Colloidal synthesis of marcasite FeS2 nanoparticles with improved electrochemical performance†
Tingting Liab,
Zuoxing Guoa,
Xiaoying Lic,
Zhennan Wub,
Kuo Zhangb,
Huiwen Liub,
Haizhu Sunc,
Yi Liub and
Hao Zhang*b
aKey Laboratory of Automobile Materials, Ministry of Education, College of Materials Science and Engineering, Jilin University, Changchun 130022, P. R. China
bState Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: hao_zhang@jlu.edu.cn
cFaculty of Chemistry, Northeast Normal University, Changchun 130024, P. R. China
First published on 13th November 2015
Abstract
Marcasite FeS2 nanoparticles were synthesized for the first time in colloidal solution via a hot-injection protocol. In comparison with the previous iron sulfides, marcasite FeS2 presents better lithium ion storage and charge–discharge performance as the anode materials in lithium ion battery application.
Iron sulfide nanomaterials are promising anode materials for lithium ion batteries (LIBs) owing to the advantages of high-capacity, environmental friendliness, low cost, and so forth.1 Although lots of works have been devoted to troilite FeS, greigite Fe3S4, and pyrite FeS2 as anode materials, the development of high performance iron sulfides anodes is still challenging.2–4 For instance, pyrite FeS2 nanoparticles (NPs) are one of the most potential anode materials for LIBs, because of the high theoretical capacity of 890 mA h g−1.5 However, the real discharge capacity of pure pyrite NPs is only half of the theoretical capacity. This is attributed to the dependence of LIBs performance upon intercalation/extraction-related lithium ion diffusion pathways, which is very sensitive to the crystalline structure/phase of electrode materials.6,7 In this scenario, pyrite FeS2 possesses cubic crystal structure (space group Pa
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , Z = 4) with Wyckoff position 4a(0,0,0) for Fe and 8c(u,u,u) for S.8 According to the previous analysis, such structure is not the best for lithium ion diffusion.9,10 To improve the LIBs performance of FeS2, the NPs with optimized crystal structure should be investigated.
, Z = 4) with Wyckoff position 4a(0,0,0) for Fe and 8c(u,u,u) for S.8 According to the previous analysis, such structure is not the best for lithium ion diffusion.9,10 To improve the LIBs performance of FeS2, the NPs with optimized crystal structure should be investigated.
Marcasite is another natural mineral of FeS2, which commonly distributes in hydrothermal systems and sedimentary rocks.11 Until now, marcasite FeS2 nanomaterials have never been synthesized by chemical method. The investigations merely rest on theoretical prediction and the direct utilization of natural marcasite.12,13 As to the orthorhombic structure of marcasite FeS2, the space group is Pnnm, Z = 2 with Wyckoff position 2a(0,0,0) for Fe and 4g(u,v,0) for S.14 Such structure is considered better than pyrite for lithium ion diffusion.10 However, limited by the rare synthesis of orthorhombic FeS2 nanomaterials, the LIBs performance of marcasite has not been tested yet. In the conventional synthesis of FeS2 NPs, oriented attachment (OA) and fusion of small FeS2 clusters is the major process for NP growth, which favors the formation of pyrite NPs with cubic structure.15 According to the reports in synthesizing II–VI semiconductor NPs, Ostwald ripening (OR) process via monomer diffusion may facilitate the growth of orthorhombic NPs.16,17 Therefore, marcasite FeS2 NPs are considered to be produced by converting the growth kinetics of FeS2 NPs from OA to OR.
In this work, we demonstrate the facile and controlled synthesis of marcasite NPs with orthorhombic crystalline structure for the first time in colloidal solution via hot-injection protocol. The as-synthesized NPs are well dispersible in non-polar solvents. The formation of marcasite FeS2 NPs rather than pyrite is attributed to the OR-determined growth of NPs, which is quite different from the previous OA-determined process. The as-synthesized marcasite NPs are further fabricated to coin cell batteries, which exhibit low electrochemical resistance and high activity in lithiation/delithiation cycling process.
As shown in the Experimental section of ESI,† marcasite FeS2 NPs are synthesized by injecting a sulfur diphenyl ether (DE) solution into the mixtures of Fe(acac)3, DE, and dodecanethiol (DT) at 220 °C and stirring for 1 h. After purification, the products are characterized in Fig. 1. As revealed by transmission electron microscopy (TEM), the products appear as spherical particles with the average diameter of 7.1 nm and a size deviation of 40% (Fig. 1a). Slight aggregation of NPs is observed by TEM, which may be attributed to two reasons. One is the high surface energy leading from the small size of NPs. The other is the intrinsic ferromagnetism of FeS2. Under high-resolution TEM (HRTEM), an interplanar spacing of 0.276 nm is observed (Fig. 1b), which corresponds to the (101) plane of marcasite FeS2.18 No the sign of oxide coating or amorphous overlay is found. The selected area electron diffraction (SAED) pattern indicates that the NPs are single-phase marcasite FeS2 without other iron sulfide signal (Fig. 1c). Low resolution TEM image and the corresponding energy-dispersive X-ray spectroscopy (EDS) elemental maps of Fe and S are displayed in Fig. 1d–f, which reveals the homogenous distribution of Fe and S elements. The products possess the Fe/S molar ratio of 1/1.97 from the EDS data (Fig. 1g), which consists well with the stoichiometric ratio of FeS2. Fig. 1h presents the X-ray diffraction (XRD) pattern. All the diffraction peaks can be exclusively attributed to the marcasite FeS2 with orthorhombic crystal phase.18 This crystal phase consists thus of an hcp stacked sulfur atoms, where the tetrahedra between a pair of hexagonal sheets is empty and the octahedra is alternatively empty or occupied by iron atom.8 Such atomic arrangement is greatly different from pyrite.
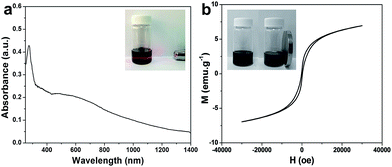
The as-synthesized marcasite NPs are well dispersible in non-polar solvents due to the capping ligands of DT. The uniform dispersion is revealed by the classical Tyndall effect (Fig. 2a).19 In addition, the NPs exhibit weak ferromagnetism that can be separated from the solution with the help of a magnet (Fig. 2b). The saturation magnetization is 6.95 emu g−1, which is among the highest level of ever reported small iron sulfides NPs. This further confirms the products are marcasite FeS2 rather than pyrite, because pyrite FeS2 has no magnetism. As revealed by TEM, the as-synthesized marcasite NPs are stable as heating at 300 °C for 2 h under Ar atmosphere (Fig. S1a†). TGA profile shows that the weight loss at 100, 217, 376, and 900 °C is 2.3, 9.8, 18.1, and 27.6%, respectively. The weight loss of the first three is attributed to water, ligand, and the phase transformation from marcasite to pyrite. Accordingly, the calculated content of marcasite FeS2 and the capping ligand of DT in the NPs is 90.2 and 7.5%, respectively.
The formation of marcasite FeS2 NPs is led from the hot-injection protocol, which converts the growth kinetics of FeS2 NPs from OA to OR, because the crystal phase of FeS2 NPs greatly depends on the formation mechanism.20 The temporal evolution of the NPs is monitored by TEM images and absorption spectra (Fig. S2 and S3†). Before the injection of sulfur solution, the nucleus already form by the reduction of Fe(acac)3 and the S released from DT decomposition (Fig. S2a†). As revealed by TEM, the average diameter of nucleus is 2.7 nm (Fig. S2b†). Subsequently, the S solution is injected into the Fe solution to supply the further growth of FeS2 NPs. Accompanied with the growth of NPs, the number of original nucleus rapidly reduces within 10 min (Fig. S2c–f and S3†). According to the numerous reports about the growth of colloidal NPs, such property is typical for OR process. Namely, the growth of bigger NPs is supplied by the dissolution of smaller ones through a monomer diffusion controlled process (Fig. S2g†).21 In comparison, OA mechanism involves the attachment of the specific lattice plane of small particles and further fusion, which are not observed in the current synthesis of marcasite FeS2 NPs.20 On the basis of OR growth mechanism, the size of marcasite FeS2 NPs is further controlled by regulating the reactivity of Fe monomers. As a dominant factor in controlling Fe reactivity, the growth temperature is altered from 180 to 260 °C, while other parameters are fixed. Accordingly, the diameter of NPs increases from 3.5, 5.0, 10, to 18 nm (Fig. S4†). Another factor that influences Fe reactivity is the amount of DT, which acts as the capping ligand (Fig. S5†). As increasing DT amount from 0.5 to 5 mL (2 to 20 mmol), the NPs size increases from 3.4 to 8.1 nm.
The good structural stability of the as-synthesized marcasite FeS2 NPs under heating treatment permits the application as LIBs anode materials (Fig. S1a†). The electrochemical properties of marcasite FeS2 NPs based electrode are further evaluated (Fig. 3). Electrochemical impedance spectroscopy (EIS) of an unused coin cell is firstly examined from 10 kHz to 10 MHz (Fig. 3a). In the higher frequency region, there is a semicircle that is attributed to the charge transfer resistance, which relates to the reaction between the active materials and electrolyte interface.3 The charge transfer resistance of the electrode is approximately 50 ohm, which is the smallest value of ever reported FeS2 materials.22 The straight line, named Warburg line, in the low frequency region is attributed to the ion diffusion from electrolyte to the electrode interface.23 On the basis of the precondition of good conductivity, marcasite FeS2 NPs should be ideal electrode materials in the coin cell.
In order to verify the performance and the reaction inside of a coin cell, the galvanostatic charge–discharge curve with the selected cycles is evaluated in the voltage window of 0.005–3 V (vs. Li+/Li) at a current density of 100 mA g−1. Fig. 3b shows that the open-circuit voltage of the cell is 2.42 V. At the reaction of the charge–discharge stage, the voltage rapidly declines at round 1.36 V to reach a plateau set until the capacity of 348 mA h g−1. Both the reduction from Fe2+ to Fe0 and the lithium transfer to FeS2 are capable to cause the discharge plateau.4 Another voltage plateau appears at about 0.8 V, which is observed only for the first cycle. It is associated with the formation of solid electrolyte interlayer (SEI) film.24 To completely understand the reaction on the electrode, the cyclic voltammetry (CV) is evaluated in the voltage range of 0.05–3.0 V with the scanning rate of 0.5 mV s−1 (Fig. 3c). The reduction peaks at 0.67, 1.16, 1.29, and 1.67 V are indicative of the single-phase insertion of Li and reduction of Fe2+, accompanying with the formation of the SEI film.5 The peaks at 1.51 and 2.83 V correspond to the oxidation of Fe0.4 At the first cycle, the internal reaction of a coin cell is complicated. Subsequently, the current intensity decreases rapidly, implying the existence of an irreversible process in the charge transfer.
The cycling performance of the coin cell at a current density of 100 mA g−1 is shown in Fig. 3d. The discharge capacity decreases continuously from 1240 mA h g−1 at the first cycle to 210 mA h g−1 at the 16th cycle. The huge first cycle loss is mainly attributed to the formation of solid electrolyte interlayer (SEI) film between the electrolyte and FeS2-based electrode, which possesses larger specific surface area due to the small size of marcasite FeS2 NPs.3 This SEI film consumes a large amount of Li+. As a result, the charge–discharge capacity decreases for the first cycle. The irreversible loss at first ten cycles is attributed to the side reaction between Li+ and active materials.5 Another reason for capacity decrease is attributed to the defects in marcasite FeS2 NPs before the 16th cycle. After the 16th cycle, the system builds a balance between active materials and Li+ diffusion. So, the capacity does not decrease any longer. Afterwards, the discharge capacity indicates a steady rising to 641 mA h g−1 until 100th cycle, accompanying with almost 100% coulombic efficiency that suggests the good cyclability of the marcasite FeS2 electrode.25 According to the previous reports, the increase of capacity from the 16th to the 100th cycle indicates an activation process in the FeS2 electrode.26 As far as we know, both the initial discharge capacity of 1240 mA h g−1 and the balance discharge capacity of 641 mA h g−1 are highest for ever reported iron sulfides as LIBs anode.2–5 Furthermore, the marcasite FeS2 NPs with different sizes are employed for electrochemical tests. The NPs with average diameter of 7.1 nm exhibit better electrochemical performance than the NPs with other sizes. The reason is attributed to the dependence of battery performance on the surface area and migration distance of the building blocks.27 Associated with the low resistance and stable charge–discharge performance, the marcasite FeS2 NPs are promising electrode materials in LIBs application.
In summary, we demonstrate the hot-injection synthesis of marcasite FeS2 NPs in colloidal solution. The formation of marcasite FeS2 rather than conventional pyrite is attributed to the OA growth mechanism. Most importantly, marcasite NPs exhibit better electrochemical performance as LIBs anode than pyrite and other iron sulfides.
Acknowledgements
This work was supported by the 973 Program of China (2014CB643503), NSFC (51425303, 21374042, 21174051), Natural Science Foundation of Jilin Province (20140101048JC), the Special Project from MOST of China, and the Fundamental Research Funds for the Central Universities.Notes and references
- X. Rui, H. Tan and Q. Yan, Nanoscale, 2014, 6, 9889 RSC.
- C. Xu, Y. Zeng, X. Rui, N. Xiao, J. Zhu, W. Zhang, J. Chen, W. Liu, H. Tan, H. H. Hng and Q. Yan, ACS Nano, 2012, 6, 4713 CrossRef CAS PubMed.
- G. Li, B. Zhang, F. Yu, A. A. Novakova, M. S. Krivenkov, T. Y. Kiseleva, L. Chang, J. Rao, A. O. Polyakov, G. R. Blake, R. A. de Groot and T. T. M. Palstra, Chem. Mater., 2014, 26, 5821 CrossRef CAS.
- L. Li, M. Cabán-Acevedo, S. N. Girard and S. Jin, Nanoscale, 2014, 6, 2112 RSC.
- J. Liu, Y. Wen, Y. Wang, P. A. van Aken, J. Maier and Y. Yu, Adv. Mater., 2014, 26, 6025 CrossRef CAS PubMed.
- S. I. Nishimura, G. Kobayashi, K. Ohoyama, R. Kanno, M. Yashima and A. Yamada, Nat. Mater., 2008, 7, 707 CrossRef CAS PubMed.
- H. Wang, X. Wang, L. Wang, J. Wang, D. Jiang, G. Li, Y. Zhang, H. Zhong and Y. Jiang, J. Phys. Chem. C, 2015, 119, 10197 CAS.
- V. K. Gudelli, V. Kanchana, S. Appalakondaiah, G. Vaitheeswaran and M. C. Valsakumar, J. Phys. Chem. C, 2013, 117, 20120 Search PubMed.
- H. Tang, J. Wang, H. Yin, H. Zhao, D. Wang and Z. Tang, Adv. Mater., 2015, 27, 1117 CrossRef CAS PubMed.
- Y. Tang, Y. Zhang, J. Deng, D. Qi, W. R. Leow, J. Wei, S. Yin, Z. Dong, R. Yazami, Z. Chen and X. Chen, Angew. Chem., Int. Ed., 2014, 53, 13488 CrossRef CAS.
- D. Rickard and G. W. Luther, Chem. Rev., 2007, 107, 514 CrossRef CAS PubMed.
- R. Sun, M. K. Y. Chan and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 235311 CrossRef.
- J. B. Murowchick and H. L. Barnes, Geochim. Cosmochim. Acta, 1986, 50, 2615 CrossRef CAS.
- M. Nespolo and B. Souvignier, Cryst. Res. Technol., 2015, 50, 442 CrossRef CAS.
- Y. Bai, J. Yeom, M. Yang, S. H. Cha, K. Sun and N. A. Kotov, J. Phys. Chem. C, 2013, 117, 2567 CAS.
- D. V. Talapin, A. L. Rogach, E. V. Shevchenko, A. Kornowski, M. Haase and H. Weller, J. Am. Chem. Soc., 2002, 124, 5782 CrossRef CAS PubMed.
- W. Niu, S. Zheng, D. Wang, X. Liu, H. Li, S. Han, J. Chen, Z. Tang and G. Xu, J. Am. Chem. Soc., 2009, 131, 697 CrossRef CAS PubMed.
- J. Puthussery, S. Seefeld, N. Berry, M. Gibbs and M. Law, J. Am. Chem. Soc., 2011, 133, 716 CrossRef CAS PubMed.
- S. Hu and X. Wang, J. Am. Chem. Soc., 2010, 132, 9573 CrossRef CAS.
- L. Zhu, B. J. Richardson and Q. Yu, Chem. Mater., 2015, 27, 3516 CrossRef CAS.
- X. Peng, J. Wickham and A. P. Alivisatos, J. Am. Chem. Soc., 1998, 120, 5343 CrossRef CAS.
- M. R. Gao, Y. F. Xu, J. Jiang and S. H. Yu, Chem. Soc. Rev., 2013, 42, 2986 RSC.
- W. Ai, Z. Luo, J. Jiang, J. Zhu, Z. Du, Z. Fan, L. Xie, H. Zhang, W. Huang and T. Yu, Adv. Mater., 2014, 26, 6186 CrossRef CAS PubMed.
- R. Mo, S. O. Tung, Z. Lei, G. Zhao, K. Sun and N. A. Kotov, ACS Nano, 2015, 9, 5009 CrossRef CAS PubMed.
- Y. Tang, Y. Zhang, W. Li, B. Ma and X. Chen, Chem. Soc. Rev., 2015, 44, 5926 RSC.
- Y. Du, Z. Yin, J. Zhu, X. Huang, X. J. Wu, Z. Zeng, Q. Yan and H. Zhang, Nat. Commun., 2012, 3, 1177 CrossRef PubMed.
- Q. Zhang, E. Uchaker, S. L. Candelaria and G. Cao, Chem. Soc. Rev., 2013, 42, 3127 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: TGA profile, absorption spectra, and TEM images of the as-synthesized FeS2 NPs. See DOI: 10.1039/c5ra22610d |
| This journal is © The Royal Society of Chemistry 2015 |