The potential cytotoxicity and mechanism of VO2 thin films for intelligent thermochromic windows†
Abstract
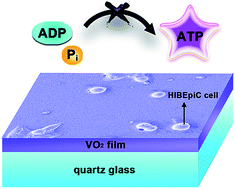
A thermochromic vanadium dioxide (VO2) film holds great promise for intelligent windows owing to marvelous semiconductor-metal transitions (SMT), an active response to external temperature stimuli and near-infrared irradiation. To date, however, its potential biological effect on human cells has not been well characterized. In this work, homogeneous high quality VO2 films with different thicknesses (30, 80, 120 nm) were prepared on a quartz glass substrate. Afterwards, for the first time, we demonstrate the time-dependent and dose-dependent cytotoxicity of the VO2 film to human cells. Speciation analysis by 51V NMR spectra and surface zeta potentials revealed the formation of vanadate(+5) and ADPV, an analogue of ATP. On the basis of energy metabolism or bioenergetics, a plausible hypothesis, i.e., “ATP dyssynthesis”, is proposed here to elucidate the potential toxicity mechanism of the VO2 nanomaterial via vanadate-phosphate antagonism including two steps: (i) vanadate speciation from VO2 surface chemistry; (ii) vanadate disturbing ATP synthesis as a phosphate analogue. ATP stores energy to carry out various life processes; once its synthesis is hindered, vital movement will be impaired. From the perspective of surface modification and bioactivation, some practical methods are recommended to compensate for the potential cytotoxicity of the VO2 material. We expect this work can stimulate scientific interest to search for more versatile material design strategies to balance the energy-saving efficiency and environmental safety of VO2 intelligent window coatings.

 Please wait while we load your content...
Please wait while we load your content...