ions from ground water, USEPA/540/5-90/005a, Cincinnati, 1990, pp. 4–5 Search PubMed.
- B. Volesky, Hydrometallurgy, 2001, 59, 203–216 CrossRef CAS.
- A. Kapoor, T. Viraraghavan and D. R. Cullimore, Bioresour. Technol., 1999, 70, 95–104 CrossRef CAS.
- M. A. Dias, I. C. A. Lacerda, P. F. Pimentel, H. F. de Castro and C. A. Rosa, Lett. Appl. Microbiol., 2002, 34, 46–50 CrossRef CAS PubMed.
- C. Sugita, K. Makimura, K. Uchida, H. Yamaguchi and A. Nagai, Med. Mycol., 2004, 42, 433–437 CrossRef CAS PubMed.
- A. Leck, Community Eye Health, 1999, 12, 24 CAS.
- E. M. Moller, G. Bahnweg, G. Sandermann and H. H. Geiger, Nucleic Acids Res., 1992, 20, 6115–6116 CrossRef CAS PubMed.
- M. Pancher, M. Ceol, P. E. Corneo, C. M. O. Longa, S. Yousaf, I. Pertot and A. Campisano, Appl. Environ. Microbiol., 2012, 78, 4308–4317 CrossRef CAS PubMed.
- Manasi, V. Rajesh, A. S. K. Kumar and N. Rajesh, Chem. Eng. J., 2014, 235, 176–185 CrossRef CAS.
- M. Boufait and H. A. Amar, Desalination, 2007, 206, 300–310 CrossRef.
- E. J. Arar and J. D. Pfaff, J. Chromatogr. A, 1991, 546, 335–340 CrossRef CAS PubMed.
- D. Naumann, Infra-red spectroscopy in microbiology, Encyclopaedia of Analytical Chemistry, ed. R. A. Meyers, John Wiley and Sons Ltd., Chichester, 2000, pp. 102–131 Search PubMed.
- U. Flessner, D. J. Jones, J. Rozière, J. Zajac, L. Storaro, M. Lenarda, M. Pavanc, A. J. López, E. R. Castellón, M. Trombetta and G. Busca, J. Mol. Catal. A: Chem., 2001, 168, 247–256 CrossRef CAS.
- L. Zhongying, R. Tang and G. Liu, Catal. Lett., 2013, 143, 592–599 CrossRef.
- Z. M. Liang, J. Yin and H. J. Xu, Polymer, 2003, 44, 1391–1399 CrossRef CAS.
- V. Singh, A. K. Sharma, P. Kumari and S. Tiwari, Ind. Eng. Chem. Res., 2008, 47, 5267–5276 CrossRef CAS.
- W. Xie, Z. Gao, W. P. Pan, D. Hunter, A. Singh and R. Vaia, Chem. Mater., 2001, 13, 2979–2990 CrossRef CAS.
- M. A. Fomina, V. M. Kadoshnikov and B. P. Zlobenko, Process Metall., 1999, 9, 245–254 Search PubMed.
- P. B. Malla and S. Komarneni, Clays Clay Miner., 1990, 38, 363–372 CAS.
- S. Gopinath and S. Sugunan, Appl. Clay Sci., 2007, 35, 67–75 CrossRef CAS.
- B. E. Wang, Y. Y. Hu, L. Xie and K. Peng, Bioresour. Technol., 2008, 99, 794–800 CrossRef CAS PubMed.
- H. K. Chung, W. H. Kim, J. Park, J. Cho, T. Y. Jeong and P. K. Park, J. Ind. Eng. Chem., 2015, 28, 241–246 CrossRef CAS.
- M. Barathi, A. S. K. Kumar, C. U. Kumar and N. Rajesh, RSC Adv., 2014, 4, 53711–53721 RSC.
- K. R. Hall, L. C. Eagleton, A. Acrivos and T. Vermeulen, Ind. Eng. Chem. Fundam., 1966, 5, 212–223 CAS.
- S. Lagergren, K. Sven. Vetenskapsakad. Handl., 1898, 24, 1–39 Search PubMed.
- Y. S. Ho and G. Mckay, Water Res., 2000, 34, 735–742 CrossRef CAS.
- W. J. Weber and J. C. Morris, J. Sanit. Eng. Div., Am. Soc. Civ. Eng., 1963, 89, 31–60 Search PubMed.
- I. A. A. Hamza, B. S. Martincigh, J. C. Ngila and V. O. Nyamori, Physics and Chemistry of the Earth, Parts A/B/C, 2013, 66, 157–166 CrossRef.
- E. Malkoc, Y. Nuhoglu and Y. Abali, Chem. Eng. J., 2006, 119, 61–68 CrossRef CAS.
- A. Baral and R. D. Engelken, Environ. Sci. Policy, 2002, 5, 121–133 CrossRef CAS.
- N. Ertugay and Y. K. Bayhan, J. Hazard. Mater., 2008, 154, 432–439 CrossRef CAS PubMed.
- B. Preetha and T. Viruthagiri, Sep. Purif. Technol., 2007, 578, 126–133 CrossRef.
- R. Kumar, N. R. Bishnoi and G. K. Bishnoi, Chem. Eng. J., 2008, 135, 202–208 CrossRef CAS.
- G. Bayramoglu, G. Celik, E. Yalcin, M. Yilmaz and M. Y. Arica, J. Hazard. Mater., 2005, 119, 219–229 CrossRef CAS PubMed.
- D. Chen, W. Li, Y. Wu, Q. Zhu, Z. Lu and G. Du, Chem. Eng. J., 2013, 221, 8–15 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |