Structural characterization of Ag-doped TiO2 with enhanced photocatalytic activity†
Lidiaine M. Santosa,
Werick A. Machadoa,
Marcela D. Françaa,
Karen A. Borgesa,
Roberto M. Paniagob,
Antonio O. T. Patrocinio*a and
Antonio E. H. Machado*a
aLaboratory of Photochemistry and Materials Science – LAFOT-CM Instituto de Química, Universidade Federal de Uberlândia, 38400-902 Uberlândia, Minas Gerais, Brazil. E-mail: aehmachado@gmail.com; otaviopatrocinio@iqufu.ufu.br
bDepartamento de Física, Universidade Federal de Minas Gerais, 31270-901 Belo Horizonte, Minas Gerais, Brazil
First published on 23rd November 2015
Abstract
Ag-doped TiO2 nanoparticles with different metallic content (0.5, 2.0, and 5.0% m/m) were prepared using a simple and cost-effective method based on the sol–gel technique, followed by thermal treatment. The addition of Ag+ ions during the hydrolysis/condensation of the Ti(IV) molecular precursor leads to homogeneous dispersion of the Ag+ cations on the titania matrix. As the amount of silver is increased, the resulting TiO2 nanoparticles exhibit smaller particle size (from 27 nm for bare TiO2 to 12 nm for TiO2–Ag 5.0%) and larger surface area. X-ray photoelectron spectroscopy (XPS) confirms that during the sintering step of the resultant powder at 400 °C for 5 hours, ca. 34% of the silver content is converted to Ag0 via thermal decomposition of Ag2O. The data also indicates the presence of highly oxidized silver species, such as Ag2+ and Ag3+. X-ray diffractograms and Raman spectroscopy confirm that the crystalline structure of the TiO2 matrix corresponds to the anatase polymorph; however, the presence of the dopant leads to an increase in the system disorder due to a higher concentration of oxygen vacancies, as also confirmed by XPS. TiO2–Ag 5.0% exhibited the highest photocatalytic activity towards mineralization of the E102 tartrazine azo-dye, being 78% faster than bare TiO2 at optimum pH conditions (pH = 6.9). Upon light excitation, the oxidized silver cations are reduced to Ag0, leading to an improvement in visible light absorption due to the surface plasmon resonance effect. The recycling of the photocatalyst showed that the enhanced photocatalytic activity is maintained, which can be associated with the reduction of charge recombination at the oxide surface and the enhanced visible light harvesting.
1. Introduction
Solar energy conversion through photocatalysis has called special attention due the possibility to produce, in a sustainable way, fuels and other valuable chemicals as well as be applied for environmental remediation. TiO2-based materials are the most investigated photocatalytic system1–5 since the breakthrough by Fujishima and Honda.6 However, TiO2 as a photocatalyst exhibits some drawbacks that have to be surpassed for commercial applications. The main ones are the low visible light absorption and the fast charge recombination rates that decrease considerably the quantum yields of the photoreactions.4,7–9Possible solutions for these limitations include doping the oxide with different species and the formation of nanocomposites with strong electronic coupling and controlled morphology.10–19 In this aspect, Ag0–TiO2 materials have been extensively investigated due to the dual function of Ag sites.20–23 Despite acting as electron acceptor centers, decreasing charge recombination, Ag0 clusters exhibit strong visible light absorption on the oxide surface due to the so called surface plasmon resonance (SPR) effect.3,24–26 Thus, it is interesting to develop relatively easy and cost-effective procedures to prepare Ag–TiO2 photocatalytic systems that result in homogeneous and highly dispersed Ag sites on the porous TiO2 matrix. Moreover, it is also necessary to gain a better understanding about the chemical environment in which the Ag sites are embedded.
In this work, a relatively simple two step methodology for the production of Ag-doped TiO2 nanoparticles with enhanced photocatalytic activity is reported. The as prepared material was fully characterized using different techniques in order to better understand the chemical and electronic interactions between the dopant and the matrix. The role of the dopant concentration and pH conditions on photocatalysis was evaluated towards the degradation of the E102 tartrazine azo-dye, which is known for its relatively high stability against direct photolysis.27,28 The results presented here provide new insights on the structure of Ag-modified TiO2 nanoparticles and the influence of preparation conditions on the photocatalytic properties of these systems.
2. Material and methods
2.1. Preparation of photocatalysts
All chemicals were of analytical or HPLC grade and were used as received. Ultrapure water was employed in all experiments. Bare and Ag-doped TiO2 nanoparticles were prepared through controlled hydrolysis of Ti(IV) isopropoxide (Aldrich, 97%). In an ultrasonic bath (135 W), 50 cm3 of water was added dropwise to 7.50 mL (25 mmol) of Ti(IV) isopropoxide previously solubilized in 20 mL of isopropanol (Sigma Aldrich). After 30 minutes, 16, 63 or 160 mg of silver nitrate (Sigma Aldrich, 99%) was added to the system to obtain respectively 0.5, 2.0 or 5.0% Ag-doped TiO2 photocatalysts. The dopant concentration refers to the theoretical Ag atomic percentage in relation to TiO2. The mixture was kept under stirring for 2 hours, and afterwards, the bare and doped photocatalysts were centrifuged, dried at 70 °C under reduced pressure and sintered at 400 °C for 5 hours.2.2. Characterization of the photocatalysts
Electronic transmission images were acquired using a Philips CM 120 microscope at 120 kV. X-ray diffraction analyses (XRD) were carried out using an XRD600 powder diffractometer (Shimadzu) operating at 40 kV and 120 mA employing Cu Kα radiation. The diffractograms were collected between 20 to 90° at 0.5° min−1. Rietveld analyses of the XRD data were done using the FullProf software. As fitting criteria, S factors were kept between 1.09 and 1.33. Fit parameters can be found in the supplementary information (Table S1†). N2 adsorption–desorption isotherms were obtained using an ASAP 2020 analyzer (Micrometrics). The sorption data were analyzed using the BET model for the surface area and the Barrett–Joyner–Halenda (BJH) model for the porous volume.29 Raman spectra were acquired at room temperature using an Alpha 300 S/Witec spectrometer using 514.5 nm Ar laser excitation (4 nW). Diffuse reflectance measurements were carried out using an UV-1650PC spectrometer (Shimadzu) with the band gap energy being estimated by the Kubelka–Munk function.30 X-Ray photoelectron spectroscopy (XPS) measurements were done using a ESCALAB 220ixL spectrometer (VG Scientific) equipped with a hemispherical electron energy analyzer using Al Kα radiation (hν = 1487 eV).31 Photoelectron spectra were recorded in constant analyzer energy (CAE) mode. The binding energies were measured in reference to the C1s peak at 284.6 eV. Peak fitting was performed using the Micronal Origin 8.0 software. The background subtraction was performed by using a polynomial function.2.3. Photocatalytic activity
The photocatalytic activity of the as prepared powders was evaluated against the UVA degradation of the E102 tartrazine dye (trisodium (4E)-5-oxo-1-(4-sulfonatophenyl)-4-[(4-sulfonatophenyl) hydrazono]-3-pyrazolecarboxylate, CI 19140, Sigma-Aldrich, 85%). The experimental setup was described in detail previously.32 A high pressure Hg lamp (400 W) was employed as the light source, providing a photonic flux of 3.3 × 10−6 Einstein s−1. In a typical experiment, 100 mg L−1 of catalyst was loaded to a 43 mg mL−1 tartrazine aqueous solution. The mixture was kept under stirring for 30 minutes in the dark to reach the adsorption equilibrium. The lamp was turned on and the photocatalytic activity was probed spectroscopically (dye absorption at 428 nm) and also using total organic carbon (TOC) measurements using a SHIMADZU TOC-VCPH/CPN analyser. The results present here are the average of at least three individual experiments. Control experiments in the absence of any photocatalyst were also carried out to evidence the role of TiO2 on the photochemical reaction.3. Results and discussion
The morphology and crystalline structure of the as prepared photocatalysts were characterized by means of transmission electron microscopy (TEM), N2 adsorption/desorption isotherms and X-ray diffraction (XRD). TEM images evidence the presence of spherical particles for both, bare and doped, TiO2-catalysts with small Ag0 islands being observed on the surface of the doped TiO2 nanoparticles (Fig. 1). The TiO2 particle size decreases as the amount of Ag increases, as can be inferred from the measurements of the particle diameters in the TEM images (the histograms of the particle size distribution can be found at ESI, Fig. S1†).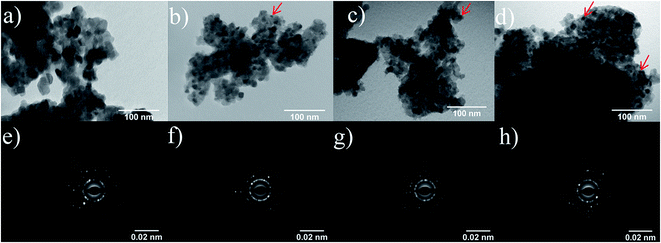 | ||
| Fig. 1 TEM micrographs and diffraction patterns of the bare TiO2 (a, e), TiO2–Ag 0.5% (b, f), TiO2–Ag 2.0% (c, g) and TiO2–Ag 5.0% (d, h) photocatalysts. | ||
The smaller particle size as the Ag concentration increases can possibly be explained by a decrease in the particle nucleation during hydrolysis/condensation of Ti(IV) isopropoxide or even due to a decrease in the grain boundaries during the sintering process. The experimental data evidences a drastic decrement in the particle size as the Ag content increases from 0.5 to 2.0%. Between 2.0% and 5.0%, only a small variation is observed. This trend may be related to a critical Ag concentration in which the influence on the particle size reaches a maximum.
SAED data (Fig. 1e–h) as well as X-ray diffractograms (Fig. 2), evidence that all samples are composed of anatase nanocrystals with (101) as the preferential exposed face. Rietveld analyses of the diffractograms (ESI, Fig. S2†) confirm the decrease of the crystallite size as the Ag amount increases, which is in agreement with the TEM images. This behavior is related to the occurrence of association defects due to the presence of the dopant, leading to distortions in the crystalline structure and smaller crystallites.10,33 The XRD data do not show any peaks related to Ag species, even for TiO2–Ag 5.0%, which is indicative of a high dispersion of the dopants in the TiO2 samples.
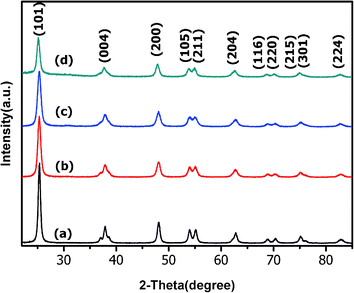 | ||
| Fig. 2 XRD patterns of the as prepared photocatalysts: (a) bare TiO2, (b) TiO2–Ag 0.5%, (c) TiO2–Ag 2.0% and (d) TiO2–Ag 5.0%. | ||
The N2 sorption isotherms of the photocatalysts follow the type IV profile according to the IUPAC classification34 (Fig. 3) which is characteristic to mesoporous materials. The calculated surface area and porosity are presented in Table 1, along with the average particle size for each sample. The diffuse reflectance spectra of the photocatalysts are shown in Fig. 4. The spectra are characterized by an intense absorption feature in the UV region attributed to the band gap excitation of TiO2. The band-gap edge absorption is red-shifted as the dopant concentration increases, as can be confirmed by the estimation of the band gap energies using the Kubelka–Munk function (Table 1). In the visible region, a broad absorption band between 380–500 nm (see the inset in Fig. 4) is observed, probably related to the plasmonic absorption bands of Ag0 clusters present on the TiO2 surface,35–37 which contributes to the improvement of visible light harvesting. Similar behavior has been observed by other authors, using different doping procedures.38–40 Specifically for the TiO2–Ag 5.0% photocatalyst, an increase in light scattering up to 800 nm is observed, which is associated to the higher concentration of the Ag species on the titania surface.
 | ||
| Fig. 3 N2-adsorption/desorption isotherms for the TiO2 photocatalysts with different silver content. | ||
| Photocatalyst | Particle size (nm) | Crystallite size (nm) | Surface area (m2 g−1) | Porosity (%) | Band gap (eV) |
|---|---|---|---|---|---|
| TiO2 | 27 ± 1 | 25 ± 1 | 54.8 ± 0.3 | 11.9 | 3.24 |
| TiO2–Ag 0.5% | 22 ± 1 | 30 ± 2 | 58.6 ± 0.3 | 13.2 | 3.18 |
| TiO2–Ag 2.0% | 14 ± 1 | 23 ± 3 | 75.0 ± 0.5 | 15.8 | 3.17 |
| TiO2–Ag 5.0% | 12 ± 1 | 14 ± 3 | 90.1 ± 0.5 | 20.5 | 3.09 |
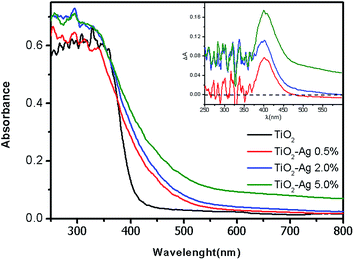 | ||
| Fig. 4 Diffuse reflectance spectra of the photocatalysts with different silver content. Inset: difference spectra obtained by subtracting the spectrum of bare TiO2 from those of the doped samples. | ||
The catalysts were also evaluated using Raman spectroscopy (Fig. 5). All samples exhibit typical anatase vibration modes (A1g + 2B1g + 3Eg), with the A1g mode being overlapped with the B1g peak at 537 cm−1.41–44 A slight shift in the signals is observed as the Ag content increases in the samples. Moreover, the peaks are broader than those observed for the bare TiO2 photocatalyst (Fig. 5 inset). This broadening of the Raman-active bands can be directly correlated to the concentration of oxygen vacancies on the photocatalysts, as previously shown by Parker and Siegel.45–47 Thus, Raman analysis indicates that both, bare and Ag-doped, TiO2 exhibit an anatase crystalline structure, but the introduction of the dopant induces the formation of oxygen vacancies on the oxide surface, increasing the system disorder.
X-ray photoelectron spectroscopy (XPS) was employed to better understand how the dopant interacts with the titania matrix. In Fig. 6, the data obtained for the TiO2–Ag 5.0% is shown. The survey spectrum (Fig. 6a) confirms the presence of only TiO2 and Ag species in the photocatalyst. The overall Ag/Ti ratio was 3.5%, indicating that ca. 30% of the initial amount of Ag+ ions was not impregnated onto the metal oxide surface. The high resolution spectrum of the Ag3d peaks (Fig. 6b) evidences the presence of the dopant in, at least, three different chemical states.
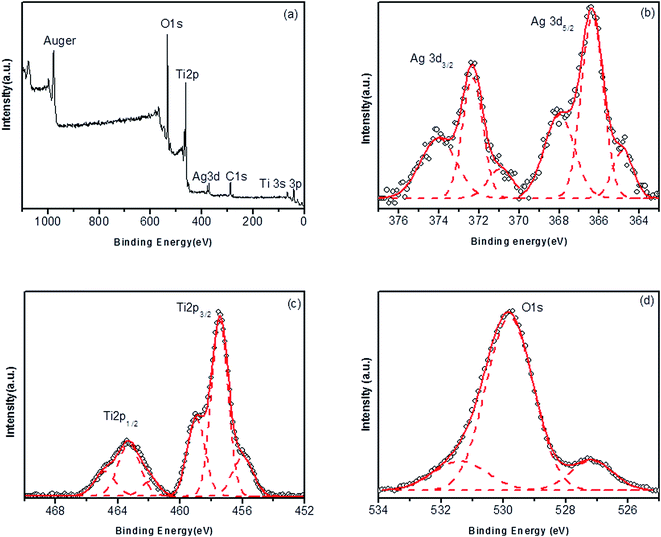 | ||
| Fig. 6 Survey (a) and high resolution XPS spectra in the Ag3d (b), Ti2p (c) and O1s (d) regions of the TiO2–Ag 5.0% photocatalyst. | ||
Gaussian deconvolution of the Ag3d5/2 signal yields three different peaks with maxima at 368.0, 366.3 and 364.8 eV. The peak at 368.0 eV is attributed to metallic silver (Ag0) according to previous studies,48,49 corresponding to 34% of the Ag content in the catalysts. The formation of Ag0 probably occurs via partial thermal decomposition of Ag2O at 400 °C, as previously reported.50 The metallic silver is on the surface of the titania particles as can be inferred from the plasmonic absorption bands observed in the diffuse reflectance spectra and from the TEM images. The two additional components at 366.3 and 364.8 eV are correlated to oxidized silver species48 and indicate that most of the silver present in the photocatalyst (66%) remains as cations, interacting with TiO2 through the Ag–O bonds. Based on previous studies involving silver oxides,39,51–54 we infer that part of the Ag+ ions should remain as Ag2O, while another part is oxidized to Ag2+ or even Ag3+.
The presence of oxidized silver species can also be confirmed through the high resolution XPS spectra in the Ti2p (Fig. 6c) and O1s (Fig. 6d) regions. In Fig. 6c, the Ti2p doublet can be seen, corresponding to 2p1/2 and 2p3/2 electrons. Each signal can be deconvoluted into three peaks. Taking Ti2p3/2, one can observe a peak at 458.9 eV, which is related to the Ti(IV) ions of bulk anatase.49 The main peak at 457.5 eV and the shoulder at 456.0 eV are correlated to reduced species of titanium. Based on a comparison with literature data,54 we can tentatively attribute these peaks to Ti(III) ions in different chemical environments. The Ti(III)/Ti(IV) ratio in the sample is ∼2.70, which is ca. thirty times higher than the values obtained by Li and coworkers for Ag/TiO2 photocatalysts prepared via different methods.54
In the O1s region, the highest intense peak at 529.8 eV is attributed to the lattice oxygen (Ti–O–Ti) in anatase, while the peak at 531.3 eV is related to surface hydroxyl groups (Ti–OH).55 A third and well resolved peak at 527.1 eV is only observed for the doped samples, confirming the presence of oxygen ions in a different chemical environment. Recently, Jones and coworkers have used DFT calculations to attribute this low O1s binding energy to atomic oxygen adsorbed on Ag0 surfaces.56 For the samples studied here, the presence of atomic oxygen on Ag0 clusters can be interpreted as a residue of the Ag2O thermal composition and it is in agreement with the high Ti(III) concentration on the composite surface.
The absence of any X-ray diffraction peak related to Ag oxides indicates that the Agn+ ions are homogenously dispersed on the TiO2 surface, acting as substituents. Moreover, the high Ti(III)/Ti(IV) ratio on the surface of the photocatalyst confirms a strong electronic interaction between the TiO2 matrix and the Ag sites. This strong interaction likely occurs due the addition of AgNO3 while the hydrolysis and condensation of the TiO2 nanoparticles are not completed. The ultrasonic stirring ensures that Ag+ ions can intercalate into the TiIV–O–TiIV chains and interact with the terminal O2− ions. Further annealing of the solid in air favors the thermal decomposition of Ag2O to Ag0 on the surface, but also the reduction of Ti(IV) by Ag(I) ions to yield Ti(III), Ag(II) and Ag(III) ions.
Therefore, the as prepared photocatalysts are constituted by anatase nanocrystals doped with both Ag0 islands and Agn+ ions, homogeneously dispersed on the semiconductor surface. The metallic silver exhibits plasmonic absorption bands in the visible region, while the Agn+ ions induce the formation of oxygen vacancies (TiIII centers) and act as electron traps by introducing new allowed electronic states below the TiO2 conduction band.57 These two effects enhance visible light absorption of the photocatalysts and possibly decrease the electron–hole recombination rate. As a result, the as prepared Ag-doped TiO2 nanoparticles exhibit suitable properties to be used as photocatalysts.
The photocatalytic activity of the different samples was evaluated against the degradation of the E102 tartrazine dye under different pH conditions. The degradation rates were measured by both absorption and TOC measurements. Control experiments in the absence of any photocatalyst reveal very low dye decolorization (4.75%) and mineralization (2.3%) after 120 minutes irradiation (ESI, Fig. S3†). The degradation efficiency for the different photocatalysts as a function of the pH is summarized in Table 2. In the three investigated pHs, TiO2–Ag 5.0% exhibited the highest degradation rates among the studied photocatalysts. The mineralization process follows Langmuir–Hinshelwood kinectics,58 with a pseudo-first order in relation to the tartrazine dye, as shown in Fig. 7 for pH = 6.9 (the data for the other pHs can be found in Fig. S4 of the ESI†). The observed rate constants are listed in Table 2.
| Bare TiO2 | TiO2–Ag 0.5% | TiO2–Ag 2.0% | TiO2–Ag 5.0% | |
|---|---|---|---|---|
| pH 3.0 | ||||
| Mineralization (%) | 18 | 17 | 19 | 23 |
| kobs (×103 min−1) | 1.34 | 1.53 | 1.85 | 2.12 |
| Decolorization (%) | 71.0 | 69.0 | 53.2 | 81.5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| pH 6.9 | ||||
| Mineralization (%) | 26 | 25 | 32 | 40 |
| kobs (×103 min−1) | 2.33 | 2.42 | 3.04 | 4.15 |
| Decolorization (%) | 62.5 | 61.0 | 72.0 | 76.0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| pH 8.0 | ||||
| Mineralization (%) | 28 | 31 | 35 | 37 |
| kobs (×103 min−1) | 2.46 | 2.90 | 3.44 | 4.03 |
| Decolorization (%) | 64.1 | 71.0 | 62.0 | 76.5 |
In all pHs, less than 2% of the dye is adsorbed by the powders, indicating that the photodegradation mechanism should involve the formation of reactive oxygen species (ROS), such as HO˙ or O2˙−, as intermediates.23,59 Better mineralization efficiencies at neutral and alkaline pHs can be observed, which is justified by the decrease of the H2O/HO˙ oxidation potential. Thus, under neutral and alkaline conditions, the thermodynamic barrier to the formation of ROS species is lower, leading to more efficient mineralization of the dye.
The improved efficiency of the TiO2–Ag 5.0% photocatalyst can be explained from the following considerations: the addition of Ag+ ions during the course of Ti(IV) hydrolysis leads to the creation of a strong interaction between the metallic ions, mediated by oxygen bridges. Further annealing promotes Ag2O decomposition into Ag0, which improves light harvesting in the visible region (SPR effect). Moreover, the presence of Ag+ ions induces the formation of oxygen vacancies on the titania surface, reducing the electron/hole recombination. The increase of surface area as a function of Ag content should also be mentioned, which favors the photocatalytic process.
During the course of photocatalysis, the oxidized silver species present on the TiO2 surface are irreversibly reduced to metallic silver, as can be inferred by the reflectance spectra of the photocatalyst exposed to different irradiation times (Fig. 8). After 1 and 2 hours of UVA irradiation, the powder was collected by filtration, washed with water and dried at 80 °C, then the diffuse reflectance spectra were acquired. One can observe the increase of the Ag0 plasmonic absorption band with a maximum at ∼440 nm due to the in situ photoreduction of Agn+ ions present on TiO2. If the catalyst is recycled and used for the degradation of a fresh dye solution (pH = 6.9), up to 85% decolorization is observed (Fig. S5, ESI†), showing that the catalyst remains highly active after the first use.
Based on the characterization of the doped catalysts and their photocatalytic activity, we can propose a mechanism, shown in eqn (1)–(9). Following light excitation ((1) and (2)), charge recombination (3) competes with electron trapping by the Ag sites (4). Electron transfer to the Ag sites reduces the charge recombination rate (5) and allows a more effective reaction between the surface trapped holes and electrons with H2O (6) and O2 (7) to yield ROS able to mineralize the dye ((8) and (9)).
| TiO2 + hν → e− + h+ | (1) |
| Ag0 + hv → Ag* | (2) |
e−(cb) + h+(vb) → TiO2 + hν (or![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) heat), fast heat), fast
| (3) |
| e−(cb) + Ag+n → Agn−1 | (4) |
| Agn−1 + h+(vb) → TiO2 + Ag+n, slow | (5) |
| h+(vb) + H2O → H+ + HO˙ | (6) |
| Agn−1 + O2 → Ag+n + O2˙− | (7) |
| O2˙− + dye → CO2 + H2O | (8) |
| HO˙ + dye → CO2 + H2O | (9) |
The presence of silver improves the light harvesting ability of the photocatalyst as well as decreases the charge recombination rate, since it acts as an effective electron acceptor. Despite that, the doping method employed here induces the formation of oxygen vacancies on TiO2, which are critical to improve charge concentration on the oxide surface. As a result, the photocatalytic activity is considerably improved.
4. Conclusions
Ag-doped TiO2 nanoparticles with improved photocatalytic activity were obtained via a sol–gel method followed by thermal treatment. The addition of Ag+ ions during hydrolysis/condensation of the Ti(IV) precursor allowed an effective doping of TiO2, leading to the formation of oxygen vacancies as well as Agn+ ions, homogeneously dispersed in the titania surface. The oxidized silver species are thermally or photochemically reduced to Ag0 clusters, resulting in an enhancement of light absorption in the visible region associated to plasmonic absorption bands. The photocatalytic tests employing the E102 tartrazine dye showed that the Agn+/0 sites act as effective electron acceptors, improving the quantum yields of the production of reactive oxygen species. As a result, the organic substrate was efficiently degraded with mineralization rates 78% faster than the bare TiO2 photocatalyst. This new methodology can be easily adapted to other metallic ion doping agents and different oxides. Further studies to improve the metal loading as well as the thermal treatment step can lead to the production of even more efficient materials for photocatalytic applications.Acknowledgements
The authors are thankful to the Brazilian research agencies FAPEMIG, CAPES, and CNPq, for funding this study and also to Mr Paulo Souza Müller Jr for technical support. This work was also funded by the Rede Mineira de Química (RQ-MG), supported by FAPEMIG (Project: CEX - RED-00010-14).References
- P. V. R. K. Ramacharyulu, J. P. Kumar, G. K. Prasad and A. R. Srivastava, RSC Adv., 2015, 5, 1309–1314 RSC
.
- H. Choi, E. Stathatos and D. D. Dionysiou, Appl. Catal., B, 2006, 63, 60–67 CrossRef CAS
.
- Y. Q. Qu and X. F. Duan, Chem. Soc. Rev., 2013, 42, 2568–2580 RSC
.
- A. E. H. Machado, A. O. T. Patrocinio, M. D. França, L. M. Santos, K. A. Borges and L. F. Paula, in Materials and processes for energy: communicating current research and technological developments, ed. A. Méndez-Vilas, Formatex, Spain, 2013, pp. 867–879 Search PubMed
.
- A. O. T. Patrocinio, J. Schneider, M. D. Franca, L. M. Santos, B. P. Caixeta, A. E. H. Machado and D. W. Bahnemann, RSC Adv., 2015, 5, 70536–70545 RSC
.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed
.
- A. E. H. Machado, L. M. Santos, K. A. Borges, P. S. Batista, V. A. B. Paiva, P. S. Müller Jr., D. F. M. Oliveira and M. D. França, in Solar Radiation, ed. E. B. Babatunde, InTech, Rijeka, Croácia, 2012, ch. 19, p. 339 Search PubMed
.
- X. J. Lang, X. D. Chen and J. C. Zhao, Chem. Soc. Rev., 2014, 43, 473–486 RSC
.
- Y. R. Liu, W. H. Hu, X. L. Yang, G. Q. Han, B. Dong, Y. M. Chai, Y. Q. Liu and C. G. Liu, Mater. Lett., 2015, 143, 181–184 CrossRef CAS
.
- A. Zielinska, E. Kowalska, J. W. Sobczak, I. Lacka, M. Gazda, B. Ohtani, J. Hupka and A. Zaleska, Sep. Purif. Technol., 2010, 72, 309–318 CrossRef CAS
.
- W. Xie, Y. Z. Li, W. Sun, J. C. Huang, H. Xie and X. J. Zhao, J. Photochem. Photobiol., A, 2010, 216, 149–155 CrossRef CAS
.
- A. O. T. Patrocinio, L. F. Paula, R. M. Paniago, J. Freitag and D. W. Bahnemann, ACS Appl. Mater. Interfaces, 2014, 6, 16859–16866 CAS
.
- A. A. Ismail, R. A. Geioushy, H. Bouzid, S. A. Al-Sayari, A. Al-Hajry and D. W. Bahnemann, Appl. Catal., B, 2013, 129, 62–70 CrossRef CAS
.
- H. H. Mohamed, R. Dillert and D. W. Bahnemann, Chem.–Eur. J., 2012, 18, 4314–4321 CrossRef CAS PubMed
.
- A. A. Ismail and D. W. Bahnemann, J. Phys. Chem. C, 2011, 115, 5784–5791 CAS
.
- R. Jaiswal, J. Bharambe, N. Patel, A. Dashora, D. C. Kothari and A. Miotello, Appl. Catal., B, 2015, 168, 333–341 CrossRef
.
- L. G. Devi, N. Kottam, B. N. Murthy and S. G. Kumar, J. Mol. Catal. A: Chem., 2010, 328, 44–52 CrossRef CAS
.
- S. W. Verbruggena, J. Photochem. Photobiol., C, 2015, 24, 64–82 CrossRef
.
- J. Lu, F. Su, Z. Huang, C. Zhang, Y. Liu, X. Ma and J. Gong, RSC Adv., 2013, 3, 720–724 RSC
.
- S. G. Kumar and L. G. Devi, J. Phys. Chem. A, 2011, 115, 13211–13241 CrossRef CAS PubMed
.
- T. X. Liu, B. X. Li, Y. G. Hao, F. Han, L. L. Zhang and L. Y. Hu, Appl. Catal., B, 2015, 165, 378–388 CrossRef CAS
.
- Z. F. Jiang, W. Wei, D. J. Mao, C. Chen, Y. F. Shi, X. M. Lv and J. M. Xie, Nanoscale, 2015, 7, 784–797 RSC
.
- M. B. Suwarnkar, R. S. Dhabbe, A. N. Kadam and K. M. Garadkar, Ceram. Int., 2014, 40, 5489–5496 CrossRef CAS
.
- M. K. Kumar, S. Krishnamoorthy, L. K. Tan, S. Y. Chiam, S. Tripathy and H. Gao, ACS Catal., 2011, 1, 300–308 CrossRef CAS
.
- M. R. Khan, T. W. Chuan, A. Yousuf, M. N. K. Chowdhury and C. K. Cheng, Catal. Sci. Technol., 2015, 5, 2522–2531 CAS
.
- Y. B. Xie, Y. Y. Jin, Y. Z. Zhou and Y. Wang, Appl. Surf. Sci., 2014, 313, 549–557 CrossRef CAS
.
- F. M. D. Chequer, G. A. R. Oliveira, E. R. A. Ferraz, J. C. Cardoso, M. V. B. Zanoni and D. P. Oliveira, in Eco-Friendly textile dyeing and finishing, ed. M. Günay, InTech, Rijeka, Croácia, 2013, ch. 6, pp. 151–176 Search PubMed
.
- P. Oancea and V. Meltzer, Chem. Pap., 2014, 68, 105–111 CAS
.
- E. P. Barrett, L. G. Joyner and P. P. Halenda, J. Am. Chem. Soc., 1951, 73, 373–380 CrossRef CAS
.
- E. M. Patterson, C. E. Shelden and B. H. Stockton, Appl. Opt., 1977, 16, 729–732 CrossRef CAS PubMed
.
- A. O. T. Patrocínio, E. B. Paniago, R. M. Paniago and N. Y. Murakami Iha, Appl. Surf. Sci., 2008, 254, 1874–1879 CrossRef
.
- D. F. M. Oliveira, P. S. Batista, P. S. Muller, V. Velani, M. D. Franca, D. R. de Souza and A. E. H. Machado, Dyes Pigm., 2012, 92, 563 CrossRef CAS
.
- A. A. Cavalheiro, J. C. Bruno, M. J. Saeki, J. P. S. Valente and A. O. Florentino, J. Mater. Sci., 2008, 43, 602–608 CrossRef CAS
.
- K. S. W. Sing, Pure Appl. Chem., 1982, 54, 2201–2218 CrossRef
.
- N. Sobana, M. Muruganadham and M. Swaminathan, J. Mol. Catal. A: Chem., 2006, 258, 124–132 CrossRef CAS
.
- L. Pinho, M. Rojas and M. J. Mosquera, Appl. Catal., B, 2015, 178, 144–154 CrossRef CAS
.
- R. Sellappan, M. G. Nielsen, F. González-Posada, P. C. K. Vesborg, I. Chorkendorff and D. Chakarov, J. Catal., 2013, 307, 214–221 CrossRef CAS
.
- S. Linic, P. Christopher and D. B. Ingram, Nat. Mater., 2011, 10, 911–921 CrossRef CAS PubMed
.
- E. P. Meliána, O. O. Díaza, J. M. D. Rodrígueza, G. Colón, J. A. Navío, M. Macías and J. P. Peña, Appl. Catal., B, 2012, 127, 112–120 CrossRef
.
- A. Bumajdad and M. Madkour, Phys. Chem. Chem. Phys., 2014, 16, 7146–7158 RSC
.
- X. B. Chen, Y. B. Lou, A. C. S. Samia, C. Burda and J. L. Gole, Adv. Funct. Mater., 2005, 15, 41–49 CrossRef CAS
.
- M. C. Mathpal, A. K. Tripathi, M. K. Singh, S. P. Gairola, S. N. Pandey and A. Agarwal, Chem.Phys.
Lett., 2013, 555, 182–186 CrossRef CAS
.
- M. N. Iliev, V. G. Hadjiev and A. P. Litvinchuk, Vib. Spectrosc., 2013, 64, 148–152 CrossRef CAS
.
- H. Fang, C. X. Zhang, L. Liu, Y. M. Zhao and H. J. Xu, Biosens. Bioelectron., 2015, 64, 434–441 CrossRef CAS PubMed
.
- J. C. Parker and R. W. Siegel, Appl. Phys. Lett., 1990, 57, 943–945 CrossRef CAS
.
- C. A. Melendres, A. Narayanasamy, V. A. Maroni and R. W. Siegel, J. Mater. Res., 1989, 4, 1246–1250 CrossRef CAS
.
- J. C. Parker and R. W. Siegel, J. Mater. Res., 1990, 5, 1246–1252 CrossRef CAS
.
- Y. C. Xu and H. You, Appl. Surf. Sci., 2014, 321, 481–487 CrossRef CAS
.
- N. D. Feng, Q. Wang, A. M. Zheng, Z. F. Zhang, J. Fan, S. B. Liu, J. P. Amoureux and F. Deng, J. Am. Chem. Soc., 2013, 135, 1607–1616 CrossRef CAS PubMed
.
- G. I. N. Waterhouse, G. A. Bowmaker and J. B. Metson, Phys. Chem. Chem. Phys., 2001, 3, 3838–3845 RSC
.
- H. Zhang, G. Wang, D. Chen, X. J. Lv and U. H. Jinghong, Chem. Mater., 2008, 20, 6543–6549 CrossRef CAS
.
- J. L. X. Baifu, Z. Ren, B. Wang and H. Fu, J. Phys. Chem., 2005, 109, 2805–2809 CrossRef PubMed
.
- K. V. B. R. Priya, S. Shukla, S. Biju, M. L. P. Reddy, K. Patil and K. G. K. Warrier, J. Phys. Chem. C, 2009, 113, 6243–6255 Search PubMed
.
- J. X. Li, J. H. Xu, W. L. Dai and K. N. Fan, J. Phys. Chem. C, 2009, 113, 8343–8349 CAS
.
- J. Y. Ruzick, F. Abu Bakar, L. Thomsen, B. C. Cowie, C. McNicoll, T. Kemmitt, H. E. A. Brand, B. Ingham, G. G. Andersson and V. B. Golovko, RSC Adv., 2014, 4, 20649–20658 RSC
.
- T. E. Jones, T. C. R. Rocha, A. Knop-Gericke, C. Stampfl, R. Schlogl and S. Piccinin, Phys. Chem. Chem. Phys., 2015, 17, 9288–9312 RSC
.
- Y. Huang, Z. Xuxu, Y. Zhongyi, T. Feng, F. Beibei and H. Keshan, Chin. J. Chem. Eng., 2007, 15, 802–807 CrossRef
.
- M. R. Hoffmann, S. T. Martin, W. Y. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS
.
- T. D. Pham and B. K. Lee, J. Colloid Interface Sci., 2014, 428, 24–31 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra22647c |
| This journal is © The Royal Society of Chemistry 2015 |















